Abstract
Ethanol-inducible cytochrome P450 2E1 (CYP2E1) contributes to increased oxidative stress and steatosis in chronic alcohol-exposure models. However, its role in binge ethanol-induced gut leakiness and hepatic injury is unclear. This study was aimed to investigate the role of CYP2E1 in binge alcohol-induced gut leakiness and the mechanisms of steatohepatitis. Female wild-type (WT) and Cyp2e1-null mice were treated with three doses of binge ethanol (WT-EtOH or Cyp2e1-null-EtOH) (6 g/kg oral gavage at 12-h intervals) or dextrose (negative control). Intestinal histology of only WT-EtOH exhibited epithelial alteration and blebbing of lamina propria while liver histology obtained at 6 h after the last ethanol dose showed elevated steatosis with scattered inflammatory foci. These were accompanied by increased levels of serum endotoxin, hepatic enterobacteria and triglycerides. All these changes including the intestinal histology and hepatic apoptosis, determined by TUNEL assay, were significantly reversed when WT-EtOH mice were treated with the specific inhibitor of CYP2E1 chlormethiazole and the antioxidant N-acetyl-cysteine, both of which suppressed the oxidative markers including intestinal CYP2E1. WT-EtOH also exhibited elevated amounts of serum TNF-α, hepatic cytokines, CYP2E1 and lipid peroxidation with decreased levels of mitochondrial superoxide dismutase and suppressed aldehyde dehydrogenase 2 activity. Increased hepatocyte apoptosis with elevated levels of pro-apoptotic proteins and decreased levels of active (phosphorylated) p-AKT, p-AMPK and peroxisome proliferator-activated receptor-alpha (PPAR-α), all of which are involved in fat metabolism and inflammation, were observed in WT-EtOH. These changes were significantly attenuated in the corresponding Cyp2e1-null-EtOH mice. These data indicate that both intestinal and hepatic CYP2E1 induced by binge alcohol seem critical in the binge alcohol-mediated increased nitroxidative stress, gut leakage, endotoxemia, and altered fat metabolism, and inflammation, contributing to hepatic apoptosis and steatohepatitis.
Keywords: Liver, binge ethanol, CYP2E1, oxidative stress, gut leakiness, steatohepatitis, apoptosis
Introduction
Alcohol (ethanol)-induced liver disease (ALD) is one of the leading causes of morbidity and mortality from hepatic complications in the United States and the World [1, 2]. Alcoholic fatty liver disease (AFLD) is a condition characterized by simple fat accumulation (steatosis) which can advance to more severe liver disease such as steatohepatitis, fibrosis, cirrhosis, hepatocellular carcinoma and liver failure, especially in people who drink more than 60 g/day [3, 4]. The “two-hit” hypothesis described the pathophysiology of advancement of non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis (NASH) and this hypothesis can be also applied to the advancement of AFLD to alcoholic steatohepatitis (ASH) [5].
Steatosis (primary insult) may prime the liver to secondary insults to promote progression of AFLD to ASH. The secondary insults include reactive oxygen species (ROS), gut bacterial overgrowth and translocation, gut-derived endotoxins, pro-inflammatory cytokines, and dysregulation of innate immunity, all contributing to ASH development [6, 7]. ASH and NASH are associated with increased levels of ROS originated from cytochrome P450 2E1 (CYP2E1), NADPH oxidase, xanthine oxidase, the mitochondrial electron transport chain, etc [8]. CYP2E1, which exhibits increased expression and activity in the livers of humans and animal models of AFLD, is involved in the metabolism and oxidation of small molecule substrates such as ethanol, fatty acids and xenobiotics [9]. In fact, CYP2E1 is one of the key players by which alcohol generates ROS in liver and other tissues [8, 9].
Although chronic alcohol consumption is the major cause of ALD, frequent and recurrent acute alcohol drinking (binge drinking) is also common and yet its potential injurious effects on the liver and the mechanism(s) involved remain elusive. The acute liver injury could result from increased gut leakiness, as demonstrated in human patients with alcoholic and nonalcoholic cirrhosis without chronic liver disease and in rats [10, 11]. Recently, using male mice and two different binge models, CYP2E1 was suggested to be essential for development of hepatic steatosis and increased oxidative stress [12, 13]. However, the role of intestinal CYP2E1 in ethanol-mediated gut leakiness, leading to hepatic inflammation, oxidative injury and apoptosis has not been fully understood.
Thus, the aims of this study were to evaluate: (1) Whether intestinal CYP2E1 is involved in enhancing gut leakiness, enterobacterial translocation to the liver and hepatic inflammation after binge alcohol exposure in wild-type (WT) or Cyp2e1-null mice; (2) whether hepatic CYP2E1 is important in promoting ASH and enhanced apoptosis through increasing oxidative stress; and (3) Whether endotoxemia and fatty liver observed in WT-EtOH can be ameliorated by treatment with an inhibitor of CYP2E1, chlormethiazole (CMZ), or an anti-oxidant, N-acetyl-cysteine (NAC).
Materials and methods
Animal treatment and histopathology analysis
Age-matched female Cyp2e1-null mice and the genetically corresponding WT mice on 129/Svj background were randomly assigned to four groups and exposed to 3 doses of ethanol (6 g/kg oral gavage) or dextrose (control) at 12-h intervals (n=5/group): (1) WT-dextrose (WT-DEX); (2) WT-ethanol (WT-EtOH); (3) Cyp2e1-null-dextrose (Cyp2e1-null-DEX); and (4) Cyp2e1-null-ethanol (Cyp2e1-null-EtOH). Some WT mice (n=4/group) were pretreated with CMZ (50 mg/kg ip injection) 15-h before the first dose of ethanol and with 50 mg CMZ/kg immediately following each of the 3 ethanol doses. Another set of animals (n=3/group) were pretreated with NAC (100 mg/kg ip injection) 24-h before the first dose of ethanol and at 1-h before each administration of the 3 ethanol doses. At 1-h or 6-h following the last dose of ethanol or dextrose, blood, intestine, and liver tissues under strict sterile conditions were collected for further evaluations. These time points were selected, based on our several preliminary studies. Weight of each mouse was recorded before the start of ethanol exposure and at the time of euthanasia. Small liver and lower intestinal sections were subjected to H&E staining for histology examinations. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Hepatic tissue extraction
Total liver homogenates were prepared in ice-cold extraction buffer (50 mM Tris-Cl, pH 7.5, 1 mM EDTA, and 1% CHAPS). Nuclear, mitochondrial, and cytosolic fractions were prepared by differential centrifugations and all extraction buffers were pre-equilibrated with nitrogen gas to remove the dissolved oxygen, as previously described [14, 15]
Please refer to Supplementary Materials and Methods for all other methods.
Results
Evaluation of hepatic steatosis, inflammation, and injury
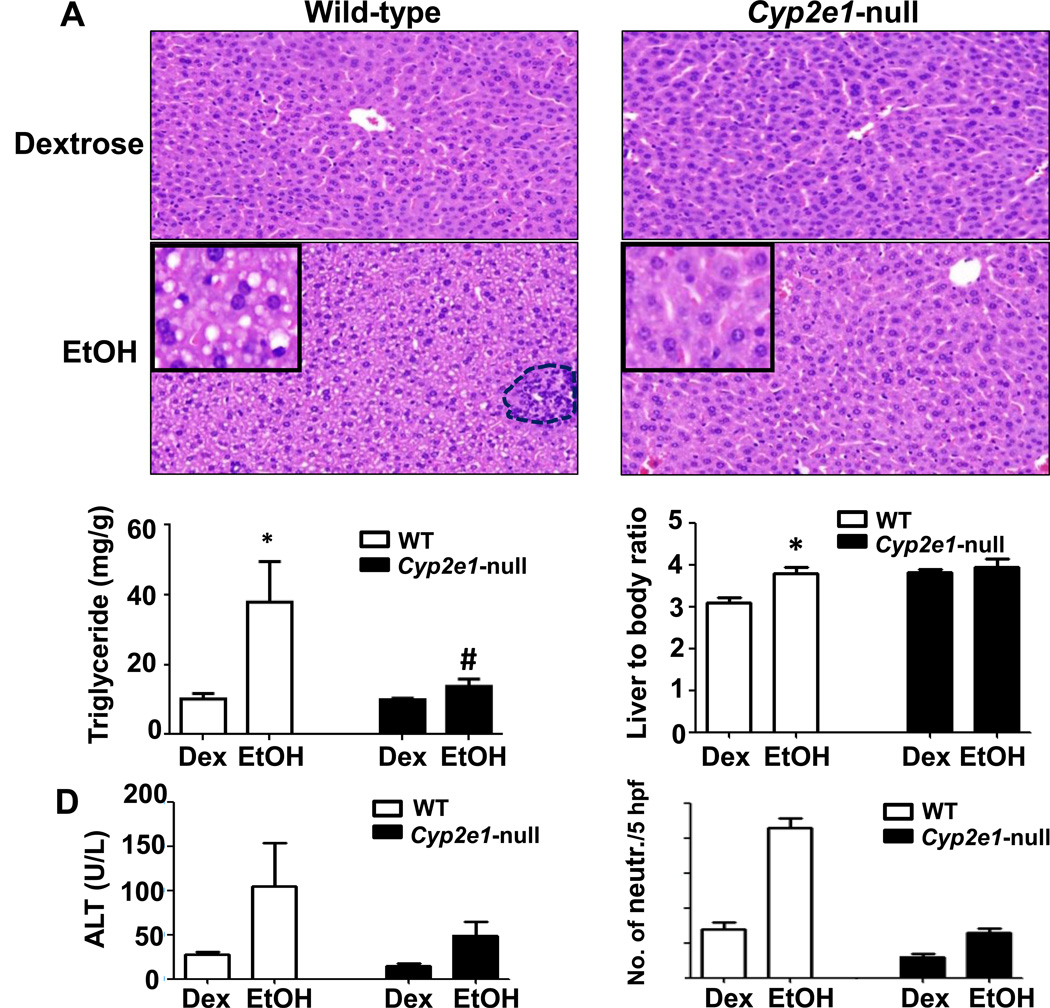
Livers of WT and Cyp2e1-null mice treated with dextrose (DEX) look grossly (not shown) and histologically normal, while the livers from WT-EtOH exhibited marked accumulation of micro-vesicular intracellular lipid droplets; livers from Cyp2e1-null-EtOH are histologically normal (Fig. 1). The histological appearance of microvesicular fat (Fig. 1, inset) was supported by the significantly higher levels of hepatic triglyceride (TG) in WT-EtOH compared to all other groups. The TG levels were also slightly, but significantly, increased in Cyp2e1-null-EtOH compared to the corresponding control (Cyp2e1-null-DEX), but significantly attenuated compared to WT-EtOH (Fig. 1B). Furthermore, the ratio of liver to body weight was significantly higher only in WT-EtOH compared to the WT-DEX control (Fig. 1C). WT-EtOH group showed the highest plasma ALT activities but did not reach the significance levels (Fig. 1D). A few scattered inflammatory foci were observed only in the WT-EtOH (Fig. 1A, dotted circle). The inflammation score, which represents the number of neutrophils per 5 high-power fields (HPF) (40x), was significantly higher in WT-EtOH compared to all other groups (Fig. 1E).
Fig. 1. Effects of binge ethanol on hepatic steatosis, and inflammation.
(A) Representative H&E staining of livers for indicated groups with inflammatory foci (a dotted circle) and (B) hepatic TG levels are shown. (C) Liver/body weight ratios, (D) plasma ALT levels at 6-h post-EtOH, and (E) summary of histochemical detection of neutrophils are presented. Data are expressed as mean ± SEM (n=5 mice/group). *p<0.05, *Significantly different from all other groups; #significantly different from the corresponding Cyp2e1-null-DEX control group.
Effects of binge ethanol on endotoxemia, hepatic enterobacterial contents, histopathological changes and markers of oxidative stress in small intestine
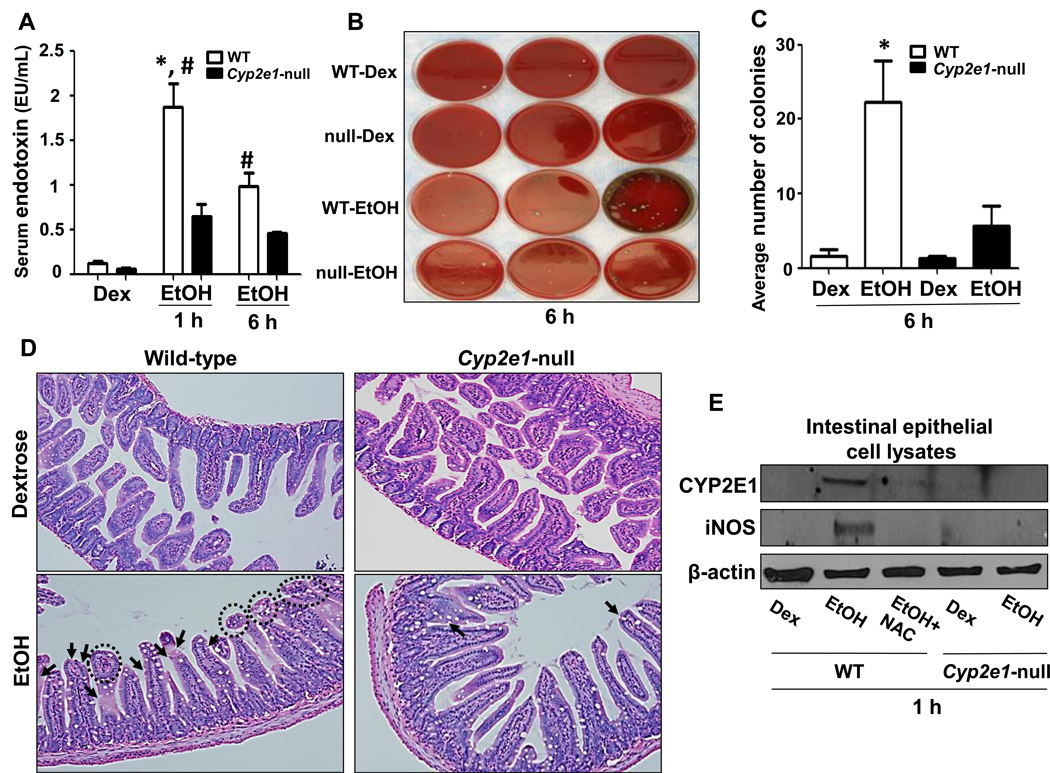
Endotoxin is an etiological factor in the development of ALD [11, 16]. Thus, we studied whether intestinal CYP2E1 plays an important role in promoting binge ethanol-induced endotoxemia by evaluating the levels of serum endotoxins and hepatic enterobacterial contents. Although serum endotoxin levels were higher in both WT-EtOH and Cyp2e1-null-EtOH than their respective corresponding controls, the endotoxin levels in WT-EtOH were significantly higher than the corresponding Cyp2e1-null-EtOH groups collected at both 1-h and 6-h post-ethanol administration (Fig. 2A). However, the highest level of endotoxin in WT-EtOH was observed at 1-h. We further examined the gut leakiness by indirectly measuring the enterobacterial contents in the liver by using the sheep blood agar plates [17]. The number of enterobacterial colonies in the fresh liver extracts from WT-EtOH was significantly higher than all other treatment groups (Figs. 2B and C), suggesting increased gut leakiness. Histopathological analysis (Fig. 2D) revealed markedly increased epithelial loss (arrows) and blebbing (dotted circles) of the lamina propria of small intestine (ileum) villi in WT-EtOH mice compared to all other groups. These histological changes were ameliorated when WT-EtOH mice were pretreated with CMZ or NAC (Supplemental Figs. 1A-D). In addition, NAC pretreatment significantly blocked the markedly elevated CYP2E1 and iNOS, oxidative stress marker proteins, in the intestines of WT-EtOH (Fig. 2E). To confirm the role of CYP2E1 in promoting oxidative stress, we determined the levels of 3-nitrotyrosine (3-NT), a foot print of peroxynitrite, as a marker of oxidative stress. Our results showed that binge ethanol markedly increased the levels of 3-NT in the intestines of WT mice compared to all other groups and that this increase was not observed in the ethanol-exposed Cyp2e1-null mice or ameliorated by CMZ, which also prevented the elevation of iNOS (Supplemental Fig. 1E). Consistent with the increased endotoxemia in alcohol-fed WT mice, serum TNF-α levels, determined by multiplex bead array assay, were significantly increased only in WT-EtOH compared to the corresponding control (Suppl. Fig. 2A). Furthermore the hepatic mRNA levels of TNF-α, INFγ and plasminogen activator inhibitor-1 (PAI-1), involved in inflammatory processes, were significantly increased in WT-EtOH than those in the Cyp2e1-null-EtOH and their corresponding controls (Suppl. Figs. 2B-D).
Fig. 2. Evaluation of the levels of serum endotoxin, hepatic enterobacteria content, intestinal histopathological changes and oxidative stress marker proteins.
(A) The serum endotoxin levels, (B) hepatic enterobacterial colony formation (n=3/group) and (C) average colony numbers were determined. (D) Representative H&E stainings of lower intestines are presented for indicated groups. WT-EtOH mice showed loss of epithelium (black arrows) and blebbing of lamina propria (dotted black circles) (n=3/group). (E) Equal amounts of intestinal lysates (n=3) were used to determine the levels of: CYP2E1 (top), iNOS (middle), and β-actin as a loading control (bottom). Data are expressed as mean ± SEM (n=4~5/group) unless otherwise indicated. *Significantly different from all other groups; #significantly different from the corresponding Cyp2e1-null-EtOH control.
Effects of CMZ or NAC on binge ethanol-induced on gut leakiness, endotoxemia, hepatic steatosis, and CYP2E1 level
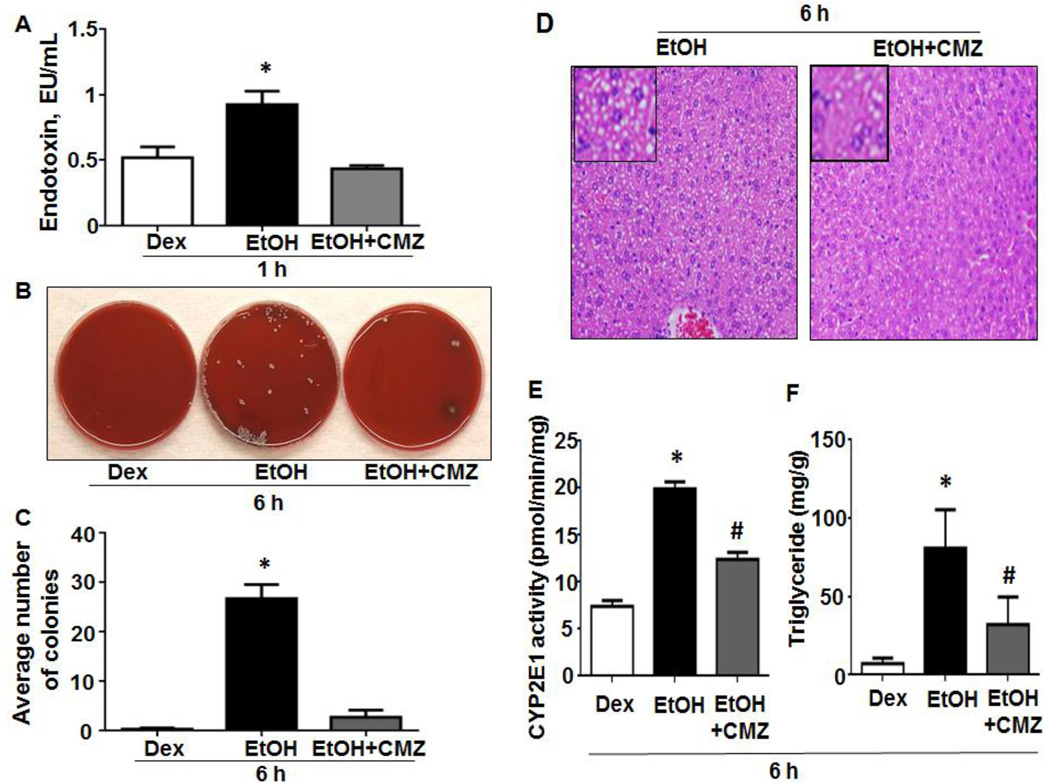
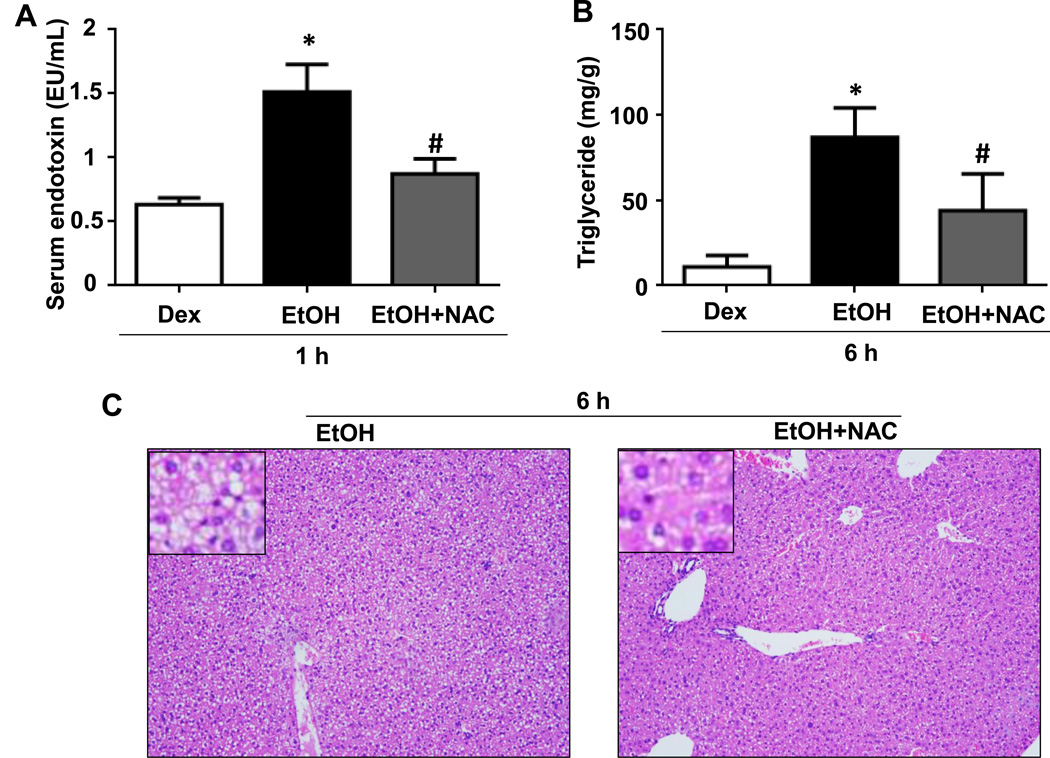
To further confirm the role of intestinal CYP2E1 and oxidative stress in promoting gut leakiness and endotoxemia, we studied the effects of CMZ or NAC treatment in our experiments. The elevated levels of serum endotoxin and hepatic enterobacterial contents in WT-EtOH mice were significantly decreased by CMZ treatment (Figs. 3A-C). CMZ treatment also reversed the elevated levels of hepatic steatosis (Fig. 3D), CYP2E1 (Fig. 3E), and triglyceride (Fig. 3F) in WT-EtOH. Similarly, NAC treatment significantly suppressed the elevated levels of serum endotoxin (Fig. 4A) as well as hepatic triglyceride (Fig. 4B) and steatosis (Fig. 4C) observed in WT-EtOH mice.
Fig. 3. Effects of CMZ on binge ethanol-mediated hepatic steatosis, CYP2E1 activity, serum endotoxin levels and hepatic enterobacterial contents.
(A) Serum endotoxin levels, (B) hepatic enterobacterial colony formation and (C) average colony numbers are presented (n=3/group). (D) Representative H&E staining of liver tissues obtained at 6-h, (E) hepatic CYP2E1 activity and (F) triglyceride levels are shown (n=4/group). Data are expressed as mean ± SEM. *Significantly different from all other groups, #significantly different from Dextrose-control.
Fig. 4. Effects of NAC on binge ethanol-mediated hepatic steatosis and serum endotoxin levels.
(A) The serum endotoxin levels and (B) hepatic triglyceride levels are presented. (C) Representative H&E staining of liver tissues obtained at 6-h as indicated. Data are expressed as mean ± SEM (n=3/group). *Significantly different from all other groups, #significantly different from Dextrose-control.
Evaluation of oxidative stress markers
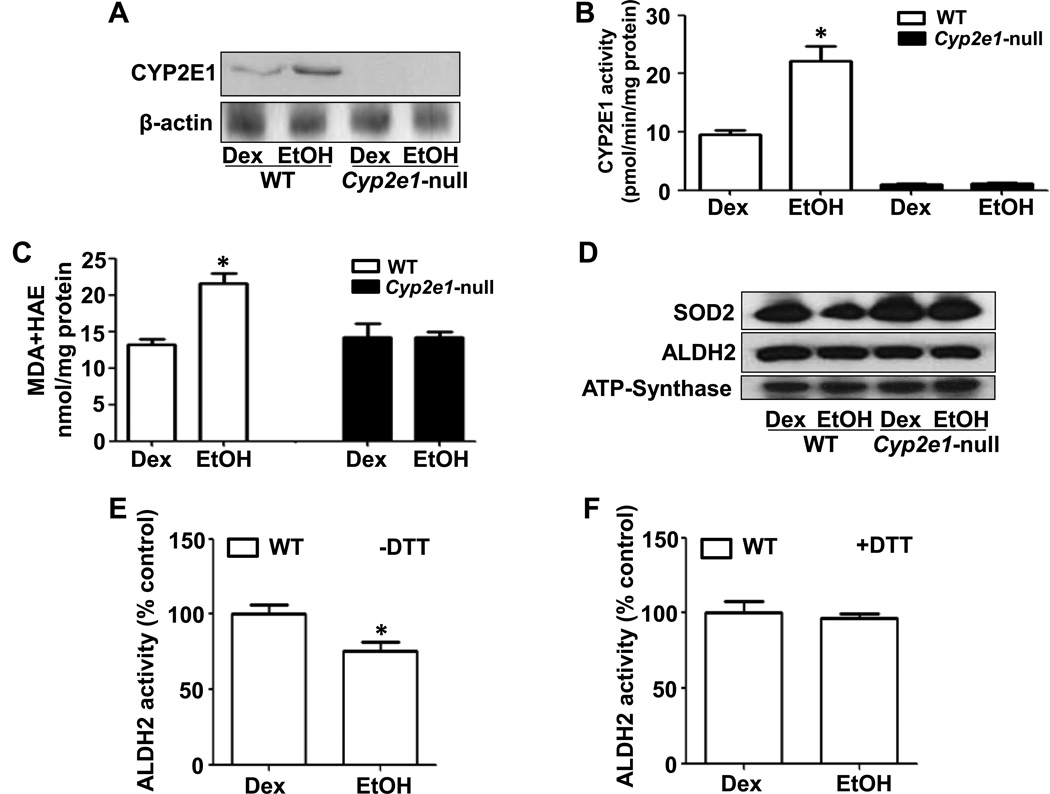
As expected, both CYP2E1 protein amount and activity were significantly increased only in the livers of WT-EtOH compared to all other groups (Figs. 5A and B). The increase of hepatic CYP2E1 levels in WT-EtOH correlated with increased levels of lipid peroxidation, as evidenced by the significant increase in the levels of MDA+HAE in WT-EtOH (Fig. 5C). We also evaluated the protein levels of mitochondrial superoxide dismutase (SOD2), an antioxidant protein, and the levels and activities of mitochondrial aldehyde dehydrogenase 2 (ALDH2), a low-Km enzyme which metabolizes both endogenous and exogenous aldehydes including lipid peroxides [18]. SOD2 was markedly decreased only in WT-EtOH (Fig. 5D). While the protein levels of ALDH2 were similar among all groups (Fig. 5D), the ALDH2 activity was significantly decreased in WT-EtOH compared to WT-DEX (Fig. 5E). The decreased ALDH2 activity was completely restored when samples were pretreated with the reducing agent DTT (15 mM) (Fig. 5F), indicating that ALDH2 was likely inhibited by increased oxidative stress [14, 15]. However, no significant decrease in ALDH2 activity was observed in Cyp2e1-null-EtOH (data not shown).
Fig. 5. Changes in the levels of hepatic oxidative parameters.
Equal amounts of cytosolic proteins were used to determine: (A) CYP2E1 protein levels (top) and β-actin (bottom); (B) CYP2E1 activity; and (C) hepatic MDA+HAE. Equal amounts of mitochondrial lysates were used to determine the levels of: (D) SOD2 (top), ALDH2 (middle), and ATP-synthase (bottom) as a loading control; (E and F) ALDH2 activity ± pretreatment with 15 mM DTT for 30 min. Data are expressed as mean ± SEM (n=5/group). *Significantly different from all other groups.
Evaluation of fat metabolism and apoptosis in WT and Cyp2e1-null mice
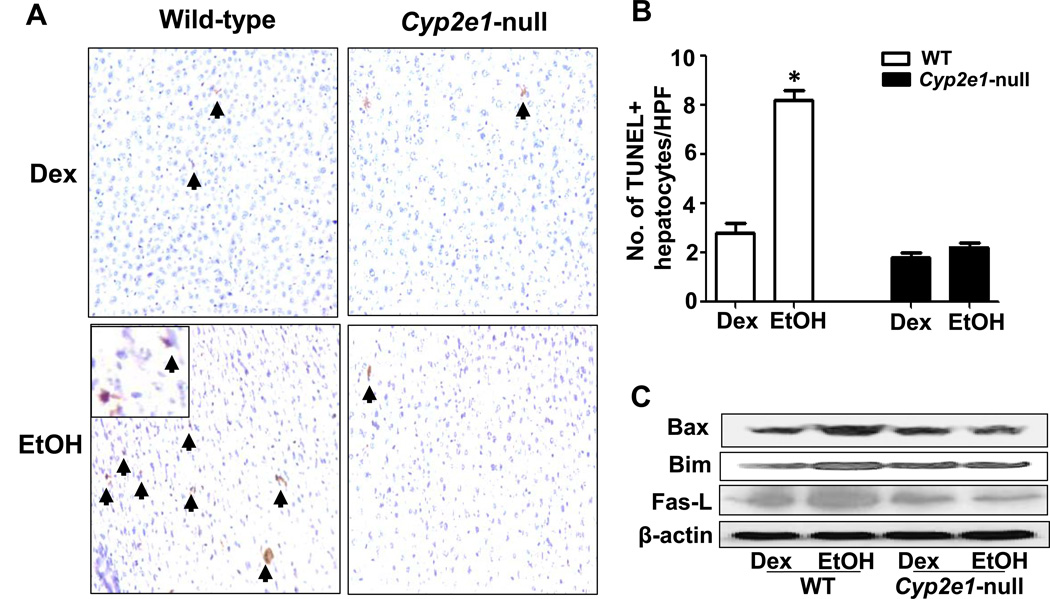
To determine whether apoptosis was increased in the EtOH-exposed mice, TUNEL assay was performed. TUNEL-positive hepatocytes were readily observed in WT-EtOH mice and were significantly higher in number than in all other groups (Figs. 6A and B), suggesting increased hepatocyte apoptosis. Further, the pro-apoptotic proteins BAX, BIM and Fas ligand were markedly increased only in WT-EtOH compared to WT-DEX (Fig. 6C). Inhibition of p-AKT was reported to cause fatty liver and apoptosis [19, 20]. Indeed, p-AKT was only markedly inhibited in WT-EtOH compared to WT-DEX (Suppl. Fig. 3A). It is noteworthy to mention that the basal levels of some proteins are different between the WT and null mice, probably because of the different genotypes, as reported [21]. In addition, the levels of phosphorylated (active) p-AMPK [22] and peroxisome proliferator-activated receptor alpha (PPAR-α), involved in accelerating fat metabolism and inflammatory process [14], were markedly decreased in WT-EtOH while increased in Cyp2e1-null-EtOH compared to their corresponding controls (Suppl. Figs. 3B and C). Finally, both CMZ and NAC treatments ameliorated ethanol-induced hepatic apoptosis in WT mice at 6-h, as determined by TUNEL assays (Suppl. Figs. 4 and 5, respectively).
Fig. 6. Evaluation of hepatic apoptosis and apoptosis-related proteins.
(A) TUNEL-positive apoptotic hepatocytes (arrowheads) in livers of indicated groups. (B) Number of TUNEL-positive hepatocytes in 20 HPFs was calculated. (C) Equal amounts of whole liver lysates were used to determine the levels of Bax, Bim, Fas ligand, and the loading control β-actin by immunoblot analysis. Data are expressed as mean ± SEM (n= 5 mice/group). *Significantly different from all other groups.
Discussion
CYP2E1 was found to be essential in the development of NAFLD and its advancement to NASH [23, 24] and alcoholic hepatitis [25] mainly through oxidative stress and inflammation-mediated events [23, 24]. In addition, CYP2E1 was reported to promote the development of steatotic liver, but not necrosis, in mice exposed to ethanol up to 4 weeks [26]. However, the role of CYP2E1 in early stage of ALD is controversial with conflicting results in the prior studies. For example, CYP2E1 was suggested to be non-essential in early alcohol-induced liver injury in mice continuously infused with an alcohol diet containing high-corn oil (37% fat-derived energy) and low carbohydrate (5% energy) for 4 weeks [27]. In contrast, recent studies showed that CYP2E1 may be important in mediating alcohol-induced hepatic steatosis in: (1) male WT mice and a total dose of ~4.7 g ethanol/kg where the first dose of ethanol was administered via i.p. injection at 0.93 g/kg and the remaining three doses were applied by gavage at 1.25 g/kg [12]; (2) male WT mice gavaged with 3 g/kg ethanol twice a day for four days [13]; and (3) CYP2E1-transgenic mice exposed to ethanol intragastrically for 4 weeks [28]. These reports suggest that CYP2E1-derived ROS leading to accumulation of hepatic lipid droplets in male mice [12, 13]. Thus, the current study sought to expand on the available information and evaluate: (1) the role of CYP2E1 in binge-alcohol induced intestinal and liver injury; (2) whether endotoxin and bacterial translocation could be involved in the development of AFLD/ASH and apoptosis; (3) whether NAC or CMZ could block intestinal and hepatic CYP2E1-mediated effects and thus prevent binge alcohol-induced gut leakiness, endotoxemia and hepatic injury, respectively, and (4) whether female WT and Cyp2e1-null mice are differentially affected from a more stringent exposure protocol of three doses of EtOH (6 g/kg each) at 12-h intervals.
In this study, female mice were used since it is essential to also evaluate female response to binge alcohol and that females are more susceptible to the damaging effect of alcohol than their male counterparts [29]. The WT-EtOH group developed hepatic steatosis as evidenced by histology, significant increases in the ratio of liver to body weights, TG levels and serum endotoxin as well as hepatic levels of enterobacteria compared to those of WT-DEX group. In contrast, alcohol binge caused only very small increase in liver TG in Cyp2e1-null mice. Neutrophil infiltration and inflammatory foci were also prominent only in WT-EtOH mice among all groups, while plasma ALT levels also exhibited a tendency to be higher in the WT-EtOH group compared to other groups although no significant levels were achieved. Thus, the first hit (i.e., steatosis) of the two-hit theory for ASH development appears to require CYP2E1 since marked steatosis with inflammation possibly via infiltrated neutrophils occur only in WT-EtOH mice but not in Cyp2e1-null-EtOH mice.
Intestine-derived endotoxins were reported to promote ALD, including binge model associated with fatty liver [30, 31]. Disruption of intestinal barrier function and endotoxemia are common features associated with alcohol-mediated liver inflammation and injury [10, 11, 16], although some alcoholics do not show clinical signs of intestinal injury possibly due to low levels of alcohol, inter-individual genetic differences, etc (Keshavarzian, personal communications). Furthermore, elevated CYP2E1 was found to prime hepatocytes to lipopolysaccharide and TNF-α via increased oxidative stress [32, 33]. These studies suggested that alcohol seems to activate macrophages for more severe liver injury, which is also supported by the increased hepatic pro-inflammatory factors observed in this study. However, the role of intestinal CYP2E1 in gut leakiness in in vivo models is unknown, despite many reports about alcohol-induced gut leakiness [11, 31, 34–36]. The present data showed that intestinal CYP2E1 and oxidative stress seem essential to the gut leakiness, as evidenced by significantly elevated levels of serum endotoxin, prominent histological changes in the small intestine and enterobacterial contents only in the liver from WT-EtOH but not in alcohol-exposed Cyp2e1-null mice (Figs. 2–4). Both iNOS and CYP2E1 were reported to increase permeability in intestinal Caco-2 cells and rat intestine via nitroxidative stress-mediated events and that inhibition of CYP2E1 using a specific siRNA or specific inhibitors of iNOS ameliorated intestinal leakiness [34–36]. Consistently with these reports [34–36], we observed that both intestine epithelial CYP2E1 and iNOS are markedly elevated only in WT-EtOH mice and that NAC treatment abolished these increases and serum endotoxin levels, indicating a critical role of nitroxidative stress in gut leakiness (Figs. 2 and 4). In addition, CMZ, which suppressed the hepatic CYP2E1 activity, prevented the elevated levels of serum endotoxin and hepatic enterobacteria and triglyceride observed in WT-EtOH mice (Fig. 3). Furthermore, CMZ ameliorated both the elevated levels of intestinal iNOS and the peroxynitrite marker 3-NT, which is known to reflect modified protein structures with increased susceptibility to protein degradation, as reported [37, 38 and references within]. It is noteworthy to mention that our data doesn’t totally exclude the induction of iNOS since the levels of protein nitration were slightly increased in Cyp2e1-null mice following binge alcohol exposure. We believe that these changes could be easily detected with longer film exposure during immunoblot analysis and with greater amounts of protein loading. However, our results simple indicate greater elevation of iNOS and 3-NT levels in the WT-EtOH than that in the corresponding Cyp2e1-null mice. Taken together, intestinal CYP2E1, induced by alcohol exposure possibly through protein stabilization [36, 39, 40], plays a central role in promoting binge alcohol-induced nitroxidative stress, gut leakiness and bacterial translocation, contributing to elevated levels of serum endotoxin and hepatic cytokines, which prime the liver to more severe injury through oxidative stress-mediated events [11, 30, 31]. Increased hepatic cytokine levels were also implicated in the development of hepatic steatosis, as recently reported in an acute binge ethanol model [41]. Thus, it is likely that increased nitroxidative stress, via elevated levels of intestinal CYP2E1 and iNOS following binge alcohol exposure, would modify critical intestinal proteins, resulting in their inactivation and apoptosis, ultimately contributing to gut leakiness. The molecular mechanisms and the potential alteration of intestinal proteins including tight junction proteins are currently under investigation.
A second hit, namely oxidative stress and/or inflammation, can also increase the severity of steatotic liver [5, 23]. Oxidation of unsaturated fatty acids produces increased lipid peroxidation products that ultimately lead to liver fibrosis through activating stellate cells [25]. Furthermore, hepatic CYP2E1 protein levels and activity have been reported to increase in cultured cells and mice exposed to ethanol [9, 26]. CYP2E1 is known to increase ROS/RNS levels which may modify the function of various proteins, contributing to more severe liver damage [8, 9, 18, 23]. Both binge and chronic ethanol administration were shown to inhibit the activity of mitochondrial ALDH2, which plays an essential role in metabolizing various aldehydes, through oxidative modification of Cys residues including its catalytic site [18, 42]. Consistent with this view, lipid peroxidation levels were highest in WT-EtOH mice. This increase was accompanied by activated CYP2E1 with suppressed levels of the anti-oxidant SOD2 and ALDH2, which may promote increased levels of superoxide levels and lipid peroxides, respectively. Thus, increased CYP2E1 with suppressed SOD2 and ALDH2 in the current model also appears to prime the liver to more severe liver injury, through oxidative stress development (another potential second hit) of the two-hit theory, as discussed [23 and references within].
Binge ethanol was shown to induce hepatocyte apoptosis [43]. Thus, we evaluated whether CYP2E1 is involved in alcohol-induced hepatocyte apoptosis. The results suggest an important role for CYP2E1 in this process. Hepatocyte apoptosis, Fas-ligand, which was reported to be a main mediator of binge ethanol induced apoptosis [43], and the pro-apoptotic proteins including Bax were markedly elevated in WT-EtOH mice compared to Cyp2e1-null-EtOH mice (Fig. 6). Collectively, these data suggests that both intestinal and hepatic CYP2E1 are key enzymes mediating a series of injurious cascades starting from gut leakage to the induction of enterobacterial translocation to the liver, contributing to the development of higher levels of steatosis, inflammation, oxidative stress, and ending with increased hepatocyte death (apoptosis) in this binge-alcohol model.
We further evaluated additional mechanisms that make liver resistant to steatotic and injurious effects of acute alcohol binge in Cyp2e1-null mice. Therefore, we also evaluated a very-well established mediators of increasing fatty acid metabolism PPAR-α and p-AMPK. Interestingly, there was a paradoxical effect of binge ethanol where the levels of both PPAR-α and active p-AMPK were decreased in WT-EtOH while they were increased in Cyp2e1-null-EtOH compared to their respective controls (Supplemental Fig. 3). Further investigation is warranted to determine the mechanisms for the paradoxical effect.
AKT was also reported to prevent fatty liver in a NASH model [19]. Indeed, AKT was markedly inhibited only in the WT-EtOH group, thus suggesting that the Akt pathway, which represents a survival pathway, may play a role in regulating the development of steatosis and apoptosis, respectively, in the WT-EtOH group. It is noteworthy to point out the different basal levels of some proteins including p-AKT (Supplemental Fig. 3A) in WT and Cyp2e1-null mice. CYP2E1 is involved in the metabolism of many small molecules and is known to induce oxidative stress [8, 9]. CYP2E1 elimination likely alters physiological status accompanied by changes in the expressed levels of various proteins that might be affected directly or indirectly by CYP2E1. It may represent a complex process for the host to adapt to genetic and/or environmental changes. However, it is difficult to dissect the exact reason why the basal levels of certain proteins are different. Taken together, the inhibition of PPAR-α, p-AKT and p-AMPK observed only in WT-EtOH mice seems to play, at least, a partial role in promoting CYP2E1-mediated steatohepatitis and apoptosis in the binge ethanol model. Another possible explanation for steatosis could result from the potential inhibition of autophagy by binge ethanol, as suggested [12, 13]. In contrast, another study of using binge alcohol model reported increased autophagy [44], suggesting the need for further study on the temporal effects of binge ethanol on autophagy.
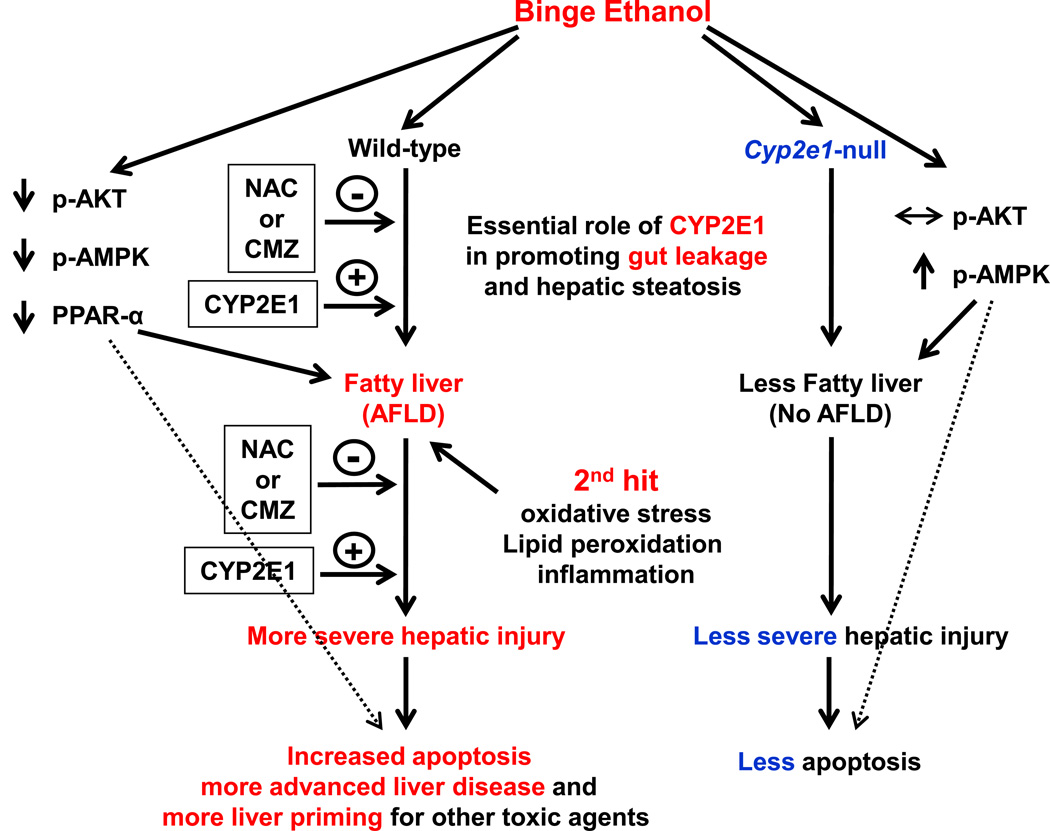
In summary, this study showed that intestinal and hepatic CYP2E1, induced by binge alcohol exposure, play an important role in promoting oxidative stress, gut leakiness, and endotoxemia, all of which could be blunted by a specific CYP2E1 inhibitor CMZ or an antioxidant NAC. The elevated levels of serum endotoxin, hepatic contents of enterobacteria, and cytokines with the suppressed survival signaling pathway, can negatively affect the vital functions of the hepatocytes and ultimately contribute to the development of inflammatory ASH and hepatic apoptosis (Fig. 7) following binge alcohol exposure.
Fig. 7. Schematic diagram for the role of CYP2E1 in binge ethanol-induced gut leakiness, hepatic steatosis and apoptosis.
The first and second hits for the development of steatohepatitis are presented. Intestinal and hepatic CYP2E1 seem to play a central role in promoting oxidative stress, gut leakiness, and endotoxemia, thereby contributing to the development of hepatic steatosis, inflammation and apoptosis.
Supplementary Material
Highlights.
-
►
Binge alcohol increased intestinal CYP2E1 and inducible nitric oxide synthase in WT mice but not in Cyp2e1-null mice.
-
►
Binge alcohol promoted increased gut leakiness, contributing to systemic endotoxemia and more severe liver injury.
-
►
Chlormethizone (CMZ), a specific inhibitor of CYP2E1, suppressed binge alcohol-mediated increases in CYP2E1 level, gut leakiness, and hepatic apoptosis.
-
►
N-acetyl-cysteine (NAC), an anti-oxidant, prevented binge alcohol-induced gut leakiness and hepatic apoptosis in WT mice.
-
►
These results strongly indicate a critical role of CYP2E1 in binge alcohol-induced gut leakiness, hepatic steatosis and apoptosis.
Acknowledgement
Funding sources: This research was supported by the Intramural Program of National Institute on Alcohol Abuse and Alcoholism. We thank Dr. Klaus Gawrisch for supporting this study.
Abbreviations
- AFLD
alcoholic fatty liver diseases
- 3-NT
3-nitrotyrosine
- ALD
alcoholic liver disease
- ALDH2
mitochondrial aldehyde dehydrogenase 2
- ALT
alanine aminotransferase
- ASH
alcoholic steatohepatitis
- CMZ
chlormethiazole
- CYP2E1
cytochrome P450 2E1
- HAE
hydroxyalkenal
- HPF
high-power field
- MDA
malondialdehyde
- NAC
N-acetyl-cysteine
- NAFLD
nonalcoholic fatty liver diseases
- NASH
nonalcoholic steatohepatitis
- null-DEX
Cyp2e1-null-dextrose
- null-EtOH
Cyp2e1-null-ethanol
- PAI-1
plasminogen activator inhibitor-1
- PBS
phosphate buffered saline
- PNP
p-nitrophenol
- PPAR-α
peroxisome proliferator-activated receptor-alpha
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD2
mitochondrial superoxide dismutase
- TG
triglyceride
- TNF-α
tumor necrosis factor-alpha
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild-type
- WT-DEX
wild-type-dextrose
- WT-EtOH
wild-type-ethanol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest
References
- 1.Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum. Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufour MC, Stinson FS, Caces MF. Trends in cirrhosis morbidity and mortality: United States, 1979–1988. Semin. Liver. Dis. 1993;13:109–125. doi: 10.1055/s-2007-1007343. [DOI] [PubMed] [Google Scholar]
- 3.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N. Engl. J. Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 4.Stickel F, Seitz HK. Alcoholic steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2010;24:683–693. doi: 10.1016/j.bpg.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Massey VL, Arteel GE. Acute alcohol-induced liver injury. Front. Physiol. 2012;3:193. doi: 10.3389/fphys.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnabl B. Linking intestinal homeostasis and liver disease. Curr. Opin. Gastroenterol. 2013;29:264–270. doi: 10.1097/MOG.0b013e32835ff948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig. Dis. 2010;28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 11.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Wu D, Wang X, Cederbaum AI. Cytochrome P4502E1, oxidative stress, JNK autophagy in acute alcohol-induced fatty liver. Free Radic. Biol. Med. 2012;53:1170–1180. doi: 10.1016/j.freeradbiomed.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic. Biol. Med. 2012;53:1346–1357. doi: 10.1016/j.freeradbiomed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic. Biol. Med. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 18.Song BJ, Abdelmegeed MA, Yoo SH, Kim BJ, Jo SA, Jo I, Moon KH. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J. Prot. 2011;74:2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JW, Zhan XR, Li XY, Xia B, Wang YY, Zhang J, Li BX. Impaired PI3K/Akt signal pathway and hepatocellular injury in high-fat fed rats. World J. Gastroenterol. 2010;16:6111–6118. doi: 10.3748/wjg.v16.i48.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al Abdulmohsen S, Platanias LC, Al-Kuraya KS, Uddin S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012;7:e39945. doi: 10.1371/journal.pone.0039945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Yeh TH, Singh VP, et al. beta-catenin is essential for ethanol metabolism and protection against alcohol-mediated liver steatosis in mice. Hepatology. 2012;55:931–940. doi: 10.1002/hep.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viollet B, Foretz M, Guigas B, et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol.-London. 2012;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelmegeed MA, Banerjee A, Yoo SH, et al. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong H, Armoni M, Harel C, et al. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012;302:E532–539. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol. Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Zhuge J, Wang X, et al. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 27.Kono H, Bradford BU, Yin M, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- 28.Butura A, Nilsson K, Morgan K, et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009;50:572–583. doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Strong MN, Yoneyama N, Fretwell AM, et al. "Binge" drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm. Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JC, Zhou Z, Wang L, et al. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J. Pharmacol. Exp. Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- 31.Bergheim I, Guo L, Davis MA, et al. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Cederbaum A. Cytochrome P4502E1 sensitizes to tumor necrosis factor alphainduced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology. 2008;47:1005–1017. doi: 10.1002/hep.22087. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Cederbaum AI. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr. 2010;5:149–167. doi: 10.1007/s12263-009-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Forsyth CB, Farhadi A, et al. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol. Clin. Exp. Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banan A, Farhadi A, Fields JZ, et al. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J. Pharmacol. Exp. Ther. 2003;305:482–494. doi: 10.1124/jpet.102.047308. [DOI] [PubMed] [Google Scholar]
- 36.Forsyth CB, Voigt RM, Shaikh M, et al. Role for Intestinal Cyp2e1 in Alcohol-Induced Circadian Gene-Mediated Intestinal Hyperpermeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome p450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem. Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction an acute liver injury. Free Radic. Biol. Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem. Biophys. Res. Commun. 1994;205:1064–1071. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- 40.Hakkak R, Korourian S, Ronis MJ, Ingelman-Sundberg M, Badger TM. Effects of diet and ethanol on the expression and localization of cytochromes P450 2E1 and P450 2C7 in the colon of male rats. Biochem. Pharmacol. 1996;51:61–69. doi: 10.1016/0006-2952(95)02154-x. [DOI] [PubMed] [Google Scholar]
- 41.Huang LL, Wan JB, Wang B, et al. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88:347–353. doi: 10.1016/j.plefa.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Moon KH, Hood BL, Kim BJ, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway. Am. J. Pathol. 2001;159:329–338. doi: 10.1016/S0002-9440(10)61699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.