Abstract
Glutathione (GSH) is a ubiquitous, redox active, small molecule that is critical to cellular and organism health. In red blood cells (RBCs), the influence of the environment (e.g. diet and lifestyle) on GSH levels has been demonstrated in numerous studies. However, it remains unknown if levels of GSH are determined principally by environmental factors, or if there is a genetic component, i.e. heritability. To investigate this we conducted a twin study. Twin studies are performed by comparing the similarity in phenotypes between mono- and di-zygotic twin pairs. We determined the heritability of GSH, as well as its oxidation product glutathione disulfide (GSSG), the sum of GSH equivalents (tGSH), and the status of the GSSG/2GSH couple (marker of oxidation status, Ehc) in RBCs. In our study population we found that the estimated heritability for the intracellular concentration of GSH in RBCs is 57 %; GSSG is 51 %, tGSH is 63 %, and Ehc is 70 %. We conclude that a major portion of the phenotype of these traits is controlled genetically. We anticipate that these heritabilities will also be reflected in other cell types. The discovery that genetics play a major role in the innate levels of redox active species in RBCs is paradigm-shifting and opens new avenues of research in the field of redox biology. Inherited RBC anti-oxidant levels may be important disease modifiers. By identifying the relative contributions of genes and the environment to anti-oxidant variation between individuals, new therapeutic strategies can be developed. Understanding the genetic determinants of these inherited traits may allow personalized approaches to relevant therapies.
Keywords: Glutathione, Red blood cells, Heritability, Twin study
Introduction
Glutathione (GSH) is an important redox active biomolecule critical in the maintenance and regulation of cellular and organismal health. Significantly lower population-mean levels of GSH in RBCs have been indicated in many disease states including but not limited to: acute exposure to drugs and toxins [1, 2, 3], protein-malnutrition [4], hormonal imbalance [5], genitourinary disease [6], gastrointestinal disease [6], cancer [6], cardiovascular disease [6], musculoskeletal disease [6], Parkinson’s disease [7], adult respiratory distress syndrome [8], diabetes mellitus [9, 10], liver disease [11], AIDS [12], cataracts [13], and aging [14, 15]. On the other hand, high availability of GSH in red blood cells (RBCs) has been correlated with longevity in mosquitos [16] and mice [17], and good health in elderly humans [18].
Major hurdles remain before levels of GSH can be used as a therapeutic target or as a diagnostic biomarker. Central questions remaining to be fully answered are: (a) does disease alter the levels of GSH in RBCs; (b) does the level of GSH influence the risk for disease of a population; and (c) what other factors determine the cellular and tissue levels of GSH. The goal of this research is to determine if there is a heritable component to observed levels of GSH in human RBCs.
Multiple studies of healthy individuals have observed a wide inter-individual range of GSH levels in RBCs (0.4 to 3.0 mM, Table 1). This large inter-individual range (coefficient of variation (CV) ≈ 36 % for the data in Table 1) is present regardless of age or method of detection. In contrast, intra-individual (i.e. within an individual) levels of GSH in RBCs are relatively stable over time (<10 % CV over a period of several months, 15 % over several years) [19, 20]. This suggests that GSH levels are maintained at an innate level that is distinct between individuals; leading to the hypothesis that: a major portion of the variability observed in GSH, glutathione disulfide (GSSG) concentrations, and Ehc status in RBCs is determined by genotypic differences.
Table 1.
Observed ranges of glutathione in human RBCs
| GSHa | GSH / mMb | Population | Methodc | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Units | Mean | SD | Range | |||
| 70 | 16.2 | 37 – 107 | mg / 100 cc pRBCsd | 1.8e | 0.4 | 0.9 – 2.6 | Healthy adults (n=117) | I | 47 |
| 71 | 16.6 | 31 – 118 | mg / 100 cc pRBCs | 1.8e | 0.4 | 0.8 – 2.9 | Non-Ashkenazi subjects | I | 48 |
| 73 | 11.9 | 42 – 114 | mg / 100 cc pRBCs | 1.9e | 0.3 | 1.0 – 2.8 | Ashkenazi subjects | I | 48 |
| 76 | 6.5 | 62 – 85 | mg / 100 cc pRBCs | 2.0e | 0.2 | 1.5 – 2.1 | G6PD normal patients (n=25) | I | 49 |
| 34 | 8.3 | 23 – 70 | mg / 100 cc pRBCs | 0.9e | 0.2 | 0.6 – 1.7 | Sensitive Iranian males(n=35) | I | 50 |
| 64 | 12.3 | 40 – 112 | mg / 100 cc pRBCs | 1.6e | 0.3 | 1.0 – 2.7 | Non-sensitive Iranian males(n=257) | I | 50 |
| 44 | 11.0 | 27 – 60 | mg / 100 cc pRBCs | 1.1e | 0.3 | 0.7 – 1.5 | Sensitive Iranian females(n=12) | I | 50 |
| 67 | 15.6 | 36 – 124 | mg / 100 cc pRBCs | 1.7e | 0.3 | 0.9 – 3.0 | Non-sensitive Iranian females(n=132) | I | 50 |
| 63 | - | 46 – 81 | mg / 100 cc pRBCs | 1.6e | - | 1.1 – 2.0 | Random samples (n=15) | II | 51 |
| 64 | - | 49 – 85 | mg / 100 cc pRBCs | 1.6e | - | 1.2 – 2.1 | Random samples (n=15) | III | 51 |
| 68 | 10.2 | 47 – 99 | mg / 100 cc pRBCs | 1.7e | 0.3 | 1.1 – 2.4 | Normal blood donors (n=85) | III | 51 |
| 80 | 12.9 | 52 – 118 | mg / 100 cc pRBCs | 2.1e | 0.3 | 1.3 – 2.9 | Normal infants (n=253) | III | 51 |
| 486 | 85 | 343 – 728 | μmole / L whole blood | 1.2f | 0.2 | 0.8 – 1.8 | Healthy adults (n=47) | IV | 52 |
| 337 | 138 | - | μmole / L whole blood | 0.8f | 0.3 | - | Elderly subjects (n=64) | IV | 52 |
| 3.5 | 1.8 | - | μM/ g Hgb | 1.2g | 0.6 | - | Young (31 ±10 years, n=33) | I | 53 |
| 2.3 | 0.9 | - | μM/ g Hgb | 0.8g | 0.3 | - | Aged (69±11 years, n=28) | I | 53 |
| 849 | 63 | 400 – 1400 | μmole /L RBCs | 0.8 | 0.6 | 0.4 – 1.4 | Healthy adults (18–73 years, n=107 men, 94 woman) | V | 54 |
| 1.0 | 0.2 | 0.7 – 1.6 | meq / L RBCs | 1.0 | 0.2 | 0.7 – 1.6 | Healthy men (n = 484) | VI | 55 |
| 1.0 | 0.2 | 0.7 – 1.9 | meq / L RBCs | 1.0 | 0.2 | 0.7 – 1.9 | Healthy woman (n = 231) | VI | 55 |
| 85 | 24.4 | 54 – 110 | mg / 100 cc pRBCs | 2.2e | 0.6 | 1.4 – 2.8 | Healthy adults (n=30) | I | 56 |
| 1549 | 133 | - | pmole / 107 cells | 1.7h | 0.1 | - | Unknown population | VII | 57 |
| 1.0 | 0.1 | 0.6 – 1.4 | mmole / L RBCs | 1.0 | 0.1 | 0.6 – 1.4 | Healthy adults (n = 10) | VI | 58 |
| 1133 | 63.5 | 1000 – 1220 | μmole / L RBCs | 1.1 | 0.1 | 1.0 – 1.2 | Healthy individuals aged 0.2 – 1 years (n=25) | VI | 59 |
| 1235 | 131 | 1038 – 1500 | μmole / L RBCs | 1.2 | 0.1 | 1.0 – 1.5 | Healthy individuals aged 2 – 11 years (n=28) | VI | 59 |
| 1257 | 99 | 1071 – 1473 | μmole / L RBCs | 1.3 | 0.1 | 1.1 – 1.5 | Healthy individuals aged 12 – 24 years (n=23) | VI | 59 |
| 1226 | 83 | 1118 – 1408 | μmole / L RBCs | 1.2 | 0.1 | 1.1 – 1.4 | Healthy individuals aged 25 – 40 years (n=40) | VI | 59 |
| 1024 | 137 | 745 – 1300 | μmole / L RBCs | 1.0 | 0.1 | 0.7 – 1.3 | Healthy individuals aged 41 – 69 years (n=60) | VI | 59 |
| Overall mean, SD and range | 1.4i | 0.5 | 0.4 – 3.0 | ||||||
Values as reported in the units provided.
Reported values converted to an estimated intracellular concentration (mM).
Legend of methods used to measure GSH: I: Beuttler [60]; II: Chlorpromazine displacement; III: Nitroprusside; IV: Capillary electrophoresis; V: HPLC with postcolumn derivatization and fluorometric detection; VI: DTNB; VII: Isotope dilution liquid chromatography-tandem mass spectrometry.
pRBCs = packed red blood cells.
Assuming packed RBCs hematocrit is 80 %.
Assuming an average hematocrit (Hct) of 40 %.
Assuming average hemoglobin (Hgb) of 140 g/L in whole blood and an Hct of 40%.
Assuming a mean corpuscular volume (MCV) of 90 fL.
Mean is not weighted.
To test this hypothesis we performed a classic twin study. Such a twin study compares the similarity of a trait in mono-zygotic (MZ) and di-zygotic (DZ) twins [21, 22]. For twin studies, this measure of similarity has been the intra-class correlation (ICC) [23]. With the ICC values for MZ and DZ twins for a given trait, the proportion of the observable inter-individual variation attributed to genotypic differences (i.e. heritability) can be estimated [24].
Materials and Methods
IRB Approval
The study was approved by the Human Subjects Office of The University of Iowa. Subjects were qualified for participation by meeting criteria for autologous blood donation according to standard operating procedures of The University of Iowa DeGowin Blood Center. Standard health history and demographic information were obtained at the time of enrollment and informed consent.
Recruitment
Twins (19 pairs; 14 MZ and 5 DZ pairs) were recruited to donate blood at the DeGowin Blood Center at the University of Iowa Hospitals and Clinics. Prior to standard blood donation, samples of whole blood for this study were collected. Twins were not required to come in as a pair, nor were there restrictions on the time of day for donations. This resulted in individuals within pairs to donate weeks apart and at different times of the day. Information on ethnicity or smoking status of subjects enrolled in the trial is not available; these topics are not queried on the standard health history form used to determine eligibility for donation of whole blood.
Zygosity testing
The zygosity of twin pairs was determined by isolating DNA from white blood cells (WBCs). White blood cells were obtained from leuko-reduction filters, utilized during the processing of the whole blood donation into components. Dulbecco's Phosphate-Buffered Saline (DPBS, 15 mL) was pushed through the filter to extract the WBCs. The DPBS with WBCs was collected into a 50 mL centrifuge tube. The 50 mL tube was centrifuged at 500 g for min. WBCs are resuspended in 2 mL of DPBS. DNA was extracted using the AutoGen (Holliston, MA) QuickGene-610L nucleic acid extraction machine with the Fuji QuickGene DNA Whole Blood Kit (AutoGen), following manufacturer’s instructions. Genotype was determined by probing for 24 single nucleotide polymorphisms (SNPs). SNP genotyping was performed with TaqMan assays (Applied Biosystems, Foster City, CA) on the EP1 SNP Genotyping System and GT48.48 Dynamic Array Integrated Fluidic Circuits (Fluidigm, San Francisco, CA). MZ twins had 90 % or greater genotype concordance out of 24 total SNPs. All other twin pairs were considered DZ. At least two independent determinations were performed on all twin pairs.
Sample preparation
Whole blood (EDTA, Vacutainer® purple top blood collection tube, 8 mL) was collected from donors prior to blood donation. The sample was centrifuged at 500 g for 5 min, followed by removal of the plasma and buffy coat. RBCs were washed twice with cold isotonic saline solution. After washing, a 30 µL aliquot of the packed red blood cells (pRBCs) was removed for complete blood count (CBC) analysis (Sysmex XE-2100™ Automated Hematology System); included in the CBC results are number per microliter, hemoglobin content, and mean volume of the RBCs. Three, 400 µL aliquots of pRBCs were each lysed with 500 µL of a 5 % perchloric acid/100 µM diethylenetriaminepentaacetic acid solution; this precipitates the protein and preserves glutathione (GSH) and glutathione disulfide (GSSG). Samples were thoroughly mixed and stored at −80 °C for a minimum of 1 day and a maximum of 2 months prior to HPLC analysis; control experiments demonstrated no significant changes in GSH or GSSG in this time. On the day of analysis, the three samples were thawed at room temperature (30 min) and centrifuged to pellet the protein (4000 g, 5 min). The supernatant (≈ 800 µL) for each sample was transferred to a separate Eppendorf tube. After an aliquot was removed for analysis, the remaining sample was stored at −80 °C.
Measurement of GSH and GSSG in RBCs with HPLC-BDD
To determine the amount of GSH and GSSG in RBCs, HPLC (ESA Coularray) with electrochemical detection (ECD) was used following the protocol outlined by Park et al. [25]. The method is based on an electrochemical detection system using a boron-doped diamond disc (BDD) electrode (Model 5040, ESA Biosciences, Chelmsford, MA, USA). Samples (65 µL) were loaded into auto sampler vials with a 100 µL glass insert. Samples were stored in the temperature controlled (+4 °C) auto sampler until analysis. At time of analysis the sample (10 µL) was loaded on the column and eluted for 60 min (number of RBCs on column = 10 – 40 million). With each set of samples, four standards containing GSH (500 – 8000 pmole on column) and GSSG (200 – 2000 pmole on column) were included; standard curves generated with each run were used to quantify the analytes for each sample set. Quantitation was performed by integrating the GSH and GSSG peaks in the BDD electrode channel with ESA Coularray® for Windows version 1.12.
Calculation of intracellular concentration for GSH and GSSG in RBCs
For each sample, the amount (mole) of GSH and GSSG per RBC was determined via the standard curve specific to each run. First, the GSH amount was divided by the number of RBCs associated with each sample (number obtained from CBC) giving a value of mole cell−1. The mole cell−1 value was then divided by the mean cell volume (MCV; value obtained from CBC) resulting in an intracellular concentration of mole L−1 for GSH and GSSG. The median of the three independently processed samples was taken to reflect the intracellular GSH and GSSG concentration of an individual. From these molar concentrations the status of the GSSG/2GSH couple was calculated using the Nernst equation Ehc = −255 – 30 log ([GSH]2/ [GSSG]) in mV [26], assuming an intracellular pH of 7.25 for RBCs and a temperature in vivo of 37 °C [27].
Calculation of ICC and Heritability
For twin studies the one-way model of intra-class correlation (ICC) is used to determine the similarity of a measure in a twin pair, ICC = (MSbetween − MSwithin) / (MSbetween + MSwithin), where MSbetween is the estimate of the mean-square variance between all twin-pairs and MSwithin is the estimate of the mean-square variance within the sets of pairs in that group [23]. The ICC is a variant of ANOVA analysis and is described on a scale of +1 to −1. An ICC that approaches +1 would be a near perfect one-to-one correlation within twin pairs and a large variation between twin pairs; an ICC of 0 would result from a set of data-points spread far apart between twin pairs with no one-to-one correlation within pairs; and an ICC of −1 would result from a data set with no one-to-one correlation within twin pairs and a small variation between twin pairs. An intra-class correlation approaching +1 is expected in MZ twins for a strongly heritable trait. The ICC of MZ and DZ pairs for GSH, GSSG and Ehc were calculated using IBM® SPPS® Statistics version 20. Heritability was estimated from the ICC values using the method derived by Newman, Freeman, and Holzinger, h2 = (ICCmz − ICCDZ) / (1 − ICCDZ) [24].
Results
Characterization of study population
Adult twins (aged 18 – 48 y) were recruited to The University of Iowa DeGowin Blood Center, Table 2. To be included, volunteers had to meet the requirements for donation of a standard unit of whole blood. Prior to donation, a blood sample was drawn as described in Materials and Methods. Fourteen MZ twin pairs (median age 24 ± 8 y; range, 18 – 48 y) and 5 DZ twin pairs (median age 22 ± 6 y; range, 18 – 33 y) were included in the study. There were no statistically significant differences in the mean height, weight or age between the MZ and DZ groups, Table 2. Zygosity was determined by DNA-based testing (See Materials and Methods.).
Table 2.
Characterization of study population
| Trait | Monozygotic (MZ) | Dizygotic (DZ) | p valuea |
|---|---|---|---|
| Age / y | 24 ± 8b | 22 ± 5 | 0.8 |
| Weight / kg | 64 ± 14 | 64 ± 8.6 | 0.6 |
| Height / m | 1.68 ± 0.07 | 1.73 ± 0.08 | 0.7 |
| Female pairs | 12 | 2 | - |
| Male pairs | 2 | 2 | - |
| Male/female pairs | - | 1 | - |
Dizygotic versus monozygotic
Median ± SD
Distribution and ranges of GSH, GSSG, tGSH, and Ehc in MZ and DZ twins
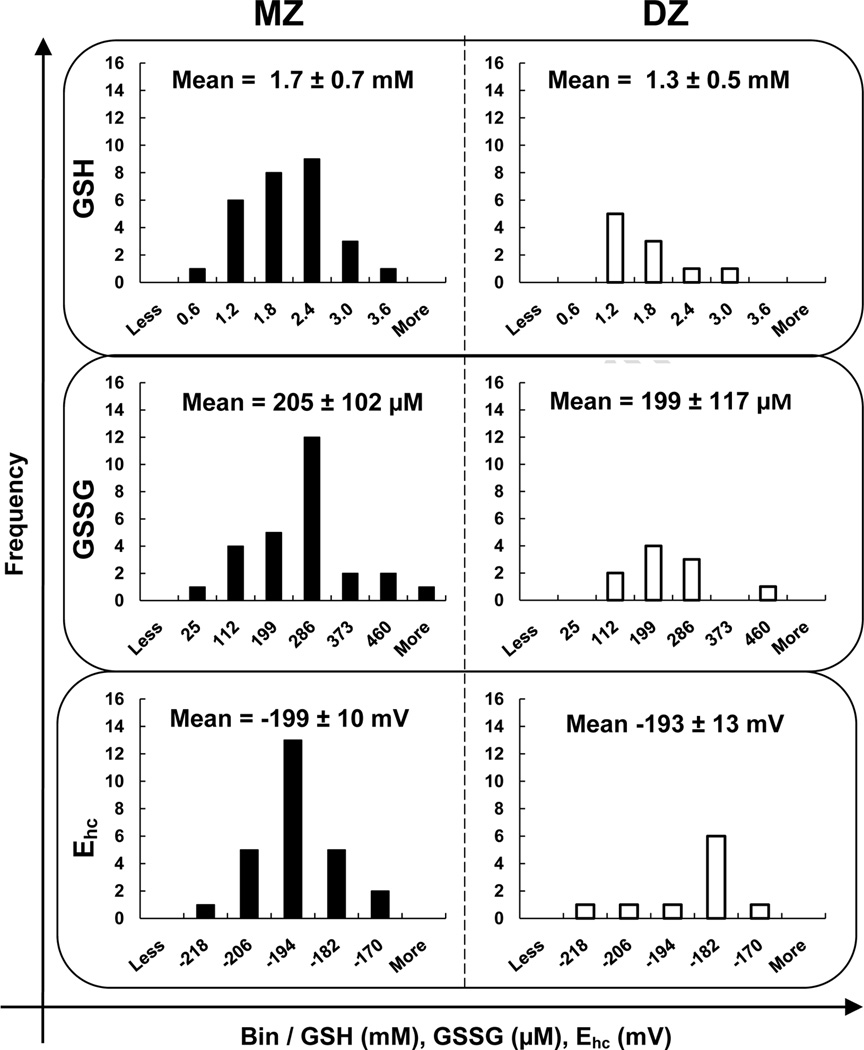
The GSH and GSSG levels in freshly collected RBCs from MZ and DZ twins were determined using high performance liquid chromatography with electrochemical detection. The median intracellular concentration of GSH in RBCs was 1.4 ± 0.7 mM with a range of 0.6 – 3.6 mM in the study population, Figure 1. This corresponds well with previous observations in healthy human RBCs (Table 1, intracellular GSH = 1.4 ± 0.5 mM, range = 0.4 – 3.0 mM). The median concentration of GSSG was 214 ± 114 µM with a range of 25 – 611 µM, Figure 1. These values for GSSG are somewhat higher, but within the range previously reported, 77 ± 38 µM [20]; observation of higher levels of GSSG may be due to the use of PCA as the precipitating acid as well as the detection methods. Total GSH equivalents (tGSH) is given by [tGSH] = [GSH] + 2[GSSG]. The median intracellular concentration of tGSH in RBCs was 2.0 ± 0.8 mM with a range of 0.7 – 4.4 mM in the study population. To determine the overall state of the GSH redox buffer in RBCs, the half-cell reduction potential (Ehc) for the GSSG/2GSH couple was determined. The median value for Ehc in RBCs was −199 ± 11 mV with a range of −173 to −223 mV, Figure 1. There were no statistically significant differences between the mean GSH, GSSG, and Ehc values for MZ and DZ twin groups.
Figure 1. The distributions of GSH, GSSG, and Ehc are very similar between MZ and DZ twins.
The intracellular concentration of GSH for MZ and DZ twins combined is: median = 1.4 mM; mean = 1.5 mM; standard deviation = 0.7 mM; and range = 0.6 – 3.6 mM. GSSG is: median = 214 µM; mean = 224 µM; standard deviation = 114 µM; and range = 25 – 611 µM. The halfcell reduction potential is: median = −199 mV; mean = −197 mV; standard deviation = 11 mV; and range = −173 to −223 mV. Bins were equally distributed between the highest and lowest values of the whole study population (MZ + DZ). The difference was divided by 5 to assign the size of the bins (e.g. GSH bin “0.6 mM” contains those individuals with a GSH concentration between 0.6 mM and 1.2 mM). There is no statistically significant difference (Students t-test) between the means of the two groups for all three analytes. This indicates that the only difference between the MZ and DZ groups is the genetic similarity of pairs within the study population.
Intra-class correlation of GSH, GSSG, tGSH, and Ehc for MZ and DZ twins
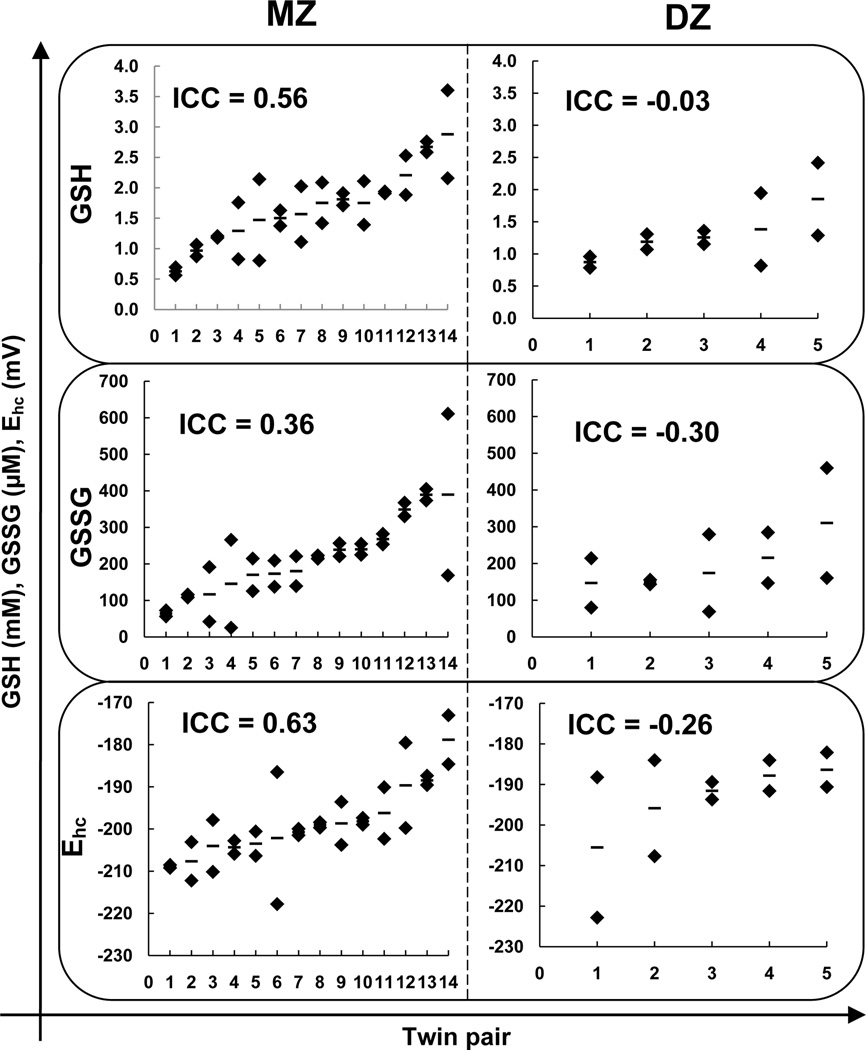
The ICC values for MZ twins were: 0.56 (95 % confidence interval 0.11–0.83) for GSH, 0.36 (95 % confidence interval −0.15–0.73) for GSSG, 0.56 (95 % confidence interval 0.10–0.83) for tGSH, and 0.63 (95 % confidence interval 0.2–0.86) for Ehc, while the ICC values for DZ twins were: −0.03 (95 % confidence interval −0.77–0.8) for GSH, −0.3 (95 % confidence interval − 0.86–0.67) for GSSG, −0.17 (95 % confidence interval −0.77–0.80) for tGSH and −0.26 (95 % confidence interval −0.85–0.70) for EhcFigure 2. The consistently stronger correlation for MZ twins over DZ twins is consistent with a heritable trait, Table 3.
Figure 2. The intra-class correlation is greater between MZ twins than DZ twins for GSH, GSSG, and Ehc.
(♦) are the measured values for GSH, GSSG or Ehc. (−) is the mean value for a twin pair. In our population, the ICC values for MZ twins were: 0.56 for GSH, 0.36 for GSSG, and 0.63 for Ehc. The ICC values for DZ twins were: −0.03 for GSH, −0.3 for GSSG, and −0.26 for Ehc. The consistently stronger correlation for MZ twins over DZ twins is consistent with a heritable trait. Values are the median of independently processed samples (n = 3).
Table 3.
Intra-class correlation coefficients for GSH, GSSG, tGSH, and Ehc
| Trait | Monozygotic (MZ) (n=14)a |
Dizygotic (DZ) (n=5)a |
p valueb |
Heritability (h2)c |
|---|---|---|---|---|
| GSH | 0.56 (0.11–0.83) | −0.03 (−0.78–0.80) | 0.39 | 57 |
| GSSG | 0.36 (−0.15–0.73) | −0.30 (−0.86–0.67) | 0.37 | 51 |
| tGSH | 0.56 (0.09–0.83) | −0.17 (−0.77–0.80) | 0.29 | 63 |
| Ehc | 0.63 (0.20–0.86) | −0.26 (−0.85–0.70) | 0.19 | 70 |
| Height | 0.94 (0.83–0.98) | −0.37 (−0.88–0.62) | 0.01 | 96 |
| BMI | 0.97 (0.93–0.99) | 0.92 (0.54–0.99) | 0.18 | 63 |
Intra-class correlation coefficients, values in parentheses are 95 % confidence intervals.
Two-tailed value for Fisher r-z transformation.
h2 = (ICCmz - ICCDZ) / (1 - ICCDZ) [24]
Heritability of GSH, GSSG, tGSH, and Ehc in RBCs
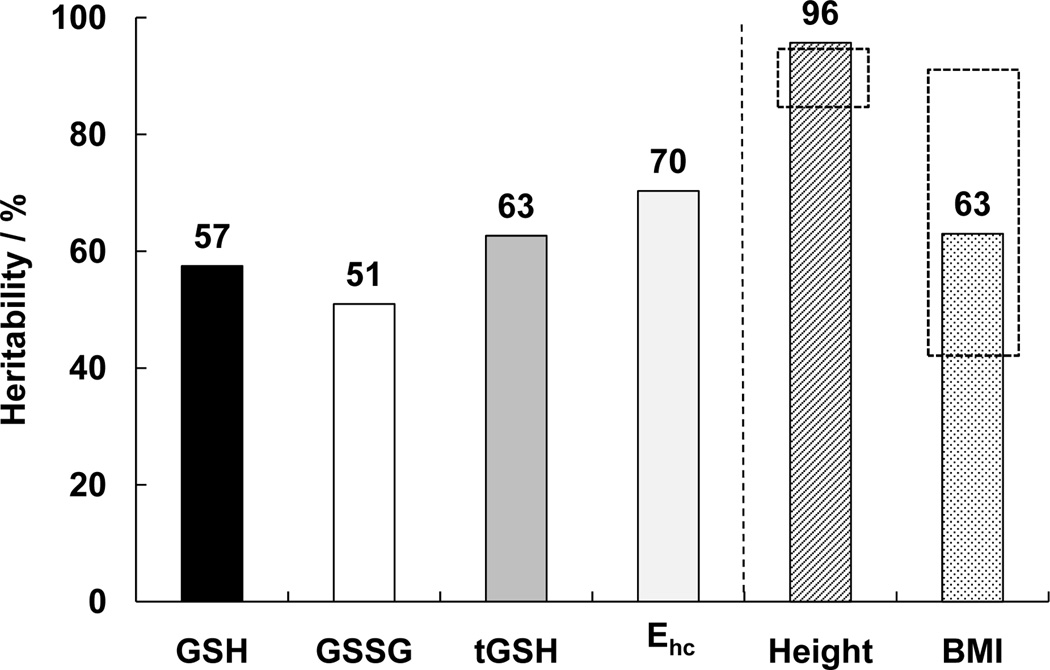
Heritability was estimated using the ICC values determined for GSH, GSSG, and Ehc (See Materials and Methods.). Figure 3 depicts the estimated heritabilities: GSH, 57 %; GSSG, 51 %; tGSH, 63 %, and Ehc, 70 %. These values for heritability indicate that a significant portion of the inter-individual variation can be explained by genotypic differences. For example, the range for inter-individual variation in GSH levels is 2.6 mM, Table 1. With heritability of GSH = 57 %, then 1.5 mM of the 2.6 mM range in inter-individual variation is due to genetic differences. The remainder is due to differences in environmental influences, e.g. diet and life style.
Figure 3. The concentrations of GSH, GSSG, Ehc and tGSH within RBCs behave as heritable traits.
Heritability was estimated using the method of Newman, Freeman, and Holzinger. The estimated heritability for GSH is 57 %, for GSSG 51 %, tGSH 63 %, and Ehc 70 van ‘t Erve et al. GSH is heritable in RBCs Page 20 of 29 %. The heritability for height (96 %) and BMI (63 %) were used as markers to compare our study population to other published reports on this established heritable trait (dashed boxes, height 87 – 93 %, BMI 39 – 91 %) [28].
Heritability of established heritable traits in this study population
To provide conformation that this study population is representative to allow reasonable heritability estimates, we determined the ICC and heritability of height as well as body-mass index, BMI. Height is a well-studied heritable trait that in affluent societies shows very high heritability, while BMI has a heritable component but will also have a significant environmental component. The ICC values for height in our study population are MZ = 0.94 and for DZ = −0.37, resulting in an estimated heritability of 96 %, Figure 3; this corresponds exceptionally well with previous reports of heritability (87 – 93 %) in the affluent societies of Western Europe [28]. The ICC values for BMI in our study population are MZ = 0.97 and for DZ = 0.92, which yields a heritability of 63 %, i.e. environmental influence is approximately 37%. Results from many studies on the heritability of body mass index yield a range of ICC from 0.39 to 0.91 in MZ twins with a weighted mean of 0.74 while the weighted mean for DZ twins is 0.32, yielding a heritability of 62 % [29].
Discussion
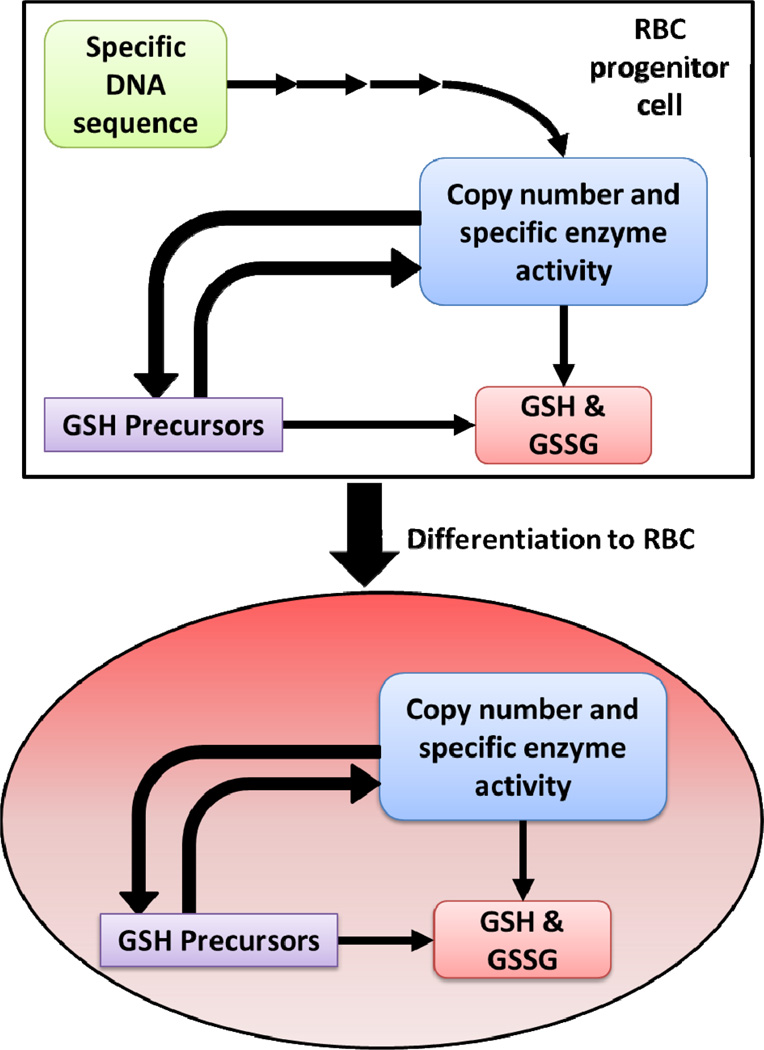
We present here compelling evidence that the intracellular concentrations of GSH, GSSG, tGSH, and Ehc in RBCs are heritable traits in our study population, i.e. the major portion of the phenotype of these traits is controlled genetically. The heritable genetic sequence of an individual will determine the specific activity and how many copies of enzymes controlling the level of GSH and GSSG are present in each cell, Figure 4. Enzymes that could be involved are: γ-glutamylcysteine synthetase, glutathione synthetase, glutathione peroxidase, glutathione disulfide reductase, glutathione-S-transferase, GSH or GSSG transport protein, cysteine or cystine transport proteins, glycine transport proteins, glutamate transport proteins, glucose-6-phosphate dehydrogenase, and many others.
Figure 4. Potential mechanisms of genetic influence for determining the levels of GSH and GSSG in RBCs.
In the progenitor cells for RBCs of each individual, specific inherited sequences of DNA are transcribed into proteins and enzymes. These enzymes have a specific activity and copy number as defined by the inherited DNA sequence. These many enzymes will directly or indirectly affect the level of GSH and GSSG. When the progenitor cells differentiate to RBCs these enzymes can no longer be renewed because the machinery for protein synthesis has been eliminated. This provides the mature RBC with a mostly locked enzyme environment. However, the set of enzymes needed to maintain the GSSG/2GSH system is fully functional; GSH turnover time is approximately 4–6 days [30, 31, 32]. The kinetic rate constants, copy number of active enzymes, and the availability of GSH precursors are at that point responsible for the steady-state levels of GSH and other small molecules.
In RBCs damaged proteins and enzymes are not discarded and replaced as the biochemical machinery for these tasks was lost upon differentiation of the progenitor cells to mature erythrocytes. However, glutathione is replaced with an estimated turnover time on the order of 4–6 days [30, 31, 32]. The intracellular concentration of GSH in RBCs will be a function of the rate of synthesis and rate of “loss” as well as associated recycling, control, and feedback mechanisms. Rate-limiting reactions involved in the turnover of GSH, such as investigated in [31, 32], would be logical starting points to probe for genetic mechanisms that lead to the observed heritability of the levels of GSH, GSSG, tGSH, and Ehc in RBCs.
Therapeutic implications
Identifying the genes regulating GSH levels in RBCs and possibly other tissues will be very important for clinical use of our finding. We would hypothesize that, since levels of GSH in RBCs are under substantial genetic control, that genes that regulate GSH may be important disease modifiers. Polymorphisms in these genes could be screened for early in life; this information could be coupled to other biomarkers of disease, informing patients on their risk for disease as well as the potential severity or progression. Based on this information, physicians can prioritize the monitoring of patients with the most substantial risk for severe, rapidly progressing disease.
Also personalized strategies to modify the levels of GSH could be implemented based on polymorphism profiles in these critical genes. By understanding the mechanism on how GSH levels are altered by disease, xenobiotic exposure, and environmental factors the variance between the innate (i.e. genetically determined) GSH level and the phenotypic GSH level could be used as a biomarker for general health and risk for secondary stresses. The levels of GSH in RBCs could be uniquely suited to reflect the status of the total GSH pool in an entire organism since it is such a diffuse organ reaching every corner of a body. This would eliminate the use of targeted approaches by e.g. biopsies.
Research implications
The heritability of GSH, GSSG, Ehc, and tGSH levels in RBCs could evolve the understanding of many more issues in human health besides direct therapeutic implications. The observed heritabilities could in part explain:
Why individuals are affected differently by aging, disease and other daily insults to our bodies;
Mechanisms of drug efficacy and adverse reactions on an individual basis (pharmacogenomics); and
Individual outcomes upon exposure to toxins and xenobiotics (toxicogenomics).
We hypothesize that the discovery of heritability will not be limited to only RBCs, but that these heritability estimates will be similar in other tissues.
Support for heritability of GSH in RBCs
There is support for our observations on heritability in published studies that determined GSH levels in RBCs. For instance, in a study of GSH in whole blood from two distinct Jewish populations, the distributions and ranges of GSH observed were quite different between the two populations but similar within the populations, consistent with a heritable trait [33]. In a twin study of toddlers (≈ 4 years old) Lang et al. estimated total GSH (GSH + GSSG) in RBCs to be 23 % heritable [34]. In a study of newborn infants, Küster et al. found an excellent correlation (R2 = 0.62) between the GSH concentration in RBCs from cord blood of newborns with the GSH concentration in the RBCs from their mothers; again, suggesting heritability of GSH in RBCs [35].
Heritability of other oxidative stress related species
Other researchers have looked at the heritability of markers of oxidative stress and compounds related to it. Broedbaek et al. investigated the influence of genetics on F2-isoprostanes, 8-oxo-7, 8-dihydro-2′-deoxyguanosine, and 8-oxo-7, 8-dihydroguanosine (lipid-, DNA-, and RNA-derived oxidation products) in a population of elderly Danish twins [36]. This study found little to no influence of genes on these markers of oxidative stress.
Chakraborty and Chaudhuri investigated the heritability of glucose-6-phosphate dehydrogenase, catalase, and glutathione peroxidase in RBCs [37]. In their study population these redox enzymes have significant heritabilities, 93, 72, and 60 % percent, respectively. These reports indicate that there might be a major genetic component to the anti-oxidant defense systems of cells; however the manifestation of oxidative stress could still be mostly dependent on environmental challenges.
Ehc in RBCs
The value of Ehc of the GSSG/2GSH couple, the principal component of the intracellular redox buffer, has been proposed as a key parameter associated with the fundamental biology of cells and tissues [26, 38]. For proliferating cells Ehc is approximately −240 mV (or more negative). Quiescent or differentiated cells have an Ehc ≈ −200 mV; cells undergoing apoptosis will have an Ehc ≈ −170 mV. If Ehc is more positive (more oxidized) than −160 mV, cells are considered necrotic. These values for Ehc and associated biology are conserved across many forms of life: from yeast [39]; to plants (including seeds) [40, 41, 42, 43, 44]; to mammals [38, 45]. The measured median value of −199 mV for Ehc is consistent with RBCs being a terminally differentiated cell; in addition the strong apparent heritability of Ehc is consistent with a fundamental biochemical parameter that is constant across individuals, as would be expected for Ehc.
Variations in human population compared to animals
Since errors of measurement in samples from individuals are relatively small (CV = 13 % across triplicate, independently processed samples), the large observed inter-individual range (GSH, CV = 37 %; GSSG, CV = 51 %) reflects the genetic diversity in this study population. Observed intra-individual variations in GSH levels are typically much smaller in animals utilized in laboratory research (CV =13 % in mosquitos and 8 % in mice) [14, 15, 17]. This smaller CV can be explained by the genetic similarity of the various strains of research animals [46].
Limitations
The estimates of heritability found here may not be reflected in all population groups. In addition, our estimates have been made on a relatively small sample size. However, estimates of heritability for two well-studied heritable traits match exceptionally well published estimates: height (estimated heritability of 96 % in this study compared to the published range of 87 – 93 % [28] and BMI (63 % in this study, published range 39 – 93 % with a weighted mean of 62 % [29]). Both papers present analyses of many studies that were carried out across many different populations, which included over 100,000 individuals. The high degree of similarity between these published values and our values for these two well-established heritable traits engender considerable confidence in our estimates on the heritability of GSH, GSSG, Ehc, and tGSH in RBCs.
Highlights.
The concentrations of GSH and GSSG in human red blood cells are genetically determined
There is a large variation between individuals in their GSH and GSSG levels
The half-cell reduction potential of the GSSG/2GSH couple is heritable
Individuals could respond differently to oxidative stress due to their genotype
Acknowledgments
The authors declare that they have no competing interests. This publication was supported by: the National Center for Advancing Translational Sciences through Grant 2UL1TR000442-06; and the National Institutes of Health (NIH) by grants R01GM073929, R01CA169046, P42ES013661, and P30ES05605. Core facilities were supported in part by the Holden Comprehensive Cancer Center, P30CA086862. The investigators thank: Dr. Jeffrey C. Murray, Allison Momany, and Dee A. Even (University of Iowa) for their technical expertise on twin studies and zygosity testing; the Widness lab (University of Iowa) and the Sysmex Corporation, Kobe, Japan for the use of the XE-2100 and XT-2000 automated hematology analyzers (P01 HL46925); the staff of The University of Iowa DeGowin Blood Center for their assistance in recruiting subjects and obtaining the blood samples. The ESR Facility at The University of Iowa provided invaluable support. The content is solely the responsibility of the authors and does not represent views of The University of Iowa or the National Institutes of Health.
Abbreviations
- BDD
boron-doped diamond electrode
- CV
coefficient of variation
- DZ
dizygotic
- Ehc
half-cell reduction potential
- GSH
glutathione
- GSSG
glutathione disulfide
- ICC
intraclass correlation coefficient
- MZ
monozygotic
- RBC
red blood cell
- tGSH
total GSH equivalents, i.e. [tGSH] = [GSH] + 2[GSSG]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Song H, Lang CA, Chen TS. The role of glutathione in p-aminophenol-induced nephrotoxicity in the mouse. Drug Chem Toxicol. 1999;22(3):529–544. doi: 10.3109/01480549909042530. [DOI] [PubMed] [Google Scholar]
- 2.Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, Zappia I, Newmark S, Gehn E, Rubin RA, Mitchell K, Bradstreet J, El-Dahr J. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: Part A--medical results. BMC Clin Pharmacol. 2009;9(17) doi: 10.1186/1472-6904-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diken H, Kelle M, Tumer C, Deniz B, Baylan Y, Sermet A. Effects of cigarette smoking on blood antioxidant status in short-term and long-term smokers. Turk J Med Sci. 2001;31:553–557. [Google Scholar]
- 4.Li J, Wang H, Stoner GD, Bray TM. Dietary supplementation with cysteine prodrugs selectively restores tissue glutathione levels and redox status in protein-malnourished mice. J Nutr Biochem. 2002;13(10):625–633. doi: 10.1016/s0955-2863(02)00218-8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CG, Nagy LE, Bray TM. Nutritional and hormonal regulation of glutathione homeostasis. Curr Top Cell Regul. 1996;34:189–208. doi: 10.1016/s0070-2137(96)80007-0. [DOI] [PubMed] [Google Scholar]
- 6.Lang CA, Mills BJ, Mastropaolo W, Liu MC. Blood glutathione decreases in chronic diseases. J Lab Clin Med. 2000;135:402–405. doi: 10.1067/mlc.2000.105977. [DOI] [PubMed] [Google Scholar]
- 7.Chinta SJ, Rajagopalan S, Butterfield DA, Andersen JK. In vitro and in vivo neuroprotection by γ-glutamylcysteine ethyl ester against MPTP: Relevance to the role of glutathione in Parkinson's disease. Neurosci Lett. 2006;402(1–2):137–141. doi: 10.1016/j.neulet.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetyl cysteine and procysteine in ARDS. Chest. 1997;112(1):164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 9.Forrester TE, Badaloo V, Bennett FI, Jackson AA. Excessive excretion and decreased levels of blood glutathione in type II diabetes mellitus. Eur J Clin Nutr. 1990;44:847–850. [PubMed] [Google Scholar]
- 10.Murakami K, Kondo T, Ohtsuka Y, Shimada M, Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989;38:753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 11.Altomare E, Vendemiale G, Albano O. Hepatic glutathione content in patients with alcoholic and non-alcoholic liver diseases. Life Sci. 1988;43:991–998. doi: 10.1016/0024-3205(88)90544-9. [DOI] [PubMed] [Google Scholar]
- 12.Buhl R, Jaffe HA, Holroyd KJ, Wells FB, Mastrangli A, Cantin A, Saltini C, Cantin AM, Crystal RG. Systemic glutathione deficiency in symptom free HIV-seropositive individuals. Lancet. 1989;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- 13.Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970;117:957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazelton GA, Lang CA. Glutathione biosynthesis in the aging adult yellow-fever mosquito [Aedes aegypti (Louisville)] Biochem J. 1983;210(2):289–295. doi: 10.1042/bj2100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. Biochem J. 1980;188(1):25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richie JP, Jr, Mills BJ, Lang CA. Correction of a glutathione deficiency in the aging mosquito increases its longevity. Proc Soc Exp Biol Med. 1987;184:113–117. doi: 10.3181/00379727-184-42454. [DOI] [PubMed] [Google Scholar]
- 17.Rebrin I, Forster MJ, Sohal RS. Association between life-span extension by caloric restriction and thiol redox state in two different strains of mice. Free Radic Biol Med. 2011;51:225–233. doi: 10.1016/j.freeradbiomed.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang CA, Mills BJ, Lang HL, Liu MC, Usui WM, Richie JP, Jr, Mastropaolo W, Murrell SA. High blood glutathione levels accompany excellent physical and mental health in women ages 60 to 103 years. J Lab Clin Med. 2002;140(6):413–417. doi: 10.1067/mlc.2002.129504. [DOI] [PubMed] [Google Scholar]
- 19.Richie JP, Jr, Abraham P, Leutzinger Y. Long-term stability of blood glutathione and cysteine in humans. Clin Chem. 1996;42(7):1100–1105. [PubMed] [Google Scholar]
- 20.Mills BJ, Richie JP, Jr, Lang CA. Glutathione disulfide variability in normal human blood. Anal Biochem. 1994;222(1):95–101. doi: 10.1006/abio.1994.1459. [DOI] [PubMed] [Google Scholar]
- 21.Newman HH, Freeman FN, Holzinger JJ. Twins: a study of heredity and environment. Chicago: The University of Chicago Press; 1937. [Google Scholar]
- 22.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3(11):872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 23.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 24.Kang KW, Christian JC, Norton JA., Jr Heritability estimates from twin studies. I. Formulae of heritability estimates. Acta Genet Med Gemellol (Roma) 1978;27:39–44. doi: 10.1017/s000156600000948x. [DOI] [PubMed] [Google Scholar]
- 25.Park HJ, Mah E, Bruno RS. Validation of high-performance liquid chromatographyboron- doped diamond detection for assessing hepatic glutathione redox status. Anal Biochem. 2010;407:151–159. doi: 10.1016/j.ab.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Schafer FQ, Buettner GR. Redox state of the cell as viewed though the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 27.Swietach P, Tiffert T, Mauritz JMA, Seear R, Esposito A, Kaminski CF, Lew VL, Vaughan-Jones RD. Hydrogen ion dynamics in human red blood cells. J Physiol. 2010;588(24):4995–5014. doi: 10.1113/jphysiol.2010.197392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silventoinen K, Sammalisto S, Perola M, Boomsma DI, Cornes BK, Davis C, Dunkel L, De Lange M, Harris JR, Hjelmborg JV, Luciano M, Martin NG, Mortensen J, Nisticò L, Pedersen NL, Skytthe A, Spector TD, Stazi MA, Willemsen G, Kaprio J. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 2003;6(5):399–408. doi: 10.1375/136905203770326402. [DOI] [PubMed] [Google Scholar]
- 29.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27(4):325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 30.Dimant E, Landsberg E, London IM. The metabolic behavior of reduced glutathione in human and avian erythrocytes. J Biol Chem. 1955;213(2):769–776. [PubMed] [Google Scholar]
- 31.Griffith OW. Glutathione turnover in human erythrocytes. Inhibition by buthionine sulfoximine and incorporation of glycine by exchange. J Biol Chem. 1981;256(10):4900–4904. [PubMed] [Google Scholar]
- 32.Raftos JE, Whillier S, Kuchel PW. Glutathione synthesis and turnover in the human erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem. 2010;285(31):23557–23567. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szeinberg A, Sheba C, Adam A. Selective occurrence of glutathione instability in red blood corpuscles of the various Jewish tribes. Blood. 1958;13:1043–1053. [PubMed] [Google Scholar]
- 34.Lang CA, Matheny AP, Jr, Mastropaolo W, Liu MC. Blood glutathione and cysteine concentrations in twin children. Exp Biol Med (Maywood) 2001;226(4):349–352. doi: 10.1177/153537020122600413. [DOI] [PubMed] [Google Scholar]
- 35.Küster A, Tea I, Ferchaud-Roucher V, Le Borgne S, Plouzennec C, Winer N, Rozé JC, Robins RJ, Darmaun D. Cord blood glutathione depletion in preterm infants: correlation with maternal cysteine depletion. PLoS One. 2011;6(11):e27626. doi: 10.1371/journal.pone.0027626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broedbaek K, Ribel-Madsen R, Henriksen T, Weimann A, Petersen M, Andersen JT, Afzal S, Hjelvang B, Roberts LJ, 2nd, Vaag A, Poulsen P, Poulsen HE. Genetic and environmental influences on oxidative damage assessed in elderly Danish twins. Free Radic Biol Med. 2011;50(11):1488–1491. doi: 10.1016/j.freeradbiomed.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty S, Chaudhuri ABD. Heritability of some important parameters of the antioxidant defense system like glucose-6-phosphate dehydrogenase, catalase, glutathione peroxidase and lipid peroxidation in red blood cells by twin study. Int J Health Geogr. 2001;1(1) [Google Scholar]
- 38.Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J Intern Med. 2010;268(5):432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruhlke MC, Portz D, Stitz M, Anwar A, Schneider T, Jacob C, Schlaich NL, Slusarenko AJ. Allicin disrupts the cell's electrochemical potential and induces apoptosis in yeast. Free Radic Biol Med. 2010;49(12):1916–1924. doi: 10.1016/j.freeradbiomed.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Kranner I, Birtić S, Anderson KM, Pritchard HW. Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radic Biol Med. 2006;40(12):2155–2165. doi: 10.1016/j.freeradbiomed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Seal CE, Zammit R, Scott P, Flowers TJ, Kranner I. Glutathione half-cell reduction potential and alpha-tocopherol as viability markers during the prolonged storage of Suaeda maritima seeds. Seed Science Research. 2010;20:47–53l. [Google Scholar]
- 42.Seal CE, Zammit R, Scott P, Nyamongo DO, Daws MI, Kranner I. Glutathione halfcell reduction potential as a seed viability marker of the potential oilseed crop Vernonia galamensis . Industrial Crops and Products. 2010;32:687–691. [Google Scholar]
- 43.Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts; definitions and applications in seed science. New Phytol. 2010;188(3):655–73. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- 44.Birtić S, Colville L, Pritchard HW, Pearce SR, Kranner I. Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing. Free Radic Res. 2011;45(9):1093–1102. doi: 10.3109/10715762.2011.595409. [DOI] [PubMed] [Google Scholar]
- 45.Buettner GR. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer. Agents Med Chem. 2011;11:341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui S, Chesson C, Hope R. Genetic variation within and between strains of outbred Swiss mice. Lab Anim. 1993;27(2):116–123. doi: 10.1258/002367793780810397. [DOI] [PubMed] [Google Scholar]
- 47.Szeinberg A, Asher Y, Sheba C. Studies on glutathione stability in erythrocytes of cases with past history of favism or sulfa-drug-induced hemolysis. Blood. 1958;13:348–358. [PubMed] [Google Scholar]
- 48.Szeinberg A, Sheba CH, Adam A. selective occurrence of glutathione instability in red blood corpuscles of the various Jewish tribes. Blood. 1958;13:1043–1053. [PubMed] [Google Scholar]
- 49.Sabine JC. Glutathione concentration and stability in the red blood cells in various disease states; and some observations on the mechanism of action of acetyl-phenylhydrazine. Br J Haematol. 1964;10:477–484. doi: 10.1111/j.1365-2141.1964.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 50.Walker DG, Bowman JE. Glutathione stability of erythrocytes in Iranians. Nature. 1959;184:1325. doi: 10.1038/1841325a0. [DOI] [PubMed] [Google Scholar]
- 51.Kum-Tatt L, Tan IK. A new colorimetric method for the determination of glutathione in erythrocytes. Clin Chim Acta. 1974;53(2):153–161. doi: 10.1016/0009-8981(74)90093-x. [DOI] [PubMed] [Google Scholar]
- 52.Serru V, Baudin B, Ziegler F, David JP, Cals MJ, Vaubourdolle M, Mario N. Quantification of Reduced and Oxidized Glutathione in whole blood samples by capillary electrophoresis. Clin Chem. 2001;47:1321–1324. [PubMed] [Google Scholar]
- 53.Matsubara LS, Machado PE. Age-related changes of glutathione content; glutathione reductase and glutathione peroxidase activity of human erythrocytes. Braz J Med Biol Res. 1991;24(5):449–454. [PubMed] [Google Scholar]
- 54.Michelet F, Gueguen R, Leroy P, Wellman M, Nicolas A, Siest G. Blood and plasma glutathione measured in healthy subjects by HPLC: relation to sex; aging; biological variables; and life habits. Clin Chem. 1995;41(10):1509–1517. [PubMed] [Google Scholar]
- 55.Richie JP, Jr, Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clinical Chemistry. 1996;42:64–70. [PubMed] [Google Scholar]
- 56.Schroter W, Schulz E, Bonn R. Die bedeutung de glutathione konzentration der erythrocyten fur die diagnose de osteomyelofibrose. Klinische Wochenschrift. 1970;48(16):1013–1014. doi: 10.1007/BF01484411. [DOI] [PubMed] [Google Scholar]
- 57.Harwood DT, Kettle AJ, Brennan S, Winterbourn CC. Simultaneous determination of reduced glutathione; glutathione disulphide and glutathione sulphonamide in cells and physiological fluids by isotope dilution liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(28):3393–3399. doi: 10.1016/j.jchromb.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Richie JP, Jr, Abraham P, Leutzinger Y. Long-term stability of blood glutathione and cysteine in humans. Clin Chem. 1996;42(7):1100–1105. [PubMed] [Google Scholar]
- 59.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–66. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 60.Buetler E, Robson MJ, Buttenwieser E. The glutathione instability of drug-sensitive red cells; a new method for the in vitro detection of drug sensitivity. J. Lab. Clin. Med. 1957;49:84–95. [PubMed] [Google Scholar]