Abstract
Background
Adolescents and adults vary in sensitivity to many effects of ethanol, although it is unknown whether they also differ in their perception of ethanol’s subjective cues. The present study characterized ethanol discrimination in adolescent and adult male rats using a rapidly acquired Pavlovian conditioned approach procedure.
Methods
Ethanol at one of three training doses (0.75, 1.0 or 1.25 g/kg) served as either a positive (POS) or negative (NOS) occasion setter. Each 20-min training session consisted of eight 15-s presentations of two cue lights located on either side of a dipper delivering chocolate Boost®. For POS-trained rats, the cue lights reliably predicted 5-s presentations of chocolate Boost during ethanol but not saline sessions, with the opposite contingencies used for NOS-trained rats. Anticipatory approach behavior (head entries into the reward delivery area) in the presence and absence of the cue lights was used to calculate discrimination scores on ethanol and saline sessions. Following acquisition, various doses of ethanol (0–1.5 g/kg) were administered to establish generalization curves.
Results
Although animals of both ages responded differentially on ethanol and saline sessions by the end of acquisition, adults met criterion more quickly and had higher discrimination scores during reinforced sessions than adolescents. Whereas adolescents failed to demonstrate any dose-dependent responding during testing when trained with the 0.75 or 1.25 g/kg ethanol doses, adults demonstrated broader ethanol generalization during testing sessions following training with all three ethanol doses. Among adolescents trained with 1.0 g/kg ethanol, less generalization occurred relative to adults.
Conclusions
Adolescents were less sensitive to ethanol’s interoceptive effects, indicating that ethanol is likely a more salient cue for adults than adolescents. These findings contribute to evidence that suggests adolescent-typical insensitivity to internal cues that typically limit ethanol consumption may contribute to the elevated intake commonly reported during this developmental period.
Keywords: ethanol, discrimination, adolescent, rat, Pavlovian conditioned approach
Introduction
Adolescence is the transition from youth to maturity during which elevations in risk taking (Kelley et al., 2004; Laviola et al., 2003), novelty seeking (Adriani et al., 1998; Kelley et al., 2004) and social interactions with peers (Harris, 1995; Varlinskaya and Spear, 2008) are commonly observed in both humans and animal models of adolescence. Adolescence also encapsulates the time during which individuals undergo hormonal changes associated with puberty. The numerous behavioral, hormonal, and neural changes that occur during this developmental period are highly conserved across species (Spear, 2000; 2010). In rodents, early-mid adolescence occurs within the age range of postnatal days (P) 28–42, with a period of late adolescence/”emerging adulthood” occurring during P42–55 (Vetter-O’Hagen and Spear, 2012).
Alcohol use is prevalent among adolescents. According to results from the 2011 Monitoring the Future study, 70% of high school seniors have tried alcohol at least once, with over 50% reporting consumption to the point of intoxication (Johnston et al., 2012). Rates of binge drinking also increase across adolescence, from 8% in 14–15 year olds to 36% in 18–20 year olds (SAMHSA, 2008). Given that ethical constraints limit human research, animal models of adolescence can be particularly helpful for examining underlying neurobiological features that may contribute to elevated ethanol consumption. Indeed, rodent studies consistently report higher ethanol consumption in adolescent rats relative to adults (Doremus et al., 2005; Vetter et al., 2007). Differential ontogenetic sensitivity to behavioral effects of ethanol may contribute to the elevated ethanol consumption often reported during adolescence. For example, adolescents appear to be more sensitive to the social facilitating effects of ethanol (Varlinskaya and Spear, 2002) and relatively insensitive to many aversive effects of ethanol, including motor impairment (White et al., 2002), sedation (Silveri and Spear, 1998), social impairment (Varlinskaya and Spear, 2002), and dysphoria (indexed by conditioned taste aversions: Anderson et al., 2010; Schramm-Sapyta et al., 2010). Given these age-related differences in sensitivity to various behavioral effects of ethanol, adolescent and adult animals likely perceive the interoceptive effects of ethanol differently.
A discriminative stimulus is a cue capable of exerting control over a particular behavior; discriminative stimulus effects of drugs reflect the interoceptive cues that accompany drug exposure. Over the past 30 years, studies employing ethanol discrimination procedures in adult animals have contributed to our understanding of the neural mechanisms underlying the subjective effects induced by ethanol administration (see Kostowski and Bienkowski, 1999). For discrimination training, food-deprived animals are typically administered either ethanol or vehicle and placed in conditioning chambers outfitted with two levers. Responses on one lever are reinforced with a food or sucrose reward following ethanol administration whereas responses on the opposite lever are reinforced following vehicle administration. Training sessions are repeated until the subjects can discriminate between the two substances with high accuracy (see Hodge et al., 2006). Unfortunately, this well-established procedure is lengthy (often requiring up to 60–90 or more training sessions) and therefore is not well-suited for testing rodents during the brief ontogenetic period of adolescence.
In contrast, a Pavlovian conditioned approach discrimination procedure in which administration of the training drug serves as a positive occasion setter that predicts contingency between a cue and reward has been used to elicit nicotine and methamphetamine discrimination (see Palmatier et al., 2004; Palmatier et al., 2005; Reichel et al., 2007; Wilkinson et al., 2009). Specifically, during drug-injected sessions, presentation of 15-sec cue lights predicts brief availability of a rewarding solution whereas no reward delivery follows the cue light presentation during vehicle-injected sessions. Eventually, the cue lights differentially elicit anticipatory approach to the reward delivery area during drug and vehicle sessions.
This procedure requires a shorter training period and may be more appropriate for testing the subjective effects of ethanol in adolescents, thereby allowing for ontogenetic comparisons of acquisition and generalization of ethanol’s discriminative stimulus effects (Besheer et al., 2012a). The present study was designed to assess the ability of ethanol to serve as an occasion setter (i.e., a discriminative stimulus) using Pavlovian conditioned approach. In order to characterize this model with ethanol, adolescent and adult animals were assigned to one of three training doses and were tested at one of two post-injection time points. For half of the subjects, ethanol served as a positive occasion setter (POS) whereas for the other half, ethanol served as a negative occasion setter (NOS). Use of both training models allowed for comparison of responding during reinforced sessions following ethanol or saline injection to ensure that ethanol administration did not hinder acquisition of conditioned approach. Following acquisition, animals were tested with various ethanol doses in order to establish a dose response curve. Thus, the design of the study allowed for the examination of both age differences in acquisition and dose response generalization across a variety of training parameters.
Materials and Methods
Subjects
Adolescent and adult male Sprague-Dawley rats bred at Binghamton University were used as subjects. On postnatal day (P) 1, litters were culled to 8–10 pups, with a ratio of six males to four females maintained whenever possible. Animals were weaned on P21, housed in pairs with a same-sex littermate and maintained in a temperature-controlled vivarium on a 12:12-hr light:dark cycle (lights on at 7AM). Water was available ad libitum, with food available as described below. Animals were at all times treated in accordance with guidelines for animal care established by the National Institutes of Health (Institute for Laboratory Animal Resources, 2011) under protocols approved by the Binghamton University Institutional Animal Care and Use Committee. On P23/P65, animals were assigned to experimental groups and individually housed. To avoid confounding litter effects, no more than one animal per litter was assigned to each experimental condition (see Holson and Pearce, 1992; Zorilla, 1997).
Apparatus
Training occurred in standard rat-sized conditioning chambers (Med Associates, St. Albans, VT) outfitted with cue lights and a liquid reward delivery system. For reward delivery, a retractable dipper arm was raised into a recessed area of the side chamber wall, flanked by two 1″ cue lights. Photocell beams counted head entries into the reward delivery area. An additional pair of photocell beams bisected the chamber and measured beam breaks in order to assess general activity. Equipment was controlled by Med PC programs.
Procedure
Boost pre-exposure and food restriction
Chocolate Boost® (Nestlé HealthCare Nutrition, Fremont, MI) served as the rewarding solution in the conditioning chambers. To avoid neophobia, subjects were given a sipper tube containing 25 ml Boost solution in the home cage one day prior to the onset of food restriction. Beginning on P25 or P67, subjects were given a daily food allotment to restrict body weight to approximately 85% of age-appropriate free-feeding weight. Weight was monitored daily, with food allotments increased across days to ensure that subjects maintained a normal growth trajectory (based on data collected previously in our lab) throughout experimentation.
Pre-training
In order to acclimate the animals to the conditioning chambers and encourage them to approach the dipper quickly when the arm was raised, pre-training sessions were conducted for two days (P26–27 or P68–69) prior to the start of acquisition sessions. At the start of each pre-training session, the dipper arm remained in the raised position (providing free access to 0.1 ml Boost) until animals first approached the delivery area. The dipper arm was then lowered and the pre-training program began. Sessions involved 25 5-s presentations of Boost in the dipper arm on a variable intermittent schedule over a span of approximately 35 min.
Acquisition
Beginning on either P28 or P70, acquisition sessions were conducted twice a day, separated by a period of at least 5 hours. Subjects were assigned to an ethanol training dose (0.75, 1.0, or 1.25 g/kg) and a post-injection testing time point (5 min or 30 min). Ethanol injections were prepared from a 12.6% (v/v) solution, with volume adjusted to achieve desired dose. For saline sessions, subjects received a volume equivalent to their ethanol injection. After intraperitoneal (i.p.) injection, subjects were returned to their home cage for either 5 or 30 min before placement in the conditioning chambers for training sessions. Sessions lasted 20 min and consisted of eight 15-s presentations of both cue lights simultaneously. Ethanol served as either a positive (POS) or negative occasion setter (NOS), counterbalanced across subjects. For animals trained with the POS-model, the cue lights reliably predicted 5-s presentations of Boost (0.1 ml in the dipper arm) following ethanol, but not saline, administration. For animals trained with the NOS training model, the cue lights predicted reward delivery following administration of saline, but not ethanol. During non-reinforced sessions, the dipper arm remained lowered following cue light presentation. One saline and one ethanol session was held each day, with within-day order randomized daily across subjects. Head entries during the cue light presentations (i.e., goal-tracking behavior) were compared on ethanol and vehicle sessions via a discrimination score. Discrimination scores reflected the difference in head entries in the presence and absence of the cue across the 8 trials (entries during 15-s cue light presentations – entries during 15 s preceding the cue light presentations) in order to control for baseline levels of exploratory or anticipatory activity. Therefore, a higher discrimination score on drug-injected days relative to vehicle-injected days indicated successful discrimination learning for POS animals. A pair of photocells counted beam breaks during each session to provide a general measure of activity.
Maintenance and testing
Following 20 acquisition trials (10 days), testing cycles began. During each testing cycle, four maintenance sessions (identical to acquisition sessions) were followed by one test session for a total of six test cycles. This design allowed within-subjects assessment of six ethanol doses for a dose response (0, 0.5, 0.75, 1.0, 1.25, and 1.5 g/kg). Sessions were conducted twice each day, such that half of the test sessions occurred in the morning and half occurred in the afternoon. Discrimination scores for the ethanol and saline sessions prior to each test were examined in order to determine whether subjects maintained discrimination criterion. POS-trained subjects must have demonstrated greater average discrimination scores (by at least 0.75) on ethanol relative to saline sessions whereas NOS-trained subjects must have demonstrated greater discrimination scores on saline sessions relative to ethanol sessions (by at least 0.75). Test session data from subjects that did not meet criterion on the preceding maintenance sessions were excluded from analysis. Test sessions lasted 4 min and involved a single, unreinforced presentation of the 15-s cue. The discrimination score for this single trial served as the dependent measure.
Blood Ethanol Concentration (BEC) assessment
One day after the final test session, subjects trained with the 1.0 g/kg ethanol dose and a separate group of ethanol-naïve rats were given an injection of 1.0 g/kg ethanol and sacrificed one hour later. Trunk blood was collected to assess BEC levels and brains were collected for tissue analysis in a separate study.
Data analysis
Acquisition
For acquisition data, average discrimination scores (across 8 trials) and first trial discrimination scores were calculated and analyzed separately by training model via 2 age × 3 training dose × 2 time point × 2 session type (ethanol or saline) × 10 days double repeated measures ANOVAs. First trial data were included to ensure that average trials discrimination scores didn’t merely reflect within-session learning. No subjects were excluded from analyses of acquisition data. Only interactions involving session type (i.e., ethanol or saline) were considered relevant findings, with significant effects further explored using Fisher’s LSD tests.
To compare responding and activity levels across POS- and NOS-trained subjects, overall head entries and beam breaks during acquisition were analyzed via 2 training model × 2 age × 3 training dose × 2 time point × 2 session type double repeated measures ANOVAs. Comparisons focused on reinforced sessions (i.e., activity during POS-trained ethanol sessions and NOS-trained saline sessions).
Dose response generalization
Nineteen (out of 164; 11.5%) subjects failed to meet criterion prior to three or more test sessions and were removed from the data set before analyzing dose response data. For subjects that failed to meet criterion prior to one or two test sessions, values from those sessions were left blank (6.8% of all data points). Remaining data were analyzed separately for each age and training model using a linear mixed model with a compound symmetry covariance structure and with dose as a repeated measure. This analysis is appropriate for data sets with missing values (e.g., Krueger and Tian, 2004). Significant interactions were further explored by examining simple effects with Bonferroni corrections to control for family-wise error rate.
BEC analysis
BEC values two standard deviations away from the group mean (two adolescents and two adults) were eliminated prior to analysis via a 2 age × 2 group (discrimination-trained or ethanol-naïve) factorial ANOVA.
Results
Average discrimination scores during acquisition
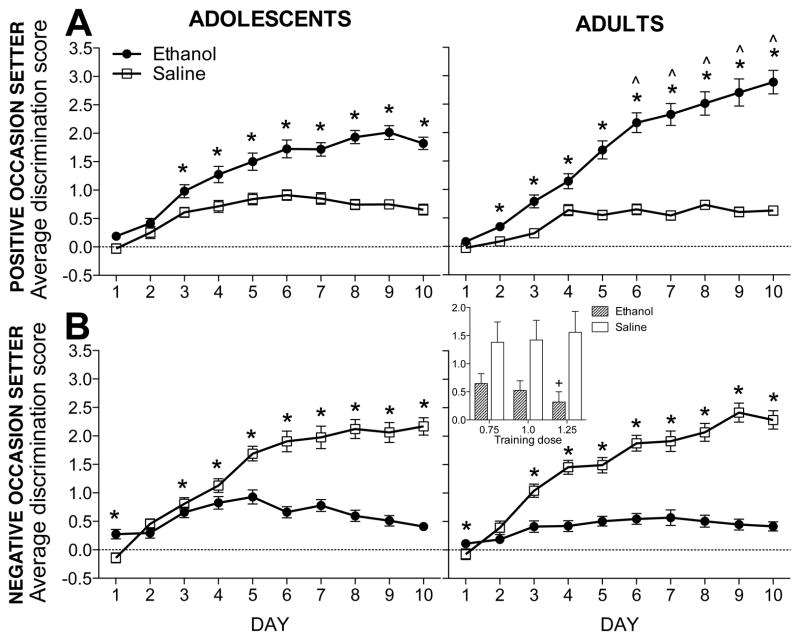
Analysis of POS-trained subjects revealed a main effect of session type that interacted with age [F(1,78) = 12.7, p < .001] and day [F(9,702) = 44.0, p < .001]. These effects were tempered by a 3-way interaction of session type × day × age [F(9,702) = 5.7, p < .001]. As shown in Figure 1A, discrimination scores differed between ethanol and saline sessions on days 3–10 for adolescents and days 2–10 for adults. Adult discrimination scores during ethanol sessions 6–10 were higher than adolescent discrimination scores on sessions 6–10; no age differences were seen during saline sessions.
Figure 1. Average discrimination scores during acquisition.
Data shown are collapsed across training dose and time point. (A) For POS-trained subjects, ethanol session discrimination scores were significantly higher than saline sessions on days 3–10 for adolescents (n=45) and days 2–10 for adults (n=46) (indicated by *s). Additionally, adult ethanol session discrimination scores were higher than adolescent ethanol session discrimination scores on days 6–10 (indicated by ^s). (B) For NOS-trained subjects of both ages (adolescent n=35; adult n=38), saline session discrimination scores were higher than ethanol session discrimination scores on days 3–10, with ethanol session scores starting higher on day 1. Collapsed across age, subjects trained with 1.25 g/kg ethanol had lower discrimination scores than subjects trained with the 0.75 g/kg dose during ethanol sessions (indicated by +, inset).
Across all days of maintenance sessions, POS-trained adults continued to have higher ethanol session discrimination scores than adolescents (adults: 3.3±0.5; adolescents: 2.2±0.6). Average discrimination scores during non-reinforced trials (i.e., saline for POS; ethanol for NOS) did not differ across age.
Analysis of NOS-trained subjects revealed a main effect of session type that interacted with training dose [F(2,61) = 4.6, p < .02] and day [F(9, 549) = 51.1, p < .001]. As shown in Figure 1B, regardless of age, discrimination scores during ethanol and saline sessions differed on days 1 and 3–10. Subjects trained with the 1.25 g/kg ethanol dose had lower discrimination scores than subjects trained with the 0.75 g/kg dose during ethanol sessions; training dose did not affect discrimination scores during saline sessions (see Figure 1B, inset). Acquisition data are shown separately for all experimental conditions in Figure S1.
First trial discrimination score during acquisition
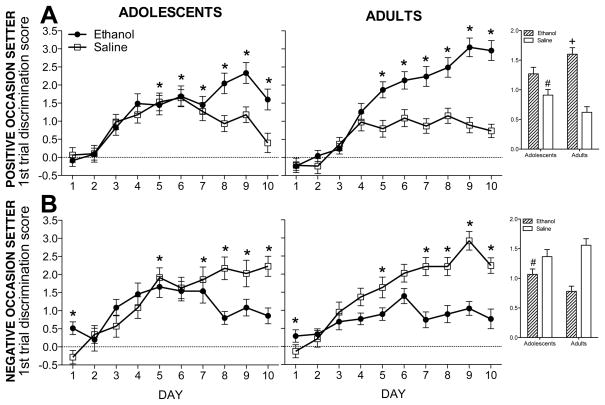
Analysis of POS-trained subjects revealed a main effect of session type along with 2-way interactions involving this variable and age [F(1,78) = 13.6, p < .001], time point [F(1,78) = 6.1, p < .02], and day [F(9,702) = 10.8, p < .001]. Collapsed across age and time point, discrimination scores were higher during ethanol sessions than saline sessions on days 5–10 (see Figure 2A). Collapsed across day and time point, adults had higher first trial discrimination scores than adolescents during ethanol sessions whereas adolescents had higher discrimination scores than adults during saline sessions (see Figure 2A, inset). Fisher’s LSD tests revealed no effects of interest for the interaction with time point.
Figure 2. First trial discrimination scores during acquisition.
Data shown are collapsed across training dose and time point. (A) For POS-trained subjects, post-hoc analyses on data collapsed across age revealed that discrimination scores were higher during ethanol sessions than saline sessions on days 5–10 (indicated by *s). Collapsed across day, adults (n=46) had higher first trial discrimination scores than adolescents (n=45) during ethanol sessions (inset; indicated by +) whereas adolescents had higher saline session discrimination scores than adults (inset; indicated by #). (B) For NOS-trained subjects, discrimination scores were higher on saline sessions than ethanol sessions on days 5, and 7–10, collapsed across age; however, on day 1, discrimination scores were higher on the ethanol session than the saline session. Collapsed across day, adolescents (n=35) had higher discrimination scores than adults (n=38) during ethanol sessions (indicated by #, see inset) whereas no age differences were evident during saline sessions.
Analysis of the NOS-trained subjects revealed a main effect of session type that interacted with age [F(1,61) = 7.3, p < .01], time point [F(1,61) = 5.4, p < .03], and day [F(9,549) = 8.5, p < .001]. Collapsed across day, adolescents had higher discrimination scores than adults during ethanol sessions whereas no age differences were evident during saline sessions. Collapsed across age, first trial discrimination scores differed between ethanol and saline sessions on days 1, 5, and 7–10. Data are shown in Figure 2B.
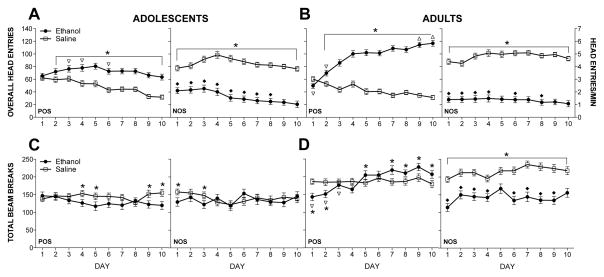
Overall head entries
Analysis of overall head entries revealed a session type × day × age × training model interaction [F(9,1251) = 5.1, p < .001]. On reinforced sessions, POS-trained (ethanol-injected) adolescents made fewer head entries than NOS-trained (saline-injected) adolescents on days 3–4 and 6. Relative to NOS-trained adults (saline-injected), POS-trained adults (ethanol-injected) made fewer head entries on days 1–2 but more head entries on days 9–10. On non-reinforced sessions, NOS-trained subjects (ethanol-injected) exhibited fewer head entries than POS-trained subjects (saline-injected) on multiple days at both ages (see Figure 3A–B for these comparisons and additional significant differences).
Figure 3. Overall head entries (A–B) and total beam breaks (C–D) during acquisition.
Data are collapsed across training dose and time point (POS n=45–46; NOS n=35–38). For both ages, significant differences on ethanol and saline sessions are indicated by *s. Values during non-reinforced sessions for NOS-trained subjects (ethanol-injected) that are lower than values during non-reinforced sessions for POS-trained subjects (saline-injected) are indicated by υs in comparisons conducted at each age. Values during reinforced sessions for POS-trained subjects (ethanol-injected) that differ from values during reinforced sessions for NOS-trained subjects (saline-injected) of the same age are indicated by σs (when lower than NOS) and ρs (when higher than NOS). For comparison to other discrimination studies that report head entry rates, the right y-axis (A–B) reflects head entries per minute.
An interaction of training dose with session type and training model [F(2,139) = 3.4, p < .04] also emerged; during non-reinforced sessions, NOS-trained subjects administered the 1.25 g/kg ethanol dose made fewer head entries (18.1±14.4) than POS-trained subjects during their non-reinforced (i.e., saline) sessions (41.5±10.9).
Total beam breaks
Analysis of beam breaks revealed a session type × day × age × training model interaction [F(9,1251) = 4.9, p < .001]. Among adolescents, no differences in activity were seen during reinforced (POS-ethanol vs. NOS-saline) or non-reinforced sessions (POS-saline vs. NOS-ethanol). During reinforced sessions among adult subjects, POS-trained (ethanol-injected) were less active than NOS-trained adults (saline-injected) on days 1–3. On non-reinforced sessions, NOS-trained adults (ethanol-injected) were less active than POS-trained adults (saline-injected) on most days. These and additional significant differences are denoted on Figure 3C–D.
A session type × training dose interaction [F(2,139) = 12.5, p < .001] revealed that regardless of age or training model, subjects injected with the 1.25 g/kg ethanol dose were less active (120±19 beam breaks) than subjects injected with lower ethanol doses (0.75: 173±19; 1.0: 155±18).
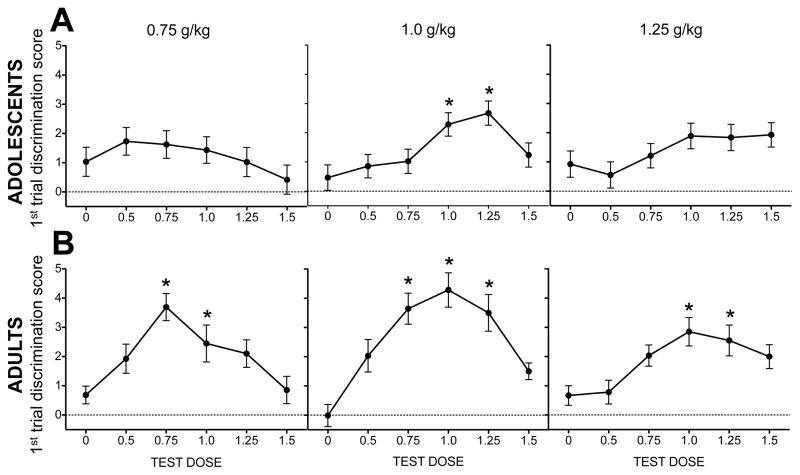
Ethanol dose response
Analysis of POS-trained adolescents revealed a significant effect of test dose that interacted with training dose [F(10, 144) = 2.32, p < .02]. For adolescents trained with 0.75 g/kg ethanol, no ethanol test dose elicited discrimination scores higher than saline. Adolescents trained with 1.0 g/kg ethanol had higher discrimination scores relative to saline following test doses of 1.0 and 1.25 g/kg. For adolescents trained with the 1.25 g/kg dose, a trend emerged for the 1.0 and 1.5 g/kg test doses to elicit higher discrimination scores than saline; however, these effects did not reach significance. Data are shown in Figure 4A.
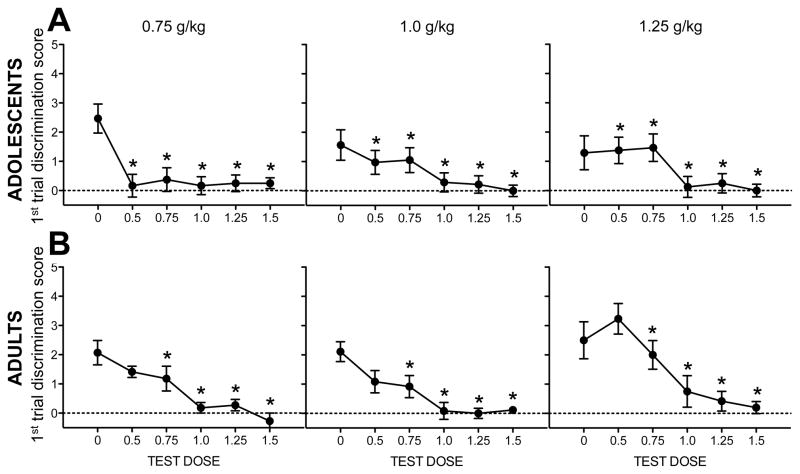
Figure 4. Test session discrimination scores (POS).
Data are collapsed across time point. Ethanol test doses that elicited discrimination scores significantly higher than saline are indicated by *s in (A) adolescents (n=11–14) and (B) adults (n=12–14).
Analysis of POS-trained adults revealed an effect of test dose that interacted with training dose [F(10,155) = 2.1, p < .03] in addition to a significant interaction of time point and training dose [F(2,32) = 4.4, p < .03]. For adults trained with the 0.75 g/kg dose, the 0.75 and 1.0 g/kg ethanol test doses elicited higher discrimination scores than saline. Adults trained with the 1.0 g/kg had higher discrimination scores than saline following the 0.75, 1.0, and 1.25 g/kg test doses. Adults trained with the 1.25 g/kg dose had higher discrimination scores relative to saline for doses of 1.0 and 1.25 g/kg. Data are shown in Figure 4B. For test session data shown separately for all experimental conditions, see Figure S2.
Analysis of test day dose response data for NOS-trained adolescents revealed a significant effect of test dose [F(5,126) = 6.4, p < .01], with all doses of ethanol eliciting lower discrimination scores than saline, regardless of training dose or time point (data shown in Figure 5A).
Figure 5. Test session discrimination scores (NOS).
Data are collapsed across time point. Ethanol test doses that elicited discrimination scores significantly lower than saline are indicated by *s in (A) adolescents (n=9–12) and (B) adults (n=11–12).
For NOS-trained adults, significant effects of test dose [F(5,142) = 17 p < .01] and training dose [F(2,29) = 4.9, p < .02] emerged during analysis of dose response data. Ethanol test doses of 0.75 g/kg and higher suppressed discrimination scores relative to saline. Collapsed across test dose, adults trained with the 1.25 g/kg training dose had higher discrimination scores relative to subjects trained with the lower ethanol doses (see Figure 5B). Data are shown separately for each experimental condition in Figure S2.
BEC analysis
Analysis revealed only a main effect of condition [F(1,53) = 5.65, p < .03], with ethanol-naïve rats (62.4±2.9 mg/dl) having higher BECs than discrimination-trained rats (56.7±1.2 mg/dl). No effects of age emerged.
Discussion
The Pavlovian drug discrimination model used in the present study revealed both similarities and differences in the sensitivity of adolescents and adults to ethanol’s interoceptive effects. Both adolescents and adults learned the task within the acquisition period, with subjects of both ages exhibiting within-session learning (evidenced by the more rapid emergence of significant differences between ethanol and saline sessions when assessing average discrimination scores than when analyzing first trial discrimination scores). Yet overall, adults demonstrated higher discrimination scores than adolescents during reinforced sessions. These results may indicate that ethanol is a more salient cue for adults than adolescents, a result that is perhaps not surprising given the reduced sensitivity to many other effects of ethanol seen in adolescents relative to adults. Adolescents are less sensitive to aversive, sedative, and motor-impairing effects of ethanol that likely contribute to the internal cues following ethanol administration. Additionally, adolescents have previously been reported to exhibit greater acute tolerance to ethanol than adults (Broadwater et al., 2011), an effect that may have also contributed to the age differences in responding to the ethanol cue.
Inclusion of both POS and NOS training models allowed systematic comparisons of beam break and overall head entry data during reinforced sessions in order to provide a more complete assessment of potential sedative effects of the ethanol training doses on discrimination acquisition. For POS-trained adults, ethanol initially suppressed responding on reinforced sessions relative to NOS-trained adults that received saline on reinforced sessions. This effect, which dissipated after the first few days of training, was not seen in adolescents. Comparisons of beam break data among NOS-trained subjects revealed that adolescents were less sensitive than adults to the activity-suppressing effects of ethanol on non-reinforced sessions. Overall head entry data suggest that ethanol did not impair discrimination performance. During non-reinforced sessions, both adolescent and adult NOS-trained subjects (ethanol injected) made fewer overall head entries than POS-trained subjects (saline-injected), again suggesting a slight sedative effect of ethanol. However, this modest sedation was overcome on reinforced sessions, where both measures generally revealed similar responses between POS- and NOS-trained subjects, regardless of age. Thus, it appears unlikely that sedative effects of ethanol influenced task performance at either age.
The extent to which these age effects may reflect age differences in learning must be considered when interpreting our results. Although previous studies have found similar levels of reward approach (i.e., goal-tracking) during cue presentation between adolescents and adults under isolate-housed, food-restricted conditions (Anderson et al., submitted), goal-tracking under the control of an occasion setter has not been compared in animals of different ages. For adolescents, the first trial discrimination scores during acquisition revealed higher scores during non-reinforced sessions and lower scores during reinforced sessions than adults. The smaller difference between discrimination scores on ethanol and saline sessions suggests that adolescent behavior is controlled less by the occasion setter than adult behavior. However, during maintenance sessions, adolescent discrimination scores on non-reinforced trials did not differ from adults, although adult discrimination scores on reinforced sessions remained higher than adolescent scores. It is also interesting that both adolescent and adult discrimination scores plateau during maintenance (i.e., with no continued escalation as seen during acquisition). These results suggest that responding may have reached a maximal levels for both ages, with that level higher for adult animals.
The age difference in discrimination scores during reinforced sessions may reflect impaired behavioral inhibition among adolescent subjects (i.e., a reduced ability to withhold head entries in the absence of the cue). Indeed, a recent study demonstrated that adolescents express diminished behavioral inhibition (‘self-control’) relative to adults in a variety of behavioral testing paradigms (Andrzejewski et al., 2011). Although ethanol doses of 1.0 g/kg and higher have been shown to impair spatial memory (assessed via water maze) in adolescents but not adults, non-spatial memory remained intact (Markwiese et al., 1998). Thus, the possibility that lower responding among adolescents is a consequence of ethanol-induced memory impairment seems unlikely. Given that BECs did not differ across age when bloods were collected one hour post-injection, age differences in responding among animals trained with the 1.0 g/kg training dose do not appear to reflect pharmacokinetic differences. Finally, the possibility of differences in adolescent and adult performance under conditions of state-dependent learning should be considered. Drug discrimination training is inherently state-dependent, but age differences in this type of learning have not been well-characterized.
Among POS-trained subjects, no effects of post-injection time point were seen during acquisition or dose-response testing for adolescents or adults. Using this model of discrimination, ethanol appears to serve as an equally salient cue for both adolescents and adults whether the interoceptive effects are assessed 5 or 30 min post-administration, time points representative of ethanol’s biphasic effects. That is not to say the interoceptive effects of ethanol are the same at these time points; across-time point generalization (e.g., Schechter, 1992; Shippenberg and Altshuler, 1985) was not examined in this study.
Dose-response curves revealed effects of training dose, age, and training model. POS training yielded more sensitive dose response testing, whereas NOS dose response curves quickly revealed floor effects with increasing test doses. Overall, among POS-trained animals, adults demonstrated more broad ethanol generalization than adolescents, with dose response curves shifting rightward with increasing training doses. The 0.75 g/kg ethanol training dose was a more salient cue for adults than for adolescents. After training with this dose, adults responded for both 0.75 and 1.0 g/kg test doses whereas adolescents did not respond more after any ethanol test dose than after saline. After training with the 1.0 g/kg ethanol dose, adults responded after several test doses whereas adolescents only showed ethanol-typical responding following 1.0 and 1.25 g/kg. Following these training conditions, adolescents appear to have a limited capacity to detect ethanol doses less than 1.0 g/kg. This reduced ability to detect the subjective effects of ethanol provides another example of reduced sensitivity among adolescents relative to adults. Given that POS-trained adolescents and adults demonstrated dose-dependent responding during test sessions and similar BECs following training with 1.0 g/kg ethanol, this dose may be particularly useful for future studies using this model. Interestingly, few effects of training dose emerged during acquisition, although the dose response generalization testing data revealed no dose-dependent responding among adolescents trained with the 0.75 and 1.25 g/kg doses.
The occasion setting ethanol discrimination model used in the present study involved a number of procedural variations that may have influenced the outcome. Discrimination studies typically employ a cumulative dose procedure to generate dose response generalization curves instead of test sessions interspersed among maintenance sessions. Besheer and colleagues have described a variant of this procedure with ethanol using the Pavlovian occasion setting model of discrimination (Besheer et al., 2012a), but this method has not been tested in adolescent subjects. Cumulative dose testing would further shorten the number of testing days and thus be desirable for future studies assessing ethanol discrimination in adolescents. Another variable in ethanol discrimination studies is route of administration. Although ethanol was delivered via i.p. injections in the present study (and others; e.g., Crissman et al., 2004), intragastric gavage is also common (e.g., Besheer et al., 2012b). These routes likely produce differing ethanol cues as a result of altered pharmacokinetic patterns. Finally, although the rats in the present study were subjected to two daily training sessions to facilitate rapid acquisition, discrimination training typically involves only one training session per day. Thus, the possibility that conducting multiple sessions per day interfered with acquisition (perhaps differentially across age) cannot be ruled out.
This study is the first to demonstrate that the Pavlovian drug discrimination model using ethanol as an occasion setter can be used in both adolescents and adults. Despite age differences in discrimination scores, animals of both ages responded differentially after ethanol and saline injections by the end of the acquisition period. Results from the present study supported our hypothesis that adolescents are less sensitive to ethanol’s interoceptive effects, effects that likely serve as internal feedback cues to limit ethanol consumption. The potential for this model of ethanol discrimination to reveal age differences in contributions of different receptor systems to ethanol’s interoceptive effects is currently being investigated.
Supplementary Material
Acknowledgments
This research was funded by NIAAA grants P50 AA017823 to LPS and F31 AA021042 to RIA. The authors thank Rick Bevins for generously providing Med PC programs that were adapted for use in the present work.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112(5):1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125(1):93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Durant B. Assessment of the interoceptive effects of alcohol in rats using short-term training procedures. Alcohol. 2012a doi: 10.1016/j.alcohol.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology (Berl) 2012b;220(4):809–22. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Different chronic ethanol exposure regimens in adolescent and adult male rats: effects on tolerance to ethanol-induced motor impairment. Behav Brain Res. 2011;225(1):358–62. doi: 10.1016/j.bbr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman AM, Studders SL, Becker HC. Tolerance to the discriminative stimulus effects of ethanol following chronic inhalation exposure to ethanol in C57BL/6J mice. Behav Pharmacol. 2004;15(8):569–75. doi: 10.1097/00008877-200412000-00005. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Harris J. Where is the child’s environment? A group socialization theory of development. Psychological Review. 1995;102(3):458–489. [Google Scholar]
- Hodge CW, Grant KA, Becker HC, Besheer J, Crissman AM, Platt DM, Shannon EE, Shelton KL. Understanding how the brain perceives alcohol: neurobiological basis of ethanol discrimination. Alcohol Clin Exp Res. 2006;30(2):203–13. doi: 10.1111/j.1530-0277.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 2011. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Bienkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17(1):63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs. 2004;6 (2):151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22(2):416–21. [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15(3):183–94. [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30(4):731–41. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Wilkinson JL, Bevins RA. Methamphetamine functions as a positive and negative drug feature in a Pavlovian appetitive discrimination task. Behav Pharmacol. 2007;18(8):755–65. doi: 10.1097/FBP.0b013e3282f14efc. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Ethanol-produced interoceptive stimuli are time dependent in selectively bred HAS and LAS rats. Alcohol. 1992;9(2):117–22. doi: 10.1016/0741-8329(92)90021-2. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34(12):2061–9. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Altshuler HL. A drug discrimination analysis of ethanol-induced behavioral excitation and sedation: the role of endogenous opiate pathways. Alcohol. 1985;2(2):197–201. doi: 10.1016/0741-8329(85)90045-x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. W. W. Norton; New York: 2010. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2008. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012;54(5):523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Li C, Bevins RA. Pavlovian drug discrimination with bupropion as a feature positive occasion setter: substitution by methamphetamine and nicotine, but not cocaine. Addict Biol. 2009;14(2):165–73. doi: 10.1111/j.1369-1600.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.