Abstract
In addition to the well-known effects of vitamin D (VitD) in maintaining bone health, there is increasing appreciation that this vitamin may serve important roles in other organs and tissues, including the brain. Given that VitD deficiency is especially widespread among the elderly, it is important to understand how the range of serum VitD levels that mimic those found in humans (from low to high) affects the brain during aging from middle-age to old-age. To address this issue, twenty-seven male F344 rats were split into three groups and fed isocaloric diets containing low (100 IU/kg food), control (1000 IU/kg food), or high (10000 IU/kg food) VitD beginning at middle-age (12 months) and continued for a period of 4–5 months. We compared the effects of these dietary VitD manipulations on oxidative and nitrosative stress measures in posterior brain cortices. The low VitD group showed global elevation of 3-nitrotyrosine (3-NT) compared to control and high VitD treated groups. Further investigation showed that this elevation may involve dysregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and NF-κB mediated transcription of inducible nitric oxide synthase (iNOS) as indicated by translocation of NF-κB to the nucleus and elevation of iNOS levels. Proteomic techniques were used to provide insights into potential mechanisms underlying these effects. Several brain proteins were found at significantly elevated levels in low VitD group compared to the control and high VitD groups. Three of these proteins, 6-phosphofructokinase, triosephosphate isomerase, and pyruvate kinase, are involved directly in glycolysis. Two others, peroxiredoxin-3 and DJ-1/PARK7, have peroxidase activity and are found in mitochondria. Peptidyl-prolyl cis-trans isomerase A (PPIA or cyclophilin A) has been shown to have multiple roles including protein folding, regulation of protein kinases and phosphatases, immunoregulation, cell signaling, and redox status. Together, these results suggest that dietary VitD deficiency contributes to significant nitrosative stress in brain and may promote cognitive decline in middle-aged and elderly adults.
Keywords: Vitamin D, nitrosative stress, 3-nitrotyrosine, proteomics, metabolism, cognitive decline
Introduction
The steroid hormone Vitamin D (VitD) can be produced by the body or obtained through the diet. VitD is synthesized in the skin from the cholesterol precursor, 7-dehydrocholesterol and is converted to cholecalciferol (VitD3) upon exposure to sunlight [1]. VitD3 can also be obtained through several dietary sources and is transported in the blood via vitamin D-binding protein. In the liver, VitD3 is converted to calcidiol, 25-hydroxy vitamin D (25-OH VitD) followed by further conversion to calcitriol, 1α, 25-dihydroxy vitamin D (1α, 25-(OH)2VitD) primarily in the kidneys where it helps to regulate calcium homeostasis[2, 3]. VitD also plays roles in autoimmunity [4], mental health [3, 5–8], and inhibition of tumor growth through reductions in proliferation and angiogenesis[9–12].
VitD deficiency has long been associated with osteoporosis, brittle bones, and muscle weakness, but recently low levels of VitD have been linked to increased overall mortality [13, 14]. VitD status is typically assessed using serum concentration of 25-OH VitD because it is longer lived than the biologically active 1α, 25-(OH)2VitD [13, 15,16].
VitD deficiency is highly prevalent in Europe and North America[1, 17] with the elderly particularly at risk [11,13,18–20]. Current estimates suggest that as many as 40–100% of the elderly populations in these areas are VitD deficient [21]. Poor diet and lower exposure to UV-B from the sun limits VitD synthesis in the skin and an age-related decrease in the VitD synthesis machinery may contribute to the observed lower VitD levels [15].
The elderly represent those at greatest risk of age-related cognitive decline and neurodegenerative disorders [22]. Recent retrospective studies on elderly human subjects provide correlative evidence that those with VitD deficiency have a much higher incidence of cognitive impairment than those with normal VitD levels [23, 24]. Thus, it appears that VitD deficiency may accelerate cognitive decline in aging [25]. A recent meta-analysis also shows that patients with Alzheimer disease (AD) typically have lower serum concentrations of VitD [26]. AD is associated with defects in amyloid-β (Aβ) processing and an upregulation of inflammatory cytokines and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)[8]. Interestingly, 1α,25(OH)2VitD helped to reverse soluble Aβ and inflammatory issues [8]. In addition to these actions, VitD is neuroprotective against Ca2+-mediated excitotoxicity, reduces biomarkers of brain aging associated with Ca2+ dyshomeostasis [3, 5], and helps to regulate glutathione levels, a primary antioxidant in the brain, by modulating γ-glutamyltranspeptidase activity [27]. VitD also prevents onset of autoimmune demyelination in animal models of multiple sclerosis [28, 29].
Here, we manipulated serum VitD status by dietary supplementation with low, moderate/control or high levels of VitD in order to identify changes in the VitD-dependent proteome in the brains of rats from middle- to old-age. Prior studies have shown that cognitively impaired subjects have significant levels of mitochondrial dysfunction and oxidative protein damage. In particular, nitration of protein resident tyrosine residues is a common marker observed in brain of cognitively impaired subjects [30–34]. Therefore, we tested the hypothesis that manipulating serum VitD levels would alter protein nitration and key protein markers of mitochondrial function. Our results identify several possible targets of VitD action that may mechanistically link circulating VitD levels with risk for age-related cognitive decline.
Methods and Materials
Chemicals
Criterion precast polyacrylamide gels, tris-glycine-SDS (TGS) and MES electrophoresis running buffers, ReadyStrip™ IPG strips, mineral oil, Precision Plus Protein™ All Blue Standards, SYPRO Ruby® Protein Stain, nitrocellulose membranes, dithiothreitol (DTT), iodoacetamide (IA), Biolytes, and urea were purchased from Bio-RAD (Hercules, CA, USA). Chemicals, proteases, protease inhibitors, and antibodies used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
Animals
All animal studies were approved by the University of Kentucky Institutional Animal Care and Use Committee and followed NIH Guidelines for the Care and Use of Laboratory Animals. Middle-aged (12 month old) male F344 rats, a standard model for studies of brain aging, were obtained from the National Institutes on Aging rodent colony. Four-to five-month dietary manipulation of VitD was carried out using cholecalciferol (VitD3) added to an isocaloric diet in the following amounts (based on pilot studies intended to mimic the range of human levels): control VitD = 1000 IU/kg food, low VitD = 100 IU/kg food, and high VitD = 10,000 IU/kg food with nine animals in each group (Table 1). Animals were weighed and food intake measured 2–3 times per week. Serum 25-OH VitD levels were monitored as a measure of circulating VitD. Dietary manipulation of VitD resulted in different serum levels of VitD. Upon conclusion of the long-term treatment, animals were euthanatized and samples isolated from the right posterior cortical area. Samples were then stored at −80°C until needed for oxidative stress and expression proteomics determinations [35, 36].
Table 1.
VitD Dietary Manipulation [*p < 0.0001 (one-way ANOVA)]
| Control | Low | High | |
|---|---|---|---|
| Weight at 12 months | 480g | 485g | 475g |
| Weight at 18 months | 538g | 565g | 548g |
| Daily Food Intake | 16.5 g/day | 16.4 g/day | 15.9 g/day |
| Daily intake of Cholecalciferol | 16.5 IU/day | 1.64 IU/day | 159 IU/day |
| *Serum levels of 25(OH)VitD | 12.7 ng/mL | 5.8 ng/mL | 31.7 ng/mL |
Sample preparation
Protein estimation was performed using the bicinchoninic acid (BCA, Pierce) assay. Homogenized cortex samples were diluted according to initial protein estimation results 20 ug sample in 140 uL of media-I buffer [0.32 M sucrose, 0.6 mM MgCl2, and 0.125 M Tris pH 8.0 with protease inhibitors, 4 μg/ml leupeptin, 4 μg/ml pepstatin A, and 5 μg/ml aprotinin].
Slot blot assay
The slot-blot method was used to determine levels of protein 3-nitrotyrosine (3-NT) in brain as previously described [37, 38]. For 3-NT determination, samples were solubilized in Laemmli buffer. Protein (250 ng) from each sample was loaded onto a nitrocellulose membrane in respective wells in a slot-blot apparatus (Bio-Rad) under vacuum. Membranes were blocked in 3% bovine serum albumin (BSA) in PBS with 0.2% (v/v) Tween-20 for 1.5 h and then incubated in primary antibody (anti-nitrotyrosine produced in rabbit, Sigma-Aldrich) for 2 h, washed three times in PBS with 0.2% (v/v) Tween-20 and then incubated for 1 h with secondary antibody (goat anti-rabbit secondary linked to alkaline phosphatase). Membranes were developed with 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) dipotassium and nitro blue tetrazolium (NBT) chloride in alkaline phosphatase activity (ALP) buffer, dried, and scanned for analysis. Image analysis was performed using Scion Image (Scion Corporation, Frederick, MD). A negative control for nitration was performed by reducing nitrotyrosine to aminotyrosine according to the method of Miyagi and Crabb et al. [39] as follows. One replicate membrane was treated with 10 mM sodium dithionite in 50 mM pyridine-acetate buffer (pH=5.0) for 1h at room temperature, rinsed thoroughly with nanopure water, and developed as above. No staining of the membrane was observed (data not shown), indicating that the anti-3NT antibody was specific for this specific posttranslational modification.
Proteomics [35]
Isoelectric focusing (IEF)
Aliquots (containing 150ug of protein) of the homogenized cortical samples prepared above were precipitated using cold 100% trichloroacetic acid (TCA) to obtain a concentration of 15% (v/v) TCA in solution and incubated on ice for 15 minutes. Samples were centrifuged at 14,000 rpm for 10 min at 4 °C. The resulting pellets were resuspended and rinsed four times in a cold ethanol: ethyl acetate (1:1 v/v) mixture. After allowing the final pellets to dry completely at room temperature, the pellets were rehydrated for 2 h in rehydration buffer [8 M urea, 2 M thiourea, 50 mM DTT, 2.0% (w/v) CHAPS, 0.2% Biolytes, Bromophenol Blue] then sonicated for 10 s at 20% power. Each entire sample was added to an 11 cm pH 3–10 ReadyStrip™ IPG strip in a lane of the IEF tray. After 45 min of run time, 2 mLof mineral oil were added to each lane to prevent evaporation. Strips were actively rehydrated at 20 °C for 18 h at 50 V, focused at a constant temperature of 20 °C beginning at 300 V for 2 h, 500 V for 2 h, 1000 V for 2 h, 8000 V for 8 h, and finishing at 8000 V for 10 h rapidly. IPG strips were stored at −80 °C until needed for the second dimension of analysis.
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)
IEF strips were allowed to come to room temperature (~30 min) and equilibrated for 10 min in the dark in 4 mL equilibration buffer A [50 mM Tris-HCl, pH 6.8, 6 M urea, 1% (w/v) SDS, 30% v/v glycerol, 0.5% DTT] and then re-equilibrated for 10 min in the dark in equilibration buffer B [50 mM Tris-HCl, pH 6.8, 6 M urea, 1% (w/v) SDS, 30% v/v glycerol, 4.5% IA]. All strips were rinsed in TGS running buffer to remove residual equilibration buffers before being placed onto Criterion precast linear gradient (8–16%) Tris-HCl polyacrylamide gels. Precision Plus Protein™ All Blue Standards and samples were run at a constant voltage of 200 V for 65 min.
SYPRO Ruby® staining
Following 2D-PAGE, gels were incubated in 50 mL fixing solution [7% (v/v) acetic acid, 10% (v/v) methanol] for 20 min at room temperature. SYPRO Ruby® Protein Gel Stain (50–55 mL) was added to gels and allowed to stain overnight at room temperature on a gently rocking platform. The stain was then removed and gels were rinsed with deionized (DI) water and stored in 50 mL DI water in the refrigerator until scanning. Gels were scanned into Adobe Photoshop 6.0 with a Molecular Dynamics STORM phosphoimager (λex/λem: 470/618 nm) and stored in DI water at 4 °C.
Image analysis: differential expression
Spot intensities from SYPRO Ruby®-stained 2D-gel images of cortex samples were quantified according to total spot density using PDQuest software (Bio-RAD, Hercules, CA, USA). Intensities were normalized to total gel densities. Only low or high VitD samples with normalized spot densities that were significantly increased or decreased by at least 1.4-fold from control were considered for mass spectrometry (MS) analysis. This is a conservative cut-off criterion, but does greatly minimize false positives.
In-gel trypsin digestion
Protein spots identified as significantly altered in VitD deficient rat brain relative to normal VitD controls were excised from 2D-gels with new, sterilized micropipette tips and transferred to Eppendorf microcentrifuge tubes. Gel plugs were then washed with 0.1 M ammonium bicarbonate (NH4HCO3) at room temperature for 15 min, followed by incubation with 100% acetonitrile at room temperature for 15 min. Solvent was removed, and gel plugs were dried in their respective tubes in a biosafety cabinet at room temperature. Gel plugs were incubated for 45 min in 20 μl of 20 mM DTT in 0.1 M NH4HCO3 at 56 °C. The DTT solution was then removed and replaced with 20 μl of 55 mM IA in 0.1 M NH4HCO3 and incubated with gentle agitation at room temperature in the dark for 30 min. Excess IA solution was removed, and the gel plugs were incubated for 15 min with 200 μl of 50 mM NH4HCO3 at room temperature. 200 μL of 100% acetonitrile was added to this solution in each tube and incubated for 15 min at room temperature. All solvent was removed, and gel plugs were allowed to dry for 30 min at room temperature in a biosafety cabinet. Gel plugs were rehydrated with 20 ng/μL of modified trypsin (Promega, Madison, WI, USA) in 50 mM NH4HCO3 in a shaking incubator overnight at 37 °C. Enough trypsin solution was added in order to completely submerge the gel plugs (approximately 10uL).
Mass spectrometry
Salts and other contaminants were removed from tryptic digest solutions using C18 ZipTips (Sigma-Aldrich, St. Louis, MO, USA), reconstituted to a volume of approximately 15 μl in a 50:50 DI water:acetonitrile solution containing 0.1% formic acid. Tryptic peptides were analyzed with an automated Nanomate electrospray ionization (ESI) [Advion Biosciences, Ithaca, NY, USA] Orbitrap XL MS (ThermoScientific, Waltham, MA, USA) platform. The Orbitrap MS was operated in a data-dependent mode whereby the eight most intense parent ions measured in the Fourier Transform (FT) at 60,000 resolution were selected for ion trap fragmentation with the following conditions: injection time 50 ms, 35% collision energy, MS/MS spectra were measured in the FT at 7500 resolution, and dynamic exclusion was set for 120 s. Each sample was acquired for a total of approximately 2.5 min. MS/MS spectra were searched against the International Protein Index (IPI) database using SEQUEST and the following parameters: two trypsin miscleavages, fixed carbamidomethyl modification, variable Methionine oxidation, parent tolerance 10 ppm, and fragment tolerance of 25 mmu or 0.01 Da. Results were filtered with the following criteria: Xcorr>1.5, 2.0, 2.5, 3.0 for +1, +2, +3, and +4 charge states, respectively, Delta CN>0.1, and P-value (protein and peptide) <0.01. IPI accession numbers were cross-correlated with SwissProt accession numbers for final protein identification [36].
One-dimensional polyacrylamide gel electrophoresis
Sample homogenates were incubated in sample buffer (0.5 M Tris (pH 6.8), 40% glycerol, 8% sodium dodecyl sulfate (SDS), 20% β-mercaptoethanol, and 0.01% bromophenol) for 5 min in a water bath at 95°C and loaded onto precast Criterion TGX (4–15%) or Criterion XT (12% Bis-Tris) Precast Gels as appropriate for the molecular weight of the protein of interest. Precision Plus Protein™ All Blue Standards and samples were run at 80 V for 15 min, increasing the voltage to 120 V and run for 90 min in TGS or MES running buffer as appropriate for the gel.
Western Blotting
In-gel proteins were transferred to a nitrocellulose membrane (0.45 μm) using a Trans-Blot® Turbo™ Blotting system at 25 V for 30 min (BioRAD, Hercules, CA, USA). After transfer, membranes were incubated in blocking solution [3% BSA in phosphate buffer (PBS) solution with 0.2% (v/v) Tween 20] at room temperature for 1.5 h. Membranes were then incubated with rabbit anti-inducible nitric oxide synthase (iNOS) antibody (Calbiochem/Millipore, Billerica, MA) or rabbit anti-NF-κB antibody (Enzo, Farmingdale, NY) for 2 h on a gentle rocking platform, followed by three rinses for 5 min each with PBS solution with 0.2% (v/v) Tween 20. Blots were then incubated for 1 h with ECL anti-rabbit IgG, horseradish peroxidase-linked whole antibody. The resulting blots were rinsed three times for 5, 10, and 10 min each in PBS solution with 0.2% (v/v) Tween 20 and signals were detected using Clarity™ Western ECL Substrate (BioRAD) and the ChemiDoc™ MP Imaging System (BioRAD). Blots were stripped using Re-blot Plus Strong Solution (Millipore, Billerica, MA, USA) according to package instructions and re-probed with mouse anti-actin antibody (Sigma-Aldrich, St. Louis, MO) or mouse anti-histone H2B antibody (Chemicon, Temecula, CA) for normalization. Analysis was performed using the ImageLab software (BioRAD).
Validation
Proteomics results were validated by IEF and 2D-PAGE as described above followed by Western blot [36] for peroxiredoxin 3 (PrxIII) and 1D-gel electrophoresis followed by Western blot for peptidyl-prolyl cis-trans isomerase A (PPIA or cyclophilin A). PrxIII blots were probed for PrxIII as described above using rabbit polyclonal to PrxIII primary antibody (Abcam, Cambridge, MA, USA), mouse anti-actin primary antibody and anti-rabbit whole molecule IgG alkaline phosphatase or anti-mouse whole molecule IgG alkaline phosphatase secondary antibody respectively. The resulting blots were developed colorimetrically with a solution of BCIP combined with NBT in ALP buffer [0.1 M Tris, 0.1 M NaCl, 5 mM MgCl2 · 6 H2O (pH 9.5)]. Developed blots were allowed to dry overnight at room temperature and scanned into Adobe Photoshop 6.0 using a Canon CanoScan 8800F scanner. The resulting images were analyzed using Image Quant software. PPIA blots were probed for PPIA by incubation with anti-cyclophilin A rabbit antiserum (Upstate/Millipore, Billerica, MA, USA,) primary antibody and ECL anti-rabbit IgG, horseradish peroxidase-linked whole antibody and developed chemiluminescently as described above. PPIA blots were stripped and re-probed for the actin. Analysis was performed using the ImageLab software (BioRAD).
Statistical analysis
All data are presented as mean±SD or mean±SEM, and statistical analyses were performed using a two-tailed Student’s t-test or ANOVA where indicated, with p<0.05 considered significant for spot comparison. A Mann–Whitney U statistical analysis was performed to determine the significance of differential expression fold-change values, where a p<0.05 was considered significant. Significance was also confirmed using a Student’s t-test. Only spots that were significant by both tests were further evaluated. Protein and peptide identifications obtained with the SEQUEST search algorithm with a p< 0.01 were considered as statistically significant. To further validate PD-Quest analysis, identities of protein spots were verified matching calculated molecular weight (MW) and isoelectric point (pI) values from MS results and SwissProt database information to spot locations on the gels (Table 2 and Figure 1).
Table 2.
Brain protein identifications based on MS/MS results and in-gel spot locations
| SSP | Protein Identification | Score | Coverage | Unique Peptides | MW [kDa] | Calc pI |
|---|---|---|---|---|---|---|
| 5201 | Triosephosphate isomerase | 67.5 | 48.6 | 10 | 26.8 | 7.24 |
| 8103 | Pyruvate kinase isozymes M1/M2 | 241 | 50.8 | 21 | 57.8 | 7.06 |
| 6701 | 6-phosphofructokinase type C | 69.2 | 19.5 | 11 | 85.7 | 7.28 |
| 3207 | Peroxiredoxin 3 | 155 | 33.8 | 6 | 28.3 | 7.55 |
| 4201 | DJ-1/PARK7 | 61.7 | 58.2 | 11 | 20.0 | 6.77 |
| 8104 | Peptidyl-prolyl cis-trans isomerase A | 56.2 | 28.7 | 7 | 17.9 | 8.16 |
Figure 1.
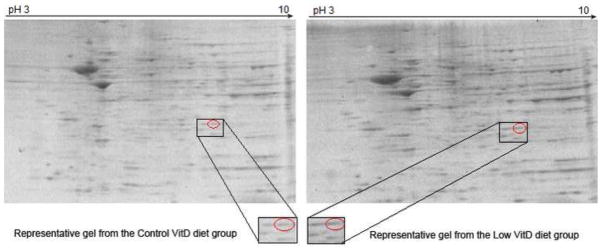
Representative 2D gels used for proteomics identification of differentially expressed proteins. Brain samples of rats fed a control-, low- or high-VitD diet from middle-age to old-age were separated by IEF using IPG strips pH 3–10 followed by SDS-PAGE using 8–16% Tris-HCl gels. Separated proteins were visualized with SYPRO Ruby protein gel stain. Acquired images were analyzed using PDQuest software and spots showing different intensities were chosen for further analysis. The figure indicates a comparison of the PrxIII spot as a representative of those found with significant expression differences in low vs. control VitD groups as identified by PDQuest analysis. Below are expansions of the spot images showing clearly that the PrxIII level is elevated in brain of rats on a chronic low VitD diet.
Results
Vitamin D deficiency leads to increased nitrosative protein damage in brain
Tyrosine nitration is a common indicator/biomarker of the aging brain and of age-related neurodegenerative disorders [30, 34], both of which typically are accompanied by different extents of cognitive deficit. Here, we tested for indicators of oxidative and nitrosative stress in brain tissue samples from rats in which we manipulated serum VitD levels from middle-age to old-age. Significantly increased global 3-NT (Figure 2) in the brains of rats on a low VitD diet compared to rats on control or high VitD diets was observed. 3-NT measures of brain samples from rats fed high VitD diets were similar to the control group. No significant differences were observed for the other oxidative stress parameters measured.
Figure 2.
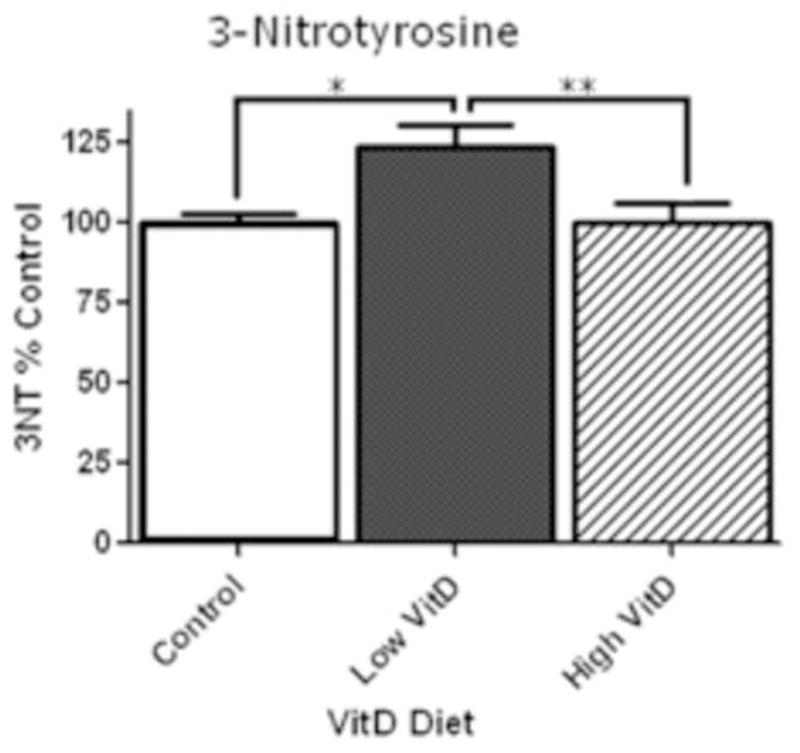
Slot-blot analysis of 3-NT levels in rat brain homogenates. Slot blot analyses were performed using brain samples of rats fed a control, low VitD or high VitD diet from middle-age to old-age (n=9). Data show a significant increase in 3-NT levels in rat brains between control and low VitD diet (*p<0.01). No difference was observed in high VitD diet when compared to control. High VitD diet decreases 3-NT levels when compared to low VitD diet (**p<0.05).
Vitamin D deficiency elevation of peroxidases, glycolytic enzymes, and PPIA
In order to determine changes, possible causes, consequences, or mechanisms for the elevated nitrosative stress, expression proteomics experiments [35] were performed on brain of rats fed a low VitD, control, or high VitD diet to determine which proteins were altered. PDQuest analysis was performed on all groups as well. Only spots that were found to be statistically different in relative intensity by statistical tests were further evaluated. Compared to control, the high VitD group was similar to control in terms of protein levels for easily discernible protein spots. In contrast, a number of protein spots with significant differences in intensity were found between the control and low VitD groups. Table 3 shows the PDQuest software generated I.D. numbers (SSP) of these protein spots. Spot intensities between control and low VitD groups were used to calculate fold changes reflecting significant increases in protein amounts. These proteins were identified by MS/MS and database interrogation as 6-phosphofructokinase (6-PFK) type C, PPIA, triosephosphate isomerase (TPI), PrxIII, DJ-1/PARK7, and pyruvate kinase (PK) isozymes M1/M2 (Table 3).
Table 3.
VitD 2D-Gel Comparison PDQuest Data: spot matching Low VitD (L) vs. Control (C), Levels.
| SSP | Fold Change | Direction of change L vs C | p-value L vs C |
|---|---|---|---|
| 6701 | 1.41 | Increased | 0.0114 |
| 8104 | 2.49 | Increased | 0.0130 |
| 5201 | 60.3 | Increased | 0.0291 |
| 3207 | 1.53 | Increased | 0.0347 |
| 8103 | 1.48 | Increased | 0.0373 |
| 4201 | 1.53 | Increased | 0.0473 |
To validate protein identity from proteomics, 2D and 1D Western blots were performed, selecting PrxIII and PPIA as representative proteins. 2D Western blots showed a 1.5-fold increase in PrxIII levels confirming the 1.5-fold increase suggested by 2D-PAGE. Further, the position of the PrxIII spot appears at approximately 26 kDa and 7.2 pI on 2D Western blot (Figure 3). 1D Western blot showed a 2.24-fold increase in PPIA levels, further validating the 2.49-fold increase seen in the proteomics results (Figure 4). These validation results provide confidence in the proteomics identification of other proteins reported in this study.
Figure 3.
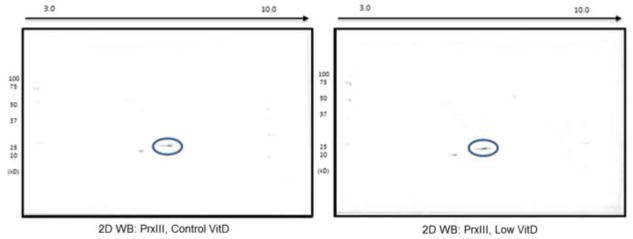
Validation of expression proteomics results for PrxIII by 2D Western blot analysis. Depicted are representative blots showing a 1.5-fold increase of PrxIII in brain samples of rats fed the low-VitD diet from middle-age to old-age compared to those fed the control diet. Circled spots are PrxIII from control VitD brain samples shown on the left and low VitD brain samples on the right.
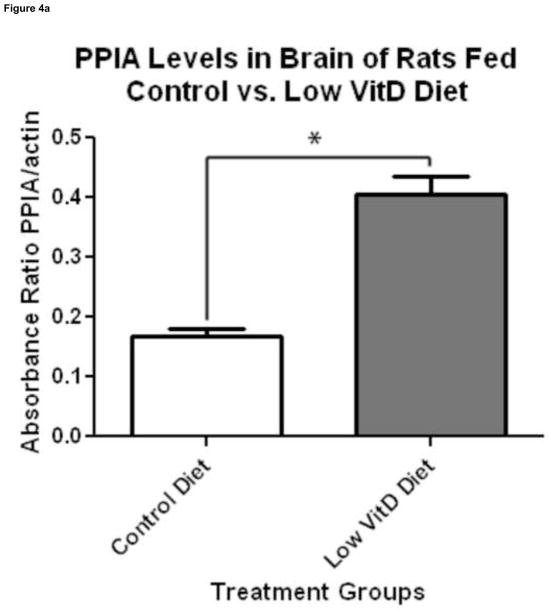
Figure 4.
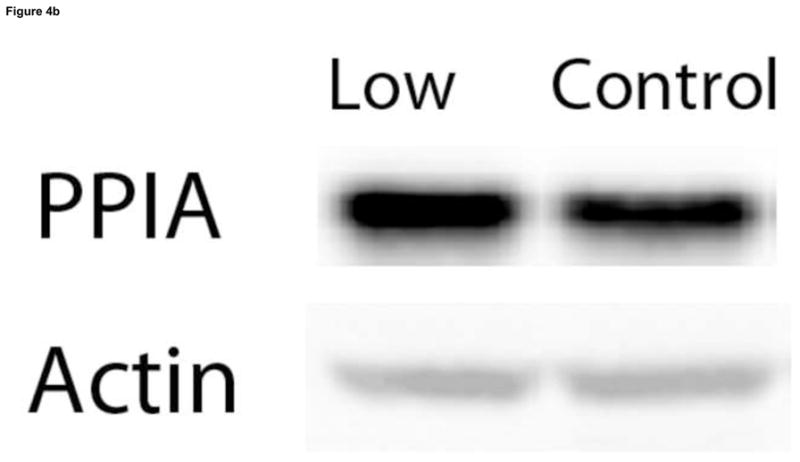
Validation of expression proteomics results by 1D Western blot analysis of PPIA levels. 1D Western blot analysis was used to determine PPIA levels of brain samples of rats fed a control and low VitD diet from middle-age to old-age (n=9). (a)Analysis using a specific antibody against PPIA showed a 2.24-fold increase in PPIA levels in brain of the low VitD group compared to control (*p-value<0.01). This result confirms the increase in PPIA levels found by expression proteomics. (b) Representative lanes from 1D Western blot showing probes for PPIA and the actin loading control.
Vitamin D deficiency leads to NF-κB activation and increased iNOS levels in brain
Preliminary results of this study coupled with previous studies in our laboratory and existing literature led us to propose a model to connect the observed increases in nitrosative protein damage and expression proteomics results showing elevated levels of the above mentioned peroxidases, glycolytic enzymes, and PPIA to VitD deficiency (Figure 5). Based on this proposed model, we examined the hypothesis that VitD deficiency would lead to increased NF-κB activation and iNOS levels, resulting in the observed increase in protein tyrosine nitration seen in the low-VitD group compared to control.
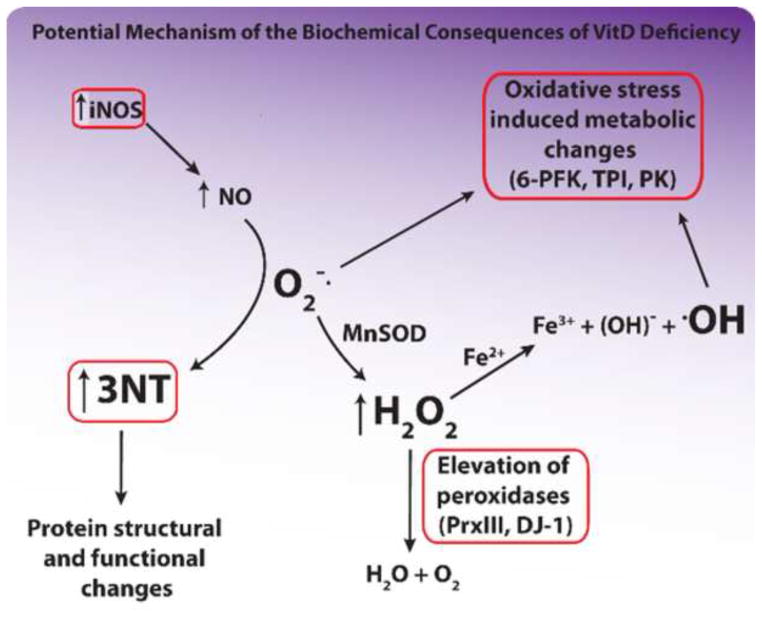
Figure 5.
Potential mechanism for the biochemical consequences of VitD deficiency. Circled areas indicate data collected in this study. Increased tyrosine nitration was found by slot-blot analysis, increased iNOS levels were found by 1D Western blot analysis, and elevation of glycolytic enzymes and peroxidases was found by expression proteomics using brain samples of rats fed a control- and low-VitD diet from middle-age to old-age. Items in large type are the most potentially damaging agents in this diagram.
NF-κB is a redox-sensitive nuclear transcription factor that can be activated by oxidizing agents such as H2O2 [40]. Hypoxic activation of NF-κB and gene transcription has been found to require mitochondrial ROS [41]. Among downstream products of NF-κB pathway activation are tumor necrosis factor-α (TNFα) and iNOS [41–43]. Using 1D Western blotting, we investigated whether NF-κB activation of iNOS expression may be a plausible reason for the nitration of tyrosine residues through overproduction of NO· [44–48] seen in these samples.
Under normal conditions, NF-κB exists in an inactive form bound to inhibitor of kB-α (IkB-α). Upon activation of a Toll-like receptor by a variety of substrates including lipopolysaccharide (LPS) and TNFα [49], phosphorylation of IkB-α occurs through IkB kinase (IKK) allowing IkB-α proteasomal degradation and NF-κB activation. NF-κB is tranlocated to the nucleus leading to bursts of iNOS activation, subsequent NO· elevation [42, 47], and further transcriptional activation of the TNFα gene[44, 50]. Pro-inflammatory cytokines such as TNFα lead to further and rapid phosphorylation of IkBs [51]. Overproduction of NO· as a consequence of iNOS activation results in protein nitration events and further stimulation of IKK [42, 52]. 1α, 25-(OH)2 VitD has been shown to interfere with these processes through decreasing TNFα levels and TNFα-induced inflammatory cytokines [53–55], down-regulating Toll-like receptors [56], up-regulating IkB-α, decreasing IkB-α phosphorylation, and decreasing translocation of NF-κB[49], thereby reducing many downstream consequences. Further decrease of NF-κB action may occur through the suppression of NF-κB-directed expression by the VitD responsive element [57]. Thus, VitD can inhibit protein nitration in the brain via its suppressive effects on the NF-κB pathway. VitD deprivation may exacerbate NF-κB-directed nitrosative damage and subsequent neurodegenerative consequences. As an estimate of NF-κB activity, subcellular fractionation was done on control and low VitD brain samples. NF-κB levels were found to be significantly decreased in the cytosolic fraction and significantly increased in the nuclear fraction of the low VitD group compared to control (Figure 6) indicating NF-κB translocation to the nucleus. Our analysis further revealed a significant increase in iNOS levels in the low VitD samples compared to control (Figure 7). These NF-κB and iNOS results provide evidence to support the proposed mechanism of protein nitration due to VitD deficiency. TNFα is one of several initiators of the NF-κB activation cascade. 1D Western blot analysis of these samples for TNFα showed a trend toward increase (data not shown) in the low VitD group compared to control consistent with NF-κB activation and iNOS elevation. Figure 8 depicts a suggested mechanism for the protein nitration regulatory effects of VitD in brain.
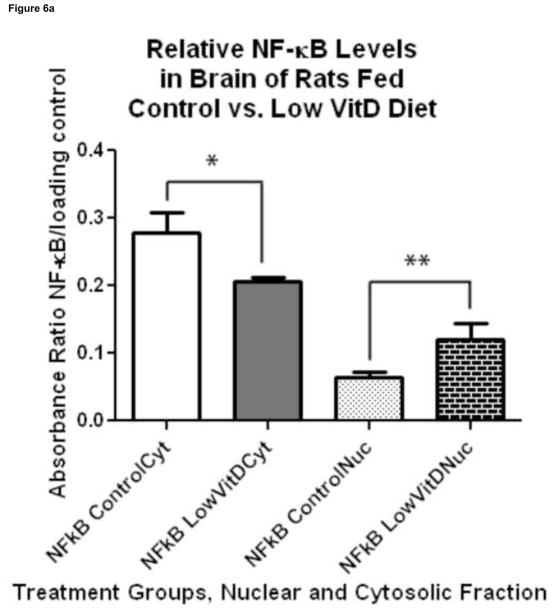
Figure 6.
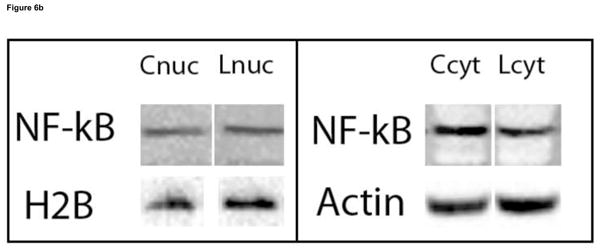
Effect of VitD deficiency on NF-κB subcellular localization in rat brain. 1D Western blots were used to determine the NF-κB (p65) levels in the cytosolic and nuclear fractions of brain samples of rats fed a control and low VitD diet from middle-age to old-age (n=9). The NF-κB (p65) subunit is common in the NF-κB signaling pathway. NF-κB (p65) levels in the cytosolic fraction were normalized to β-actin, while NF-κB (p65) levels in the nuclear fraction were normalized to histone H2B. (a) A decrease in NF-κB (p65) in the cytosolic fraction (*p<0.05) and increase in the nuclear fraction (**p<0.05) in brain samples from the low VitD group compared to control is observed indicative of NF-κB activation and translocation. (b) Representative bands from 1D western blot are shown here side by side for comparison. Nuclear and cytosolic fractions were alternated across all gels. Blots were probed for NF-κB (p65), stripped, probed for β-actin, then stripped and probed for histone H2B.
Figure 7.
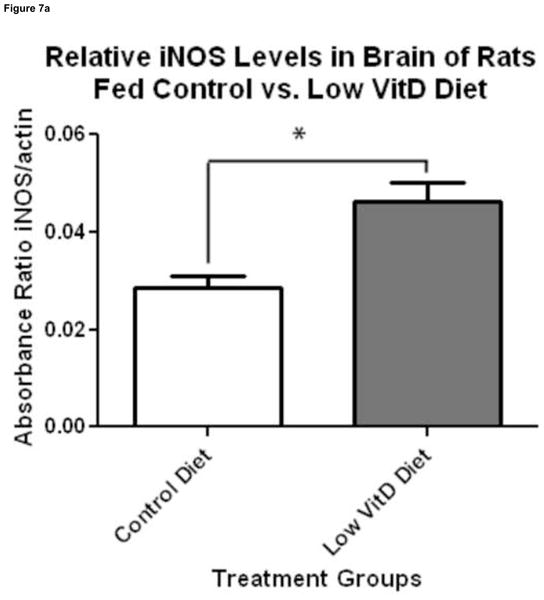
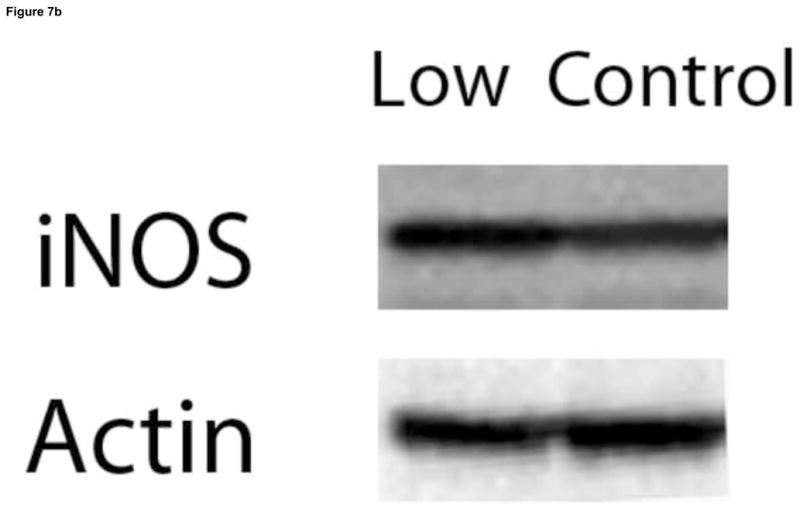
Effect of VitD deficiency on iNOS levels in rat brain. 1D Western blot was performed to compare iNOS levels between brain samples of rats fed a control and low VitD diet from middle-age to old-age (n=9). iNOS signals were normalized to β-actin. (a) iNOS levels were found to be significantly higher in brain samples from the low VitD group compared to control (*p<0.01). (b) Representative lanes from 1D Western blot.
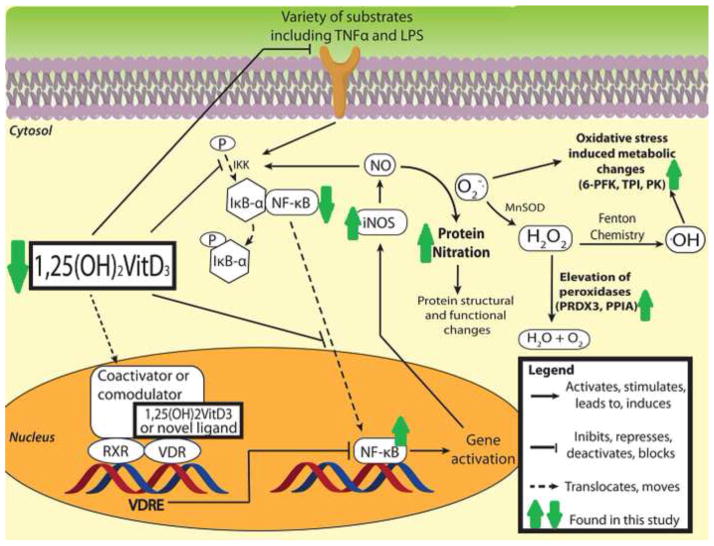
Figure 8.
Proposed mechanism for the protein nitration regulatory effects of VitD in brain based on recent literature and results from the current study. Results obtained from this study are marked with dark green arrows. The deficient serum levels of VitD in the low VitD group led in brain to decreased NF-κB in the cytosolic fraction, increased NF-κB in the nuclear fraction, iNOS elevation, increased protein tyrosine nitration, and elevations in mitochondrial and regulatory proteins.
Discussion
We have previously shown that tyrosine nitration occurs early in neurodegenerative processes, i.e., in mild cognitive impairment (MCI), arguably the earliest form of AD [31]. Nitration of tyrosine occurs from the reaction NO· with the O2−· through the reactive intermediate ONOO− in the presence of CO2 [32, 58] leading to tyrosine nitration by NO2· radical. Nitrosative stress measures on these cortical samples showed approximately a 25% elevation of 3-NT globally in brain protein in the low VitD group versus the control and high VitD-treated groups.
Glycolytic enzymes targeted by low VitD
The brain proteins identified by proteomics in the present study to be at increased levels (Table 2) fall primarily in the following functional categories: glycolysis, mitochondrial peroxidase activity, and protein folding. Three of these proteins, 6-PFK, TPI, and PK, are enzymes involved in the production of ATP in the cell during glycolysis in response to the need for energy. PK catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate transferring a phosphate group from PEP to ADP to produce ATP. Pyruvate, NADH, and H+ from glycolysis continue onto the tricarboxylic acid (TCA) cycle for further ATP production. PK has been identified by redox proteomics as oxidatively modified in brain regions, such as cortex and hippocampus, during brain aging [22, 59] and neurodegeneration resulting in reduced enzymatic activity [60]. 6-PFK catalyzes a key regulatory step, the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate at the expense of ATP, committing the path of glucose through glycolysis. This rate-limiting glycolytic enzyme was found by Tang and colleagues to be upregulated in several biological systems as part of the oxidative stress response including the response to H2O2 induced oxidative stress [61]. TPI catalyzes the interconversion of the trioses, glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). Significant increases of TPI have been found by our group using proteomic analysis in the Alzheimer’s disease hippocampus [62]. Further studies identified TPI as significantly nitrated in the inferior parietal lobe (IPL) in early AD brain [63] and in both AD hippocampus and IPL [64]. Dysfunction of this enzyme leads to the buildup of a toxic species, methylglyoxal, leading to neurodegenerative consequences [65, 66]. Thus, metabolic effects in brain are evident from the effect of a low-VitD diet.
Other identified targets of VitD deficiency (DJ-1, PPIA, PRXIII)
Mutations of the DJ-1 gene are believed to be one possibility that leads to Parkinson disease (PD) [67]. DJ-1 is thought to be involved in cell cycle regulation, gene transcription, spermatogenesis, and the cellular stress response [68] among other functions. Evidence exists that DJ-1, in response to oxidative stress, may have roles as an anti-oxidant [69, 70] and a redox sensitive chaperone [71, 72]. Accordingly, elevation of DJ-1 in brain could be a consequent cell-stress response to elevated protein nitration.
PPIases regulate activity of target proteins to which they bind by catalyzing the cis-trans isomerization of proline of these target proteins [73, 74], typically a rate limiting step in protein folding. PPIA, or cyclophilin A, is also known to play a variety of roles from immunoregulation to cellular signaling and proliferation, but more recently has been identified as a potential marker of inflammation in a variety of disease states [75–79] and may play a regulatory role in the NF-κB pathway in some cell types [80, 81]. In addition, PPIA treatment was shown to reduce reactive oxygen species (ROS) and alleviate some forms of Aβ-induced neurotoxicity in PC12 cell culture while maintaining activities of certain key anti-oxidant enzymes including SOD [82]. PPIA binds Prxs and activates their peroxidase activity acting as an immediate electron donor [83]. PPIA may also indirectly stimulate PrxIII, a downstream consequence of tumor necrosis factor-α (TNFα) [84].
PrxIII is a mitochondria resident, thioredoxin-dependent peroxide reductase that acts to scavenge as much as 90% of H2O2 in mitochondria, with GPx 1 and 4 and PrxV thought to account for the rest [85–90]. Rhee and colleagues elegantly showed that peroxiredoxin overexpression inhibited NF-κB [85], and overexpression of PrxIII has been shown to protect against H2O2-dependent apoptosis in cancer cells [91]. Further, PrxIII was shown to be essential in maintaining normal mitochondrial homeostasis [92]. Dysfunctional PrxIII leads to increased ROS and subsequent DNA damage and apoptosis linked to loss of peroxidase activity in mitochondria [93]. Prxs have been shown to detoxify peroxynitrite, thereby decreasing biomolecule damage caused by more reactive products of ONOO− and protecting human cells in culture from iNOS-related cell death [94].
Tyrosine nitration can and does have functional consequences on the affected proteins [33, 58, 95]. Steric hindrance by the NO2 group in the 3-position of tyrosine may prevent phosphorylation at the 4-position thereby causing dysregulation of activation/deactivation processes in the affected proteins [33, 45, 95–98]. Such considerations have implications for cognitive dysfunction following low dietary vitamin D.
Consequences of low dietary VitD
Based on this experimental evidence, the biochemical consequences of low dietary VitD include increased 3-NT in the brain as well as increases in certain proteins with peroxidase activity. A likely link between these two pathways is O2−· (Figure 5). The high O2 usage in energy metabolism coupled with age-related decreases in the efficiency of these reactions results in the production and leakage of O2−· from the electron transport chain. MnSOD in the mitochondria react with O2−· producing H2O2. NO· reacts with O2−· by radical-radical recombination producing the potentially more reactive species, ONOO−, that leads to the nitration of tyrosine [32, 99–101] residues, hindering protein activity regulation by sterically blocking the phosphorylation site. The findings of this study are consistent with current literature hypothesizing that oxidative damage during the aging process leads to mitochondrial dysfunction [59, 61]. Increased glycolytic enzymes could be a compensatory mechanism reflecting changes in energy production, changes in mitochondrial redox status, and oxidative stress [61] reminiscent of the Warburg effect in cancer [102–105].
Collectively, these results reflect a change in mitochondrial redox potential, glucose metabolism, and structural changes in proteins. Data from the current study are consistent with recent findings that VitD: a) increases SOD activity, b) decreases levels of endogenous oxidants, c) attenuates H2O2 induced changes, d) decreases release of inflammatory cytokines, and e) may have natural anti-oxidant and anti-inflammatory properties [106, 107]. In contrast, VitD deficiency contributes to inflammation through increased production of inflammatory cytokines, effects that are attenuated by 1,25(OH)2VitD supplementation [6, 13, 108, 109].
More recently, VitD has been shown to play potential roles in CNS homeostasis [110]. The VitD receptor (VDR) is expressed in microglia, and 1α-hydroxylase, the enzyme that converts 25-OH VitD to 1α, 25-(OH)2 VitD, is present in activated but not resting microglia [28, 111–113]. In activated microglia, 1α,25(OH)2VitD suppresses the production of NO· and the inflammatory mediators, TNFα and interleukin-6 (IL-6), in a dose-dependent manner suggesting direct anti-inflammatory roles for VitD in the brain [110]. Local conversion of VitD to the active 1α,25(OH)2VitD in the brain may be a direct neuroprotective response to CNS inflammation followed by inhibition of NF-κB related iNOS induction [112]. NF-κB leads to mitochondrial dysfunction inhibition of MnSOD through nitration via activation of iNOS, an effect that is absent in iNOS knockout animal models [46].
Our data are consistent with existing evidence that sufficient VitD is known to be antiinflammatory and to suppress the NF-κB cellular stress response pathway [49, 57, 114–116]. Hydrogen peroxide (H2O2) has been shown to modulate NF-κB activity thereby helping to regulate NF-κB-dependent processes including inflammation [40, 117–119]. During inflammatory events in the CNS, iNOS generates excessive amounts of NO· This increased activity of iNOS and resulting overproduction of NO· may occur in brief bursts. In rat models with experimental allergic encephalomyelitis (EAE), Garcion and colleagues showed that 1α,25(OH)2VitD inhibited the iNOS increases [120]. VitD restriction led to slight worsening of clinical symptoms [121]. Nitration of protein resident tyrosine residues also occurs during brain aging leading to mitochondrial dysfunction and neurodegeneration [45, 96, 122].
Conclusions
This study is the first to demonstrate that a chronic low-VitD diet and consequential low levels of VitD in the bloodstream result in significant increases in tyrosine nitration in brain proteins, alterations in glucose metabolism and mitochondrial changes in brain of elderly rats, an animal model of brain in older human subjects (Fig. 8). A shift from the TCA cycle to glycolysis may be indicative of metabolic dysfunction. Further, ATP generated from glycolysis is important for maintaining a proper resting membrane potential in neurons (n., via NA+/K+ATPase), which is, in turn, important for neurotransmission. The results of this study are consistent with the notion that nitration of brain proteins occurs via NF-κB activation of iNOS. These results provide biochemical evidence to support the conclusions, consistent with other studies, that suggest that higher serum VitD levels may have direct and indirect anti-oxidant properties and be beneficial to modulate damaging effects of brain aging. Based on evidence from the present study and the literature as noted above, it is our opinion that current daily VitD intake in the general adult population is too low and should be increased.
The present study used brain tissue from animals as part of a larger study examining the effects of serum VitD status and brain aging. A component of these larger studies examined learning and memory and a preliminary report indicated that low VitD animals displayed poorer performance, but that performance improved with high VitD (Latimer CS, et al., personal communication, 2011). Hence, these data support the present findings and conclusions.
As people age, their lifestyles become more sedentary, physically and mentally. Perhaps due to limited access, physical or financial limitations, or lack of motivation, nutritional status often declines. Concurrently, time spent outside decreases. Less sun exposure further decreases circulating VitD levels. Our studies, together with those of others, indicate that higher VitD may be beneficial for older individuals and, thus, it would appear that further studies are warranted to determine whether VitD supplementation can offset some of the changes associated with unhealthy brain aging. Further studies to address this issue are ongoing in our laboratory.
Highlights.
VitD deficiency and cognitive decline are highly prevalent in the elderly
Low VitD during brain aging leads to elevated 3-NT, glycolytic enzymes, peroxidases
Results correlate with VitD involvement in VDR-RXR and NF-κB signaling
VitD supplementation may help protect against cognitive decline in adults.
Acknowledgments
This work was supported by the following grants from the National Institute on Aging: AG05119 (DAB), AG010836(PW Landfield, DAB, NMP), AG034605(PW Landfield, NMP) and T32 AG0000242 (CSL, G Gerhardt).
Abbreviations
- 3-NT
3-nitrotyrosine
- BCIP
5-bromo-4-chloro-3-indolyl-phosphate
- 6-PFK
6-phosphofructokinase
- ALP
alkaline phosphatase activity buffer
- Aβ
amyloid beta peptide
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- JNK
c-jun N-terminal kinase
- VitD3
cholecalciferol
- 25-OH VitD, calcidiol
25-hydroxy vitamin D
- 1α
25-(OH)2VitD, calcitriol, 1α, 25-dihydroxy vitamin D
- DI
deionized
- DHAP
dihydroxyacetone phosphate
- DTT
dithiothreitol
- ESI
electrospray ionization
- VitD2
ergocalciferol
- FT
Fourier Transform
- F1
6BP, fructose-1, 6-bisphosphate
- F6P
fructose-6-phosphate
- GPx
glutathione peroxidase
- G3P
glyceraldehyde-3-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IPL
inferior parietal lobule
- iNOS
inducible nitric oxide synthase
- ICAD
International Conference on Alzheimer’s Disease
- IPI
International Protein Index
- IA
iodoacetamide
- IEF
Isoelectric focusing
- pI
isoelectric point
- MnSOD
manganese superoxide dismutase
- MS
mass-spectrometry
- MCI
mild cognitive impairment
- MW
molecular weight
- NBT
nitro blue tetrazolium
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PD
Parkinson disease
- PPIA
peptidyl-prolylcis-trans isomerase A
- PrxIII
peroxiredoxin 3
- ONOO−
peroxynitrite
- PEP
phosphoenolpyruvate
- PK
pyruvate kinase
- ROS
reactive oxygen species
- SSP
software generated I.D. numbers
- TCA cycle
tricarboxylic acid cycle
- TCA
trichloroacetic acid
- TPI
triosephosphate isomerase
- TGS
tris-glycine-SDS
- TNFα
tumor necrosis factor-α
- 2D-PAGE
Two-dimensional polyacrylamide gel electrophoresis
- VitD
vitamin D
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 2.Nezbedova P, Brtko J. 1alpha,25-dihydroxyvitamin D3 inducible transcription factor and its role in the vitamin D action. Endocr Regul. 2004;38:29–38. [PubMed] [Google Scholar]
- 3.Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O. Chronic 1alpha,25-(OH)2 vitamin D3 treatment reduces Ca2+-mediated hippocampal biomarkers of aging. Cell calcium. 2006;40:277–286. doi: 10.1016/j.ceca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 5.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farid K, Volpe-Gillot L, Petras S, Plou C, Caillat-Vigneron N, Blacher J. Correlation between serum 25-hydroxyvitamin D concentrations and regional cerebral blood flow in degenerative dementia. Nucl Med Commun. 2012;33:1048–1052. doi: 10.1097/MNM.0b013e32835674c4. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34(Suppl 1):S265–277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, Mahanian M, Weitzman R, Hayden EY, Rosenthal MJ, Nemere I, Ringman J, Teplow DB. 1alpha,25-Dihydroxyvitamin D3 and Resolvin D1 Retune the Balance between Amyloid-beta Phagocytosis and Inflammation in Alzheimer’s Disease Patients. J Alzheimers Dis. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29:361–368. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinol. 2012;4:183–190. doi: 10.4161/derm.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zittermann A, Gummert JF, Borgermann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. 2009;12:634–639. doi: 10.1097/MCO.0b013e3283310767. [DOI] [PubMed] [Google Scholar]
- 14.Semba RD, Houston DK, Ferrucci L, Cappola AR, Sun K, Guralnik JM, Fried LP. Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res. 2009;29:525–530. doi: 10.1016/j.nutres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginter E, Simko V. Vitamin D deficiency, atherosclerosis and cancer. Bratisl Lek Listy. 2009;110:751–756. [PubMed] [Google Scholar]
- 16.Weaver CM, Fleet JC. Vitamin D requirements: current and future. Am J Clin Nutr. 2004;80:1735S–1739S. doi: 10.1093/ajcn/80.6.1735S. [DOI] [PubMed] [Google Scholar]
- 17.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. The vitamin D deficiency pandemic: a forgotten hormone important for health. Public Health Rev. 2010;32:267–283. [Google Scholar]
- 19.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D cognitive impairment in the elderly US population. J Gerontol A Biol Sci Med Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2013;33:659–674. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 27.Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem. 1999;73:859–866. doi: 10.1046/j.1471-4159.1999.0730859.x. [DOI] [PubMed] [Google Scholar]
- 28.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrin Met. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 29.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 31.Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 33.Feeney MB, Schoneich C. Tyrosine modifications in aging. Antioxid Redox Signal. 2012;17:1571–1579. doi: 10.1089/ars.2012.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza JM, Chen Q, Blanchard-Fillion B, Lorch SA, Hertkorn C, Lightfoot R, Weisse M, Friel T, Paxinou E, Themistocleous M, Chov S, Ischiropoulos H. Reactive nitrogen species and proteins: biological significance and clinical relevance. Adv Exp Med Biol. 2001;500:169–174. doi: 10.1007/978-1-4615-0667-6_22. [DOI] [PubMed] [Google Scholar]
- 35.Butterfield DA, Perluigi M, Reed T, Muharib T, Hughes CP, Robinson RA, Sultana R. Redox proteomics in selected neurodegenerative disorders: from its infancy to future applications. Antioxid Redox Signal. 2012;17:1610–1655. doi: 10.1089/ars.2011.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson RA, Lange MB, Sultana R, Galvan V, Fombonne J, Gorostiza O, Zhang J, Warrier G, Cai J, Pierce WM, Bredesen DE, Butterfield DA. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-beta peptide of amyloid precursor protein. Neuroscience. 2011;177:207–222. doi: 10.1016/j.neuroscience.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultana R, Butterfield DA. Slot-blot analysis of 3-nitrotyrosine-modified brain proteins. Methods Enzymol. 2008;440:309–316. doi: 10.1016/S0076-6879(07)00820-8. [DOI] [PubMed] [Google Scholar]
- 38.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK, Butterfield DA. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50:1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi M, Sakaguchi H, Darrow RM, Yan L, West KA, Aulak KS, Stuehr DJ, Hollyfield JG, Organisciak DT, Crabb JW. Evidence that light modulates protein nitration in rat retina. Mol Cell Proteomics. 2002;1:293–303. doi: 10.1074/mcp.m100034-mcp200. [DOI] [PubMed] [Google Scholar]
- 40.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 41.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 42.Feo F, Frau M, Tomasi ML, Brozzetti S, Pascale RM. Genetic and epigenetic control of molecular alterations in hepatocellular carcinoma. Exp Biol Med (Maywood) 2009;234:726–736. doi: 10.3181/0901-MR-40. [DOI] [PubMed] [Google Scholar]
- 43.Griscavage JM, Wilk S, Ignarro LJ. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JI, Ju WK, Choi JH, Choi E, Carp RI, Wisniewski HM, Kim YS. Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res Mol Brain Res. 1999;73:17–27. doi: 10.1016/s0169-328x(99)00229-6. [DOI] [PubMed] [Google Scholar]
- 45.Tangpong J, Sompol P, Vore M, St Clair W, Butterfield DA, St Clair DK. Tumor necrosis factor alpha-mediated nitric oxide production enhances manganese superoxide dismutase nitration and mitochondrial dysfunction in primary neurons: an insight into the role of glial cells. Neuroscience. 2008;151:622–629. doi: 10.1016/j.neuroscience.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100:191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 47.Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald DC, Meade KG, McEvoy AN, Lillis L, Murphy EP, MacHugh DE, Baird AW. Tumour necrosis factor-alpha (TNF-alpha) increases nuclear factor kappaB (NFkappaB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells. Vet Immunol Immunopathol. 2007;116:59–68. doi: 10.1016/j.vetimm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Chagas CE, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell metabolism. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Molecular cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 52.Mendes AF, Carvalho AP, Caramona MM, Lopes MC. Role of nitric oxide in the activation of NF-kappaB, AP-1 and NOS II expression in articular chondrocytes. Inflamm Res. 2002;51:369–375. doi: 10.1007/pl00000317. [DOI] [PubMed] [Google Scholar]
- 53.Furman I, Baudet C, Brachet P. Differential expression of M-CSF, LIF, and TNF-alpha genes in normal and malignant rat glial cells: regulation by lipopolysaccharide and vitamin D. J Neurosci Res. 1996;46:360–366. doi: 10.1002/(SICI)1097-4547(19961101)46:3<360::AID-JNR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 54.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile 1,25-Dihydroxyvitamin D(3) works as antiinflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Diaz L, Noyola-Martinez N, Barrera D, Hernandez G, Avila E, Halhali A, Larrea F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009;81:17–24. doi: 10.1016/j.jri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 57.Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66:S98–112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 58.Labetoulle M, Capelli C, Dubreuil F, Kirsch O, Perlemuter G, Buffet C, Quillard J, Offret H, Frau E. Orbital metastasis of hepatocellular carcinoma. Gastroenterol Clin Biol. 2001;25:914–915. [PubMed] [Google Scholar]
- 59.Perluigi M, Di Domenico F, Giorgi A, Schinina ME, Coccia R, Cini C, Bellia F, Cambria MT, Cornelius C, Butterfield DA, Calabrese V. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res. 2010;88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 60.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Tang H, Lee M, Sharpe O, Salamone L, Noonan EJ, Hoang CD, Levine S, Robinson WH, Shrager JB. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. Faseb J. 2012;26:4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- 63.Reed TT, Pierce WM, Jr, Turner DM, Markesbery WR, Butterfield DA. Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J Cell Mol Med. 2009;13:2019–2029. doi: 10.1111/j.1582-4934.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Pun PB, Murphy MP. Pathological significance of mitochondrial glycation. Int J Cell Biol. 2012:843505. doi: 10.1155/2012/843505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J Bioenerg Biomembr. 2009;41:441–446. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, Snijders PJ, Testers L, Breedveld GJ, Horstink M, Sandkuijl LA, van Swieten JC, Oostra BA, Heutink P. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonifati V, Oostra BA, Heutink P. Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson’s disease. J Mol Med (Berl) 2004;82:163–174. doi: 10.1007/s00109-003-0512-1. [DOI] [PubMed] [Google Scholar]
- 69.Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 70.Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 71.Foti R, Zucchelli S, Biagioli M, Roncaglia P, Vilotti S, Calligaris R, Krmac H, Girardini JE, Del Sal G, Gustincich S. Parkinson disease-associated DJ-1 is required for the expression of the glial cell line-derived neurotrophic factor receptor RET in human neuroblastoma cells. J Biol Chem. 2010;285:18565–18574. doi: 10.1074/jbc.M109.088294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zucchelli S, Vilotti S, Calligaris R, Lavina ZS, Biagioli M, Foti R, De Maso L, Pinto M, Gorza M, Speretta E, Casseler C, Tell G, Del Sal G, Gustincich S. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 2009;16:428–438. doi: 10.1038/cdd.2008.169. [DOI] [PubMed] [Google Scholar]
- 73.Andreotti AH. Native state proline isomerization: an intrinsic molecular switch. Biochemistry. 2003;42:9515–9524. doi: 10.1021/bi0350710. [DOI] [PubMed] [Google Scholar]
- 74.Sarkar P, Reichman C, Saleh T, Birge RB, Kalodimos CG. Proline cis-trans isomerization controls autoinhibition of a signaling protein. Molecular cell. 2007;25:413–426. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramachandran S, Venugopal A, KS, GR, Charles S, GD, Chandran NS, Mullassari A, Pillai MR, Kartha CC. Proteomic profiling of high glucose primed monocytes identifies cyclophilin A as a potential secretory marker of inflammation in type 2 diabetes. Proteomics. 2012;12:2808–2821. doi: 10.1002/pmic.201100586. [DOI] [PubMed] [Google Scholar]
- 76.Satoh K, Shimokawa H, Berk BC. Cyclophilin A: promising new target in cardiovascular therapy. Circ J. 2010;74:2249–2256. doi: 10.1253/circj.cj-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satoh K, Matoba T, Suzuki J, O’Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang LH, Youn HD, Liu JO. Inhibition of cell cycle progression by the novel cyclophilin ligand sanglifehrin A is mediated through the NFkappa B-dependent activation of p53. J Biol Chem. 2001;276:43534–43540. doi: 10.1074/jbc.M104257200. [DOI] [PubMed] [Google Scholar]
- 79.Bannon JH, O’Donovan DS, Kennelly SM, Mc Gee MM. The peptidyl prolyl isomerase cyclophilin A localizes at the centrosome and the midbody and is required for cytokinesis. Cell Cycle. 2012;11:1340–1353. doi: 10.4161/cc.19711. [DOI] [PubMed] [Google Scholar]
- 80.Bahmed K, Henry C, Holliday M, Redzic J, Ciobanu M, Zhang F, Weekes C, Sclafani R, Degregori J, Eisenmesser E. Extracellular cyclophilin-A stimulates ERK1/2 phosphorylation in a cell-dependent manner but broadly stimulates nuclear factor kappa B. Cancer Cell Int. 2012;12:19. doi: 10.1186/1475-2867-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez-Tillo E, Wojciechowska M, Comalada M, Farrera C, Lloberas J, Celada A. Cyclophilin A is required for M-CSF-dependent macrophage proliferation. Eur J Immunol. 2006;36:2515–2524. doi: 10.1002/eji.200535270. [DOI] [PubMed] [Google Scholar]
- 82.Ge YS, Teng WY, Zhang CD. Protective effect of cyclophilin A against Alzheimer’s amyloid beta-peptide (25–35)-induced oxidative stress in PC12 cells. Chin Med J. 2009;122:716–724. [PubMed] [Google Scholar]
- 83.Lee SP, Hwang YS, Kim YJ, Kwon KS, Kim HJ, Kim K, Chae HZ. Cyclophilin a binds to peroxiredoxins and activates its peroxidase activity. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]
- 84.Regent A, Dib H, Ly KH, Agard C, Tamby MC, Tamas N, Weksler B, Federici C, Broussard C, Guillevin L, Mouthon L. Identification of target antigens of anti-endothelial cell and anti-vascular smooth muscle cell antibodies in patients with giant cell arteritis: a proteomic approach. Arthritis Res Ther. 2011;13:R107. doi: 10.1186/ar3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors. 1999;10:207–209. doi: 10.1002/biof.5520100218. [DOI] [PubMed] [Google Scholar]
- 86.Netto LES, Chae HZ, Kang SW, Rhee SG, Stadtman ER. Removal of hydrogen peroxide by thiol-specific antioxidant enzyme (TSA) is involved with its antioxidant properties TSA possesses thiol peroxidase activity. J Biol Chem. 1996;271:15315–15321. doi: 10.1074/jbc.271.26.15315. [DOI] [PubMed] [Google Scholar]
- 87.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 88.Bentley AR, Emrani P, Cassano PA. Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: a systematic review. Thorax. 2008;63:956–961. doi: 10.1136/thx.2007.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 91.Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- 92.Wonsey DR, Zeller KI, Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc Natl Acad Sci U S A. 2002;99:6649–6654. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 95.Ischiropoulos H. Protein tyrosine nitration--an update. Arch Biochem Biophys. 2009;484:117–121. doi: 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 96.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 97.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite Mediates Active Site Tyrosine Nitration in Manganese Superoxide Dismutase. Evidence of a Role for the Carbonate Radical Anion. J Am Chem Soc. 2010;132:17174–17185. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frau M, Simile MM, Tomasi ML, Demartis MI, Daino L, Seddaiu MA, Brozzetti S, Feo CF, Massarelli G, Solinas G, Feo F, Lee JS, Pascale RM. An expression signature of phenotypic resistance to hepatocellular carcinoma identified by cross-species gene expression analysis. Cell Oncol (Dordr) 2012;35:163–173. doi: 10.1007/s13402-011-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butterfield DA, Reed T, Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic Res. 2011;45:59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- 100.Koppal T, Drake J, Yatin S, Jordan B, Varadarajan S, Bettenhausen L, Butterfield DA. Peroxynitrite-induced alterations in synaptosomal membrane proteins: insight into oxidative stress in Alzheimer’s disease. J Neurochem. 1999;72:310–317. doi: 10.1046/j.1471-4159.1999.0720310.x. [DOI] [PubMed] [Google Scholar]
- 101.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 102.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 103.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochem Z. 1924;152:309–344. [Google Scholar]
- 105.Warburg O. The metabolism of tumours. London: Constable; 1930. [Google Scholar]
- 106.Tukaj S, Trzonkowski P, Tukaj C. Regulatory effects of 1,25-dihydroxyvitamin D(3) on vascular smooth muscle cells. Acta Biochim Pol. 2012;59:395–400. [PubMed] [Google Scholar]
- 107.Halicka HD, Zhao H, Li J, Traganos F, Studzinski GP, Darzynkiewicz Z. Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3. Aging (Albany NY) 2012;4:270–278. doi: 10.18632/aging.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–512. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 109.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–224. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 110.Lefebvre d’Hellencourt C, Montero-Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003;71:575–582. doi: 10.1002/jnr.10491. [DOI] [PubMed] [Google Scholar]
- 111.Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabedian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994;38:214–220. doi: 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- 112.Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22:282–294. [PubMed] [Google Scholar]
- 113.McCarty MF. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med Hypotheses. 2006;67:251–269. doi: 10.1016/j.mehy.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 114.Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1995;92:10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 116.Minelli A, Grottelli S, Mierla A, Pinnen F, Cacciatore I, Bellezza I. Cyclo(His-Pro) exerts anti-inflammatory effects by modulating NF-kappaB and Nrf2 signalling. Int J Biochem Cell Biol. 2012;44:525–535. doi: 10.1016/j.biocel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Q, Sanlioglu S, Li S, Ritchie T, Oberley L, Engelhardt JF. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal. 2001;3:415–432. doi: 10.1089/15230860152409068. [DOI] [PubMed] [Google Scholar]
- 119.Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid Redox Signal. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- 120.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45:255–267. doi: 10.1016/s0169-328x(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 121.Garcion E, Sindji L, Nataf S, Brachet P, Darcy F, Montero-Menei CN. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003;105:438–448. doi: 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 122.Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am J Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]