Abstract
Objective
Targeting HIV antigens directly to dendritic cells using monoclonal antibodies against cell-surface receptors has been shown to evoke potent cellular immunity in animal models. The objective of this study was to configure an anti-human CD40 antibody fused to a string of five highly conserved CD4+ and CD8+ T-cell epitope-rich regions of HIV-1 Gag, Nef and Pol (αCD40.HIV5pep), and then to demonstrate the capacity of this candidate therapeutic vaccine to target these HIV peptide antigens to human dendritic cells to expand functional HIV-specific T cells.
Methods
Antigen-specific cytokine production using intracellular flow cytometry and multiplex bead-based assay, and suppression of viral inhibition, were used to characterize the T cells expanded by αCD40.HIV5pep from HIV-infected patient peripheral blood mononuclear cell (PBMC) and dendritic cell/T-cell co-cultures.
Results
This candidate vaccine expands memory CD4+ and CD8+ T cells specific to multiple epitopes within all five peptide regions across a wide range of major histocompatibility complex (MHC) haplotypes from HIV-infected patient PBMC and dendritic cell/T-cell co-cultures. These in vitro expanded HIV antigen-specific CD4+ and CD8+ T cells produce multiple cytokines and chemokines. αCD40.HIV5pep-expanded CD8+ T cells have characteristics of cytotoxic effector cells and are able to kill autologous target cells and suppress HIV-1 replication in vitro.
Conclusion
Our data demonstrate the therapeutic potential of this CD40-targeting HIV candidate vaccine in inducing a broad repertoire of multifunctional T cells in patients.
Keywords: CD40, dendritic cell, HIV, T cell, vaccine
Introduction
Several studies have shown that strong host immune responses are associated with the control of human immunodeficiency type 1 virus (HIV) replication [1,2]. Rare patients, named long-term nonprogressors (LTNPs, 5–15% of HIV-infected patients) [3,4] or elite controllers (less than 1%) [5], can maintain their CD4+ T-cell counts and/or have undetectable viral replication levels [6] in the absence of HAART. The mechanisms involved in this control imply several factors influencing immune response (reviewed in [6]). These patients exhibit strong HIV-specific polyclonal CD4+ T-cell proliferation, maintain a pool of HIV-specific central memory CD4+ T cells [7], and cytotoxic [8] and polyfunctional CD8+ T-cell responses [9]. In early HIV infection, CD8+ T-cell responses seem to be key determinants of disease progression [1]. This is supported in the rhesus macaque model in which elimination of CD8+ T cells results in dramatic increase of simian immunodeficiency virus (SIV) load [10,11]. Moreover, the rapid appearance of cytotoxic T lymphocyte (CTL) escape mutations in transmitted/founder virus during acute infection highlights the important role of CD8+ T cells in HIV containment [12]. Thus, effective generation of CTL during natural infection or eliciting potent HIV-specific CD8+ T-cell responses following vaccination is likely to be important for eliminating HIV replication in vivo or controlling viral load in the absence of sterilizing immunity [13]. However, the maintenance of functional memory CD8+ T cells [14] and effective CTL responses [15] requires CD4+ T-cell help. CD4+ T cells themselves could also contribute to the control of HIV replication [16–18]. This has implications for HIV vaccine development. Thus, in a therapeutic setting, immunization strategies which induce both CD4+ and CD8+ T-cell responses may lead to more durable CD8+ T-cell activity against HIV-infected cells, resulting in reduced viral load [19,20].
Currently, vaccine strategies combining DNA, viral vectors, or proteins in prime-boost vaccination regimens are being explored to enhance the poor immunogenicity of the individual vaccine components. One way to increase immunogenicity of proteins is to improve their delivery to the antigen-presenting cells (APCs), especially dendritic cells. Dendritic cells play a key role in inducing and regulating antigen-specific immunity. They capture antigens, process and present them to T cells as peptides bound to both major histocompatibility complex (MHC) class I and II [21–23]. Antigens can be targeted efficiently and specifically to dendritic cells using monoclonal antibodies (mAbs) directed against cell-surface receptors. For example, an anti-DEC-205 mAb fused to HIV Gag p24 induced strong CD4+ T-cell immunity in mice that was protective against challenge with recombinant vaccinia-Gag virus, but only when co-administered with an activating anti-CD40 mAb in combination with poly(I:C) [24]. The anti-DEC-205-Gag p24 fusion mAb plus poly(I:C) generated Gag-specific T cells in non-human primates (NHPs) [25] and, when targeted to HIV-infected patient dendritic cells and peripheral blood mononuclear cells (PBMCs), mediated HIV Gag p24 presentation to CD8+ T cells across a wide spectrum of MHC class I haplotypes in vitro [26].
An epitope-based vaccine composed of a set of HIV peptides which bear multiple and highly conserved CD4+ and CD8+ T-cell epitopes has been developed. This candidate vaccine, which uses five 19–32-amino acid long peptides from HIV-1 Gag, Nef, and Pol proteins covalently linked to a lipid tail [27] to facilitate uptake by APCs, is well tolerated [28] and elicits HIV-specific CD4+ and CD8+ T-cell responses in healthy volunteers [29,30] and HIV-infected individuals [19,31]. As a component of a therapeutic vaccination strategy, these HIV lipopeptides contributed to the containment of viral replication after HAART interruption [19,20].
We have developed a candidate HIV vaccine for cellular immunity based on targeting the above-mentioned HIV peptides (called herein HIV5pep) to APCs. This candidate vaccine is based on a recombinant anti-human CD40 antibody (rAb) fused via the heavy chain C-terminus to a string of the five HIV peptides (αCD40.HIV5pep). CD40 is a potent activating receptor expressed by a range of APCs, including dendritic cells, B cells and monocytes [32]. Thus, targeting CD40 offers the potential advantage of inducing dendritic cell maturation without the need for additional stimuli [33] and delivery of antigen to CD40 induced antigen-specific antibody [34,35] and protection against tumor [36]. Here, we demonstrate that αCD40.HIV5pep can effectively expand HIV antigen-specific multifunctional helper CD4+ and cytotoxic CD8+ T cells in HIV-infected patient PBMC and autologous dendritic cell/T-cell co-cultures. These cytotoxic CD8+ T cells can control HIV replication in vitro. Thus, our results provide a preclinical rationale for further testing this dendritic cell-targeting HIV candidate vaccine in nonhuman primates and humans.
Methods
Cloning and production of recombinant anti-human CD40 antibody fused to five HIV peptide regions
Using previously established mammalian cell vectors directing the secretion of chimeric mouse anti-human dendritic cell receptor antibody variable regions fused to human IgG4 heavy and light chain constant regions [37], we engineered in-frame insertions at the heavy chain C-terminus of five HIV peptide antigens and flexible linker-coding cassettes. Screening for secreted protein production in transiently transfected 293F cells was used to establish an optimal configuration of five concatenated HIV peptides interspersed with flexible linker sequences (Supplemental Fig. 1a, http://links.lww.com/QAD/A351). This optimized HIV5pep coding region was then transferred to vectors for stable transfection of CHO-S cell lines for expression and subsequent purification of αCD40.HIV5pep and hIgG4.HIV5pep fusion proteins using methods described in [38].
Cell culture and cytokine assay
PBMCs or monocyte-derived IFNα-dendritic cells loaded with HIV5pep fusion proteins were co-cultured with autologous T cells for 10 days in the presence of IL-2, then rechallenged with the individual five HIV long peptides and analyzed after 6 h for cell surface markers and intracellular cytokine production, or at 48 h for cytokine secretion into the culture supernatants.
Cytotoxic T-lymphocyte assay
Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell line (BLCL) target cells were loaded o/n with αCD40.HIV5pep, αCD40 rAb or HIV peptides at 10 μmol/l, labeled with 51Cr, and then the cytolytic activity of autologous effectors cells was determined at various E:T ratios in a 5 h 51Cr-release assay.
Inhibition of HIV-1 replication assay
Phytohaemagglutinin (PHA, Sigma)-stimulated CD4+ T cells were superinfected with a CXCR4-tropic laboratory strain (HIV-1. LAV-1, clade B) virus at a multiplicity of infection of 0.001 and autologous CD8+ T cells enriched from PBMCs cultured with HIV5pep fusion proteins were added at various CD8+ T-cell/CD4+ T-cell ratios in the presence of 100 IU/ml IL-2 (Bayer Healthcare). Supernatants were collected every 3–4 days over a 10-day period for Gag p24 quantification by ELISA (HIV-1 p24 Antigen Capture Assay; Advanced Biosciences Laboratories).
Results
Targeting APCs with αCD40.HIV5pep results in presentation of HIV peptide epitopes
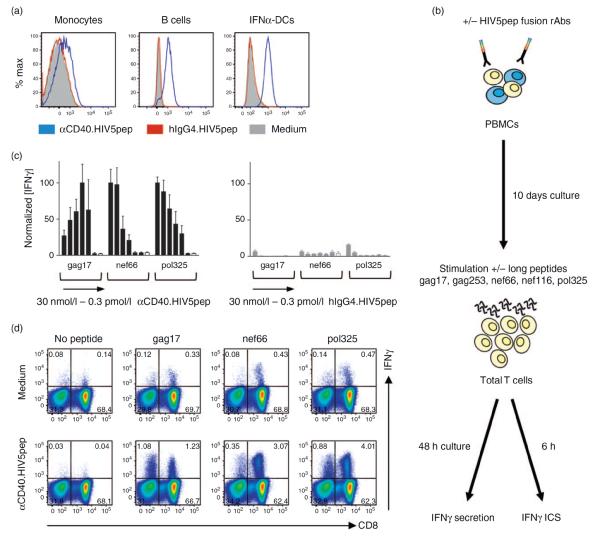
αCD40.HIV5pep, a recombinant anti-human CD40 antibody fused via the heavy chain C-terminus to a string of five epitope-rich HIV peptides (Supplemental Fig. 1, http://links.lww.com/QAD/A351), bound specifically to HIV-infected patient monocytes, B cells, and monocyte-derived IFNα-dendritic cells (Fig. 1a). αCD40.HIV5pep did not activate monocyte-derived IFNα-dendritic cells in vitro as measured by cytokine and chemokine secretion (Supplemental Fig. 2, http://links.lww.com/QAD/A351) and upregulation of surface markers (data not shown). However, the stimulatory capacity of these dendritic cells was not impaired in response to various toll-like receptor ligands (Supplemental Fig. 2, http://links.lww.com/QAD/A351). To study the ability of αCD40.HIV5pep to mediate presentation of HIV peptides, we incubated PBMCs from an HIV-infected individual with a wide dose range (0.3 pmol/l – 30 nmol/l) of αCD40.HIV5pep, as well as control hIgG4.HIV5pep. After 10 days, the cultures were stimulated for 48 h with each of the five individual HIV-long peptides, or no peptide, and then secreted IFNγ was measured to assess expansion of HIV peptide-specific T cells within the bulk PBMC population (Fig. 1b). In this patient, αCD40.HIV5pep expanded T cells specific to the gag17, nef66, and pol325 peptides. Some peptide-specific T cells could be expanded with as little as 3 pmol/l of αCD40.HIV5pep, but in general 3–30 nmol/l gave the maximum response (Fig. 1c, left panel). We observed the suppression of the gag17-specific T-cell response when the antigen-presenting cells were loaded with high concentrations of αCD40.HIV5pep (Fig. 1c, left panel). This is most likely due to the induction of antigen-specific activation-induced cell death in gag17-specific activated memory T cells in vitro. However, this effect was not seen in all the patients responding to the gag17 peptide (data not shown). The control hIgG4.HIV5pep elicited only weak pol325-specific T responses at the highest dose (Fig. 1c, right panel). In similar experiments, hIgG4.HIV5pep resulted in no or weak responses comparable to the medium control.
Fig. 1. HIV peptide-specific T-cell responses to αCD40.HIV5pep candidate vaccine stimulation.
(a) Binding of αCD40.HIV5pep to monocytes (left panel), B cells (middle panel) and monocyte-derived IFNα-dendritic cells (DCs) (right panel) from HIV-infected patients. Cells were treated with 30 nmol/l of αCD40.HIV5pep-Alexa Fluor 555 conjugated rAb (blue traces) and analyzed by flow cytometry. Red traces (staining by 30 nmol/l hIgG4.HIV5pep-Alexa Fluor 555) and solid traces (unstained cells) are the control staining. Data are representative of two different patients. (b–d), αCD40.HIV5pep candidate vaccine expands HIV peptide-specific T cells. (b) Experimental procedure. ICS, intracellular staining. (c) PBMCs from an HIV-infected individual (patient A17) were cultured for 10 days with a dose range of αCD40.HIV5pep (■) or of the matching control hIgG4.HIV5pep ( ) or left unstimulated (□), and then rechallenged for 48 h with the gag17, nef66 and pol325 individual long peptides. The culture supernatants were then harvested and IFNγ secreted by total T cells was analyzed by multiplex bead-based assay. From left to right, concentrations are 30 nmol/l, 3 nmol/l, 0.3 nmol/l, 30 pmol/l, 3 pmol/l and 0.3 pmol/l. Error bars represent SD of two independent experiments from one HIV-infected individual with seven replicates per concentration. Data were combined and IFNγ levels were normalized relative to the maximum average value for each peptide response. The average values for the three positive peptide responses to αCD40.HIV5pep for each experiment were for gag17, nef66 and pol325, respectively: 7.9, 5.7, 7.9 ng/ml and 49, 15, 26 ng/ml. These individual values were designated as 100% max response for each peptide response for each experiment performed on the same patient. (d) PBMCs from an HIV-infected patient (patient A17) were cultured for 10 days in medium alone (top panel), or with 10 nmol/l αCD40.HIV5pep (lower panel) and then rechallenged for 6 h with or without the gag17, nef66 and pol325 long peptides in the presence of brefeldin A prior to intracellular IFNγ staining. The flow cytometry profiles are gated on the overall CD3+ cell population. Data are representative of five independent experiments. PBMCs stimulated with αCD40.HIV5pep and stained with CFSE indicated that IFNγ-producing T cells proliferated, but B cell expansion was not observed (data not shown).
) or left unstimulated (□), and then rechallenged for 48 h with the gag17, nef66 and pol325 individual long peptides. The culture supernatants were then harvested and IFNγ secreted by total T cells was analyzed by multiplex bead-based assay. From left to right, concentrations are 30 nmol/l, 3 nmol/l, 0.3 nmol/l, 30 pmol/l, 3 pmol/l and 0.3 pmol/l. Error bars represent SD of two independent experiments from one HIV-infected individual with seven replicates per concentration. Data were combined and IFNγ levels were normalized relative to the maximum average value for each peptide response. The average values for the three positive peptide responses to αCD40.HIV5pep for each experiment were for gag17, nef66 and pol325, respectively: 7.9, 5.7, 7.9 ng/ml and 49, 15, 26 ng/ml. These individual values were designated as 100% max response for each peptide response for each experiment performed on the same patient. (d) PBMCs from an HIV-infected patient (patient A17) were cultured for 10 days in medium alone (top panel), or with 10 nmol/l αCD40.HIV5pep (lower panel) and then rechallenged for 6 h with or without the gag17, nef66 and pol325 long peptides in the presence of brefeldin A prior to intracellular IFNγ staining. The flow cytometry profiles are gated on the overall CD3+ cell population. Data are representative of five independent experiments. PBMCs stimulated with αCD40.HIV5pep and stained with CFSE indicated that IFNγ-producing T cells proliferated, but B cell expansion was not observed (data not shown).
Using pools of overlapping 15-mer peptides to restimulate expanded antigen-specific T cells, we could map in more detail the most likely epitopes involved in the response to the individual HIV long peptides (Supplemental Fig. 3, http://links.lww.com/QAD/A351). In similar experiments with PBMCs from two healthy volunteers, no HIV peptide-specific T cells were detected in response to the αCD40.HIV5pep stimulation (data not shown). Finally, in PBMC cultures from two HIV-infected patients, no responses were seen against long peptides, or against pools of individual 15-mer overlapping peptides, corresponding to the five flexible linker sequences interspersing the HIV peptide regions (Supplemental Fig. 4, http://links.lww.com/QAD/A351), indicating the absence of memory T cells with specificities to possible epitopes in the linker sequences. Thus, αCD40.HIV5pep mediates efficient presentation of HIV peptides and expansion of antigen-specific memory T cells from HIV-infected individuals.
αCD40.HIV5pep elicits broad HIV peptide-specific CD4+ and CD8+ T-cell responses
To ascertain T-cell types expanded by αCD40.HIV5pep, we stimulated the 10-day expansion cultures with each HIV long peptide and then stained for cell surface marker and intracellular IFNγ expression (Fig. 1b). We observed expansion of both CD8+ and CD8− HIV peptide-specific IFNγ-producing T cells (Fig. 1d). In this patient, αCD40.HIV5pep expanded gag17, nef66, and pol325-specific CD8− T cells and gag17, nef66, and pol325-specific CD8+ T cells.
We further examined whether αCD40.HIV5pep could elicit T-cell responses using PBMC and autologous IFNα-dendritic cell/T-cell co-cultures from eight other HIV-infected individuals. Our data show that αCD40.HIV5pep expanded HIV peptide-specific IFNγ-producing memory CD4+ and CD8+ T cells in all the patients tested (Fig. 2). The breadth and relative magnitude of individual peptide-specific T-cell responses were consistent in replicate experiments (Supplemental Fig. 5, http://links.lww.com/QAD/A351), but varied between patients (Fig. 2). Importantly, αCD40.HIV5pep could present MHC class I and class II epitopes from all five HIV peptide regions across a wide range of MHC haplotypes (Supplemental Table 1, http://links.lww.com/QAD/A351). Moreover, we observed that the αCD40.HIV5pep-enhanced T-cell responses require physical linkage of the HIV5pep antigen to the CD40 targeting antibody, as combination of the uncoupled activating αCD40 recombinant antibody with the hIgG4.HIV5pep did not enhance the responses (Supplemental Fig. 6, http://links.lww.com/QAD/A351). HIV-specific T-cell responses elicited with αCD40.HIV5pep were mainly dependent on dendritic cells within the PBMCs as depletion of BDCA-1+, BDCA-2+ and BDCA-3+ cells greatly reduced the responses (Supplemental Fig. 7a, http://links.lww.com/QAD/A351). Also, we obtained an identical range of T-cell responses to this candidate vaccine in PBMC cultures depleted of monocytes or B cells (Supplemental Fig. 7b, http://links.lww.com/QAD/A351), as well as in the co-cultures of autologous IFNα-dendritic cells and T cells from the same HIV-infected individuals (Supplemental Fig. 7c, http://links.lww.com/QAD/A351). Thus, αCD40.HIV5pep mainly acts via dendritic cells to expand memory CD4+ and CD8+ T cells which are specific to multiple epitopes within all five HIV peptide regions.
Fig. 2. αCD40.HIV5pep elicits a broad spectrum of HIV peptide-specific CD4+ and CD8+ T cell responses.
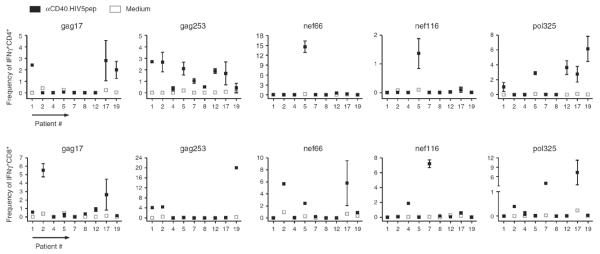
Summary from nine HIV-infected individuals of the frequencies of IFNγ+CD4+ (upper panel) and IFNγ+CD8+ (lower panel) T cells obtained from PBMC or IFNα-dendritic cell/T-cell co-cultures stimulated for 10 days with 10 nmol/l αCD40.HIV5pep (■) or in medium alone (□). Samples were then rechallenged for 6 h with or without the 5 individual HIV long peptides in the presence of brefeldin A prior to intracellular IFNγ staining. Net frequency of IFNγ+ cells = [IFNγ+CD4+ or IFNγ+CD8+ frequency in peptide-treated group] – [background IFNγ+CD4+ or IFNγ+CD8+ frequency without peptide rechallenge]. Mean frequencies of two to five independent experiments on the same donors are presented. Duplicate assays were performed in each experiment and the ranges of the replicates are presented.
HIV peptide-specific CD4+ and CD8+ T cells expanded with αCD40.HIV5pep are multifunctional
Characteristics that are considered important for antigen-specific CD4+ T-cell function in the control of HIV infection include the production of effector cytokines and β-chemokines. Thus, antigen-specific cytokine production profiles of the αCD40.HIV5pep-expanded T cells were determined. In PBMC cultures from one representative HIV-infected patient, αCD40.HIV5pep-expanded CD4+ T cells co-expressed IFNγ with TNFα, MIP-1β, as well as CD154 (CD40 ligand), in response to stimulation with gag253 and pol325 peptides (Fig. 3a–c). Similar analysis within multiple HIV peptide responses across several HIV-infected individuals showed that from 30 to 94% of the αCD40.HIV5pep-expanded IFNγ-producing CD4+ T cells co-expressed TNFα (N=9) and 83–95% MIP-1β (N=3). Intracellular IL-2 was not detectable (data not shown).
Fig. 3. HIV peptide-specific T cells expanded with αCD40.HIV5pep candidate vaccine are multifunctional.
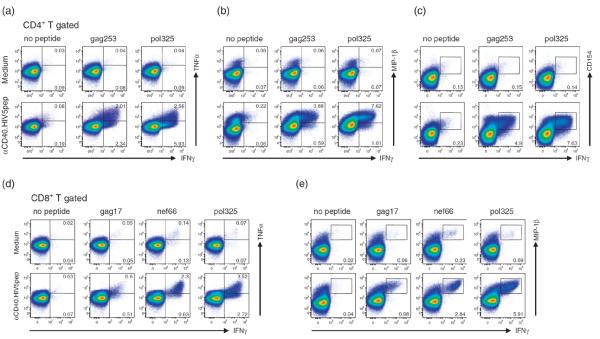
PBMCs from two HIV-infected patients were cultured for 10 days in medium alone, or with 10 nmol/l αCD40.HIV5pep and then rechallenged for 6 h with or without HIV long peptides in the presence of brefeldin A prior to intracellular cytokine staining. The profiles are gated on the overall CD4+ (panels a, b, c, patient A6) or CD8+ (panels d, e, patient A17) T-cell populations, with the percentages of populations responding to the designated peptides shown in each plot. The FACS plots show coordinate analysis of IFNγ versus TNFα (a,d), MIP-1β (b,e), or CD154 (c) expression. Data are representative of two to three independent experiments using at least three different patients. The scales are log from 0 to 105, and in some cases bi-exponential transformation was applied.
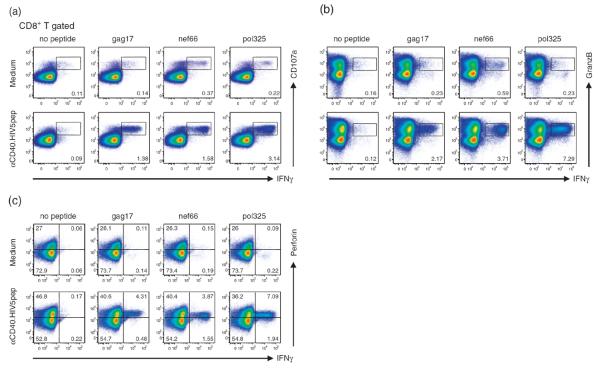
To control virus dissemination, virus-specific CD8+ T cells exert several effector functions, mediated through the production of soluble factors and cytolytic mechanisms. We therefore assessed the functional capacity of the expanded HIV peptide-specific CD8+ T cells by measuring the expression of effector cytokines, β-chemokines, and cytolytic factors, as well as by measuring degranulation capacity as determined by externalization of CD107a. In αCD40.HIV5pep-treated PBMC cultures from one representative HIV-infected patient, most gag17, nef66, and pol325-specific CD8+ T cells co-expressed intracellular TNFα and MIP-1β with IFNγ (Fig. 3d,e). Also, in response to peptide challenge, most HIV peptide-specific CD8+ T cells displayed CD107a and coexpressed granzyme B and perforin with IFNγ (Fig. 4). We noticed an increase of the preformed perforin in the total CD8+ T cells expanded with αCD40.HIV5pep (Fig. 4c), but not with non-antigen linked αCD40 rAb (data not shown) and this observation was consistent among several donors examined.
Fig. 4. HIV peptide-specific CD8+ T cells expanded with αCD40.HIV5pep candidate vaccine have CTL characteristics.
PBMCs from an HIV-infected patient (patient A17) were cultured for 10 days in medium alone, or with 10 nmol/l αCD40.HIV5pep and then rechallenged for 6 h with or without gag17, nef66 and pol325 HIV long peptides in the presence of brefeldin A prior to intracellular cytokine staining. The FACS plots show coordinate analysis of IFNγ versus CD107a externalization (a), or IFNγ versus granzyme B (b), or IFNγ versus perforin (c). The profiles are gated on the overall CD8+ T-cell population, with the percentage of populations responding to the designated peptides shown in each plot. Data are representative of two to three independent experiments using three different patients. The scales are log10 from 0 to 105, and in some cases bi-exponential transformation was applied.
Similar analysis within multiple HIV peptide responses across several HIV-infected individuals showed that from 20 to 87% (N=9) of the αCD40.HIV5pep-expanded IFNγ-producing CD8+ T cells coexpressed TNFα; 96–99% MIP-1β; 82–97% CD107a; 72–90% perforin; 84–100% granzyme B, (N=3). Overall, these results show that αCD40.HIV5pep expands a broad range of HIV peptide-specific memory CD4+ and CD8+ T cells that are multifunctional by the criteria we have examined and the pattern of antigen-specific coexpression of intracellular cytokines observed in αCD40.HIV5pep-elicited CD4+ and CD8+ T-cell responses was similar and consistent within multiple HIV peptide responses across several HIV-infected individuals tested.
αCD40.HIV5pep-expanded CD8+ T cells kill target cells and suppress HIV-1 replication in vitro
To assay the cytolytic activity of the αCD40.HIV5pep-expanded HIV-specific CD8+ T cells, we used as target cells HIV peptide-loaded autologous EBV-transformed B lymphocytes (BLCL) labeled with 51Cr. To this end, we mapped in greater detail the epitopes of CD8+ T-cell responses from two HIV-infected patients. In PBMC cultures from one patient, in which αCD40.HIV5pep expanded CD8+ T-cell responses specific to epitopes within the nef66 long peptide, responses to individual overlapping 15-mer peptides from the nef66 region identified a previously known A*1101 (84–92) epitope contained within two overlapping peptides (Supplemental Fig. 3, http://links.lww.com/QAD/A351). The CD8+ T cells expanded in PBMC cultures with αCD40.HIV5pep were able to kill autologous BLCL loaded with a 15-mer Nef (80–94) peptide containing the (84–92) epitope, but did not kill BLCL treated with a control Nef (56–70) peptide (Fig. 5a) or an irrelevant 9-mer prostate-specific antigen peptide (data not shown). Interestingly, the αCD40.HIV5pep-expanded CD8+ T cells were also able to efficiently kill autologous BLCL loaded with αCD40.HIV5pep, but did not kill autologous targets incubated with non-antigen linked αCD40 rAb (Fig. 5a). In PBMC cultures from a second patient, in which αCD40.HIV5pep expanded CD8+ T-cell responses against the gag17, gag253, and nef66 long peptides, mapping with individual overlapping 15-mer peptides identified responses corresponding to epitopes A*0301 Gag p17 (18–26/20–29), A*0301 Nef (73–82), A*0301 Nef (84–92), B*0801 Nef (90–97), B*0811 Gag p24 (128–135), and B*1501 Gag p24 (137–145) (Supplemental Fig. 3, http://links.lww.com/QAD/A351). CD8+ T cells expanded in these PBMC cultures with αCD40.HIV5pep were equally potent in killing autologous BLCL treated with a 15-mer Gag p17 (17–31) peptide containing the (18–26/20–29) epitope or with an 8-mer Nef peptide containing the (90–97) epitope or with a 15-mer Gag p24 (137–151) peptide containing the (137–145) epitope, but did not kill BLCL treated with a control Gag p17 (5–19) peptide (Fig. 5b).
Fig. 5. αCD40.HIV5pep-expanded CD8+ T cells kill autologous target cells and suppress HIV-1 replication in vitro.
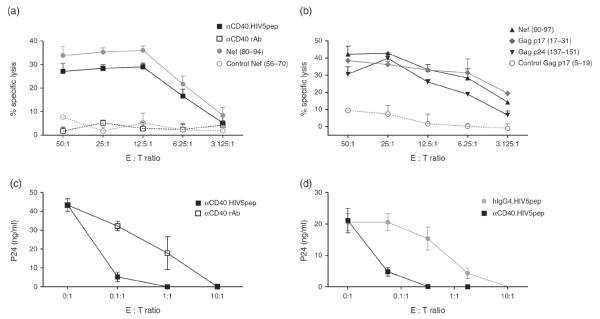
(a, b) PBMCs from two HIV-infected individuals were cultured for 10 days with 30 nmol/l αCD40.HIV5pep and then the expanded CD8+ T cells were tested at different E : T ratios for their capacity to kill an autologous EBV-transformed B lymphoblastoid cell line (BLCL). 51Cr-labeled BLCL were previously loaded with 30 nmol/l αCD40.HIV5pep (■), or 30 nmol/l αCD40 rAb (□), or 15-mer Nef peptides ( and
and  ) (a, patient A17); or were previously loaded with an 8-mer Nef peptide (▲), or 15-mer Gag p17 peptides (
) (a, patient A17); or were previously loaded with an 8-mer Nef peptide (▲), or 15-mer Gag p17 peptides ( and
and  ) and Gag p24 peptides (▼) (b, patient A2). The Nef (56–70) and Gag p17 (5–19) peptides are irrelevant HIV peptides. Triplicate assays were performed in each experiment. (c, d) PBMCs from an HIV-infected patient (patient A17) were cultured for 10 days with αCD40.HIV5pep (■) or αCD40 rAb (□) (c); or with αCD40.HIV5pep (■), or hIgG4.HIV5pep (
) and Gag p24 peptides (▼) (b, patient A2). The Nef (56–70) and Gag p17 (5–19) peptides are irrelevant HIV peptides. Triplicate assays were performed in each experiment. (c, d) PBMCs from an HIV-infected patient (patient A17) were cultured for 10 days with αCD40.HIV5pep (■) or αCD40 rAb (□) (c); or with αCD40.HIV5pep (■), or hIgG4.HIV5pep ( ) (d) at 30 nmol/l and then co-cultured at different ratios with autologous LAV-1-superinfected CD4+ T cells. The line graphs represent the mean change in the p24 levels in the supernatants after 10 days of co-culture in wells containing CD4+ T cells alone (0 : 1) and CD8+ T cells and autologous CD4+ T cells at different E : T ratios. Six replicate assays were performed in each experiment. Data are representative of two independent experiments using two different patients.
) (d) at 30 nmol/l and then co-cultured at different ratios with autologous LAV-1-superinfected CD4+ T cells. The line graphs represent the mean change in the p24 levels in the supernatants after 10 days of co-culture in wells containing CD4+ T cells alone (0 : 1) and CD8+ T cells and autologous CD4+ T cells at different E : T ratios. Six replicate assays were performed in each experiment. Data are representative of two independent experiments using two different patients.
We further assessed the ability of αCD40.HIV5pep-expanded HIV-specific CD8+ T cells to suppress HIV-1 propagation in CD4+ T-cell cultures. Purified activated autologous CD4+ T cells were superinfected with HIV-1 and coincubated over a period of 10 days with different effector/target (E/T) ratios of purified CD8+ T cells previously expanded with αCD40.HIV5pep or αCD40 rAb alone. In PBMC cultures of one HIV-infected patient, αCD40.HIV5pep expanded gag17, nef66, and pol325-specific CD8+ T cells (Supplemental Fig. 5, http://links.lww.com/QAD/A351), but control hIgG4.HIV5-pep did not efficiently expand HIV peptide-specific CD8+ T cells (data not shown). When HIV-1-superinfected CD4+ T cells were co-cultured with HIV-specific CD8+ T cells previously expanded with αCD40.HIV5pep or with αCD40 rAb at a 10 : 1 E/Tratio, viral replication was undetectable (Fig. 5c). Moreover, αCD40.HIV5pep-expanded CD8+ T cells still controlled viral replication at a ratio of 0.1 : 1 E/Tratio, whereas the antiviral activity of CD8+ T cells expanded with the control αCD40 rAb was rapidly lost when less effector CD8+ T cells were present (Fig. 5c). In a replicate experiment, similar data was obtained, in which CD8+ T cells previously expanded with αCD40.HIV5pep suppressed HIV-1 propagation even at a 0.01 : 1 E/T ratio, while a similar degree of inhibition was only observed at 1 : 1 E/Tratio with CD8+ T cells expanded with hIgG4.HIV5pep (Fig. 5d). Comparable results were obtained in another patient (data not shown). Collectively, these data demonstrate that HIV-specific CD8+ T cells expanded with αCD40.HIV5pep are capable of lysing autologous target cells in an HIV peptide-specific manner and effectively suppress HIV-1 replication in vitro.
Discussion
Antigens can be targeted to dendritic cells via different endocytic receptors, and this presents exciting possibilities for vaccine design. For example, targeting HIV Gag p24 to human dendritic cells via DEC-205 in HIV-infected patient PBMC cultures expanded IFNγ-producing Gag p24-specific CD8+ T cells across a wide spectrum of MHC class I haplotypes [26]. Although, Gag-specific CD8+ T cells are associated with lower viremia [39–41], vaccines eliciting responses to conserved regions of other immunogenic HIV proteins, such as Nef, may also be important to force escape pathways that reduce HIV fitness. We developed a candidate dendritic cell-targeting vaccine composed of a recombinant anti-human CD40 antibody fused to five concatenated HIV peptides. The addition of flexible glycosylated spacers between the five HIV peptide regions fused to the anti-CD40 rAb was critical to enabling production of such a complex dendritic cell-targeting antigen candidate vaccine. αCD40.HIV5pep can effectively bind and deliver the HIV5pep antigens to APCs in PBMC and autologous dendritic cell/T-cell co-cultures from HIV-infected individuals. Our candidate vaccine was designed to specifically expand T cells focused to highly conserved CD4+ and CD8+ T-cell epitope-rich regions from Gag, as well as Nef and Pol. Although the range of the epitope specificities varied for each patient, epitopes within all five HIV peptide regions of our αCD40.HIV5pep were effectively presented across the entire HIV-infected individual sample set we surveyed, which was heterogeneous for MHC class I and II alleles. This must likely reflect the presence of different repertoires of memory T cells in each patient analyzed as responses were largely consistent with predicted HLA-restricted class I and II epitopes [31,42,43]. Our study demonstrated a need for physical linkage between the HIV5pep antigen and the CD40 targeting antibody to activate HIV-specific T cells in vitro. This might be explained by the CD40 antibody internalization being mediated by a specific surface receptor while the non-targeted antigen (hIgG4.HIV5-pep) probably enters via pinocytosis, in a non-receptor-dependent manner since it is based on a non-Fc receptor binding hIgG4. Thereafter, they may traffic to different endocytic compartments or to the same compartment at different rates. The in vitro potency of our αCD40.HIV5-pep prototype vaccine in eliciting expansion of HIV-specific memory T cells does not result from CD40 activation per se, but rather from the efficiency of high affinity binding to CD40 via the antibody combining sites and the associated favorable internalization and processing pathway. This is supported by a recent study showing that CD40 is internalized and degraded slowly into early endosomes leading to efficient MHC class I and II presentation [44].
Our aim was to develop a candidate vaccine capable of expanding potent effector CD8+ T cells, as well as helper CD4+ T cells for maintaining functional memory CD8+ T cells [14] and potentially reinforcing HIV control through secretion of chemokines that inhibit HIV replication [7]. αCD40.HIV5pep elicits in vitro expansion of a broad repertoire of multifunctional HIV antigen-specific memory CD4+ T cells, by the criteria of simultaneous expression of IFNγ with TNFα, MIP-1β, and CD154, from HIV-infected individual PBMC and dendritic cell/T-cell co-cultures. In contrast to mouse and NHP studies with targeting HIV Gag p24 via DEC-205 [24,25,45], no antigen-specific expansion of CD4+ T cells was detected in the αDEC-205-Gag p24-treated cultures [26]. αCD40.HIV5pep was also very effective at expanding a broad repertoire of HIV-specific memory CD8+ T cells with characteristics of potent multi-functional cytotoxic effector cells, which co-express IFNγ with TNFα, MIP-1β, granzyme B, perforin and externalization of CD107a. Results from studies in which different parameters of CTL function were analyzed have shown that while HIV-specific CD8+ T cells from progressors typically secrete just IFNγ, HIV-specific CD8+ T cells from LTNPs produce multiple cytokines and undergo degranulation [9]. These defective functions are not restored by HAART [46].
In vitro, αCD40.HIV5pep induced upregulation of both perforin expression in the global CD8+ T-cell population, as well as de novo synthesis of perforin by the HIV antigen-specific CD8+ T cells in response to peptide stimulation. This perforin-enhancing property could be critical for the in vivo potency of such a candidate vaccine since, compared to progressors, HIV-specific CD8+ T cells from LTNPs have higher proliferative capacity and greater ability to upregulate perforin and granzyme B expression levels upon stimulation [47–49]. Furthermore, αCD40.HIV5pep-expanded CD8+ T cells efficiently killed autologous BLCL loaded with relevant HIV peptides, and furthermore they controlled HIV-1 replication in vitro within CD4+ T-cell cultures. In contrast, in the αDEC-205-Gag p24 study [26], critical features of cytotoxic CD8+ T-cell effectors or control of HIV-1 replication were not addressed. This is important since studies have shown that HIV-specific CD8+ T cells from LTNPs, but not from progressors, have a greater ability to suppress the replication of HIV in autologous infected CD4+ T cells during extended culture [8]. This role of CD8+ T cells for the control of HIV replication in vivo is also supported by studies in the NHP challenge model where vaccination inducing SIV-specific CD8+ T-cell responses partially contained SIV replication [40].
Taken together, our data demonstrated that all desirable T-cell effector qualities were elicited by αCD40.HIV5-pep in the HIV-patient PBMC and dendritic cell/T-cell co-cultures. A current phase I clinical trial involving ex vivo HIV-patient IFNα-dendritic cells loaded with five individual HIV lipopeptides [50] (ClinicalTrials.gov Identifier: NCT00796770), using the same HIV peptide sequences that are present on αCD40.HIV5pep is ongoing and this gives support to further develop αCD40.HIV5pep as a therapeutic vaccination strategy and to test this candidate vaccine in animal models and humans. Our data also open the pathway to the development of vaccines based on this CD40-targeting vaccine approach for other viral or cancer antigens, for which induction of potent cellular immunity will be required.
Supplementary Material
Acknowledgements
We thank Dr A.K. Palucka for discussions, Dr C. Harrod for help with regulatory requirements, P. Klucar for some vector constructs and protein purifications, the Luminex core and L. Walters for the processing of the cells.
A.L.F. designed, did and analyzed all experiments and wrote the article with major input from S.O., G.Z. and Y.L.; J.B., Y.L. and G.Z. provided intellectual input; G.Z. designed experiments and contributed to the molecular biology; Y.X. provided technical assistance for the inhibition of viral replication experiments; S.M.Z. validated and provided reagents; S.O. provided reagents and intellectual input; M.M. contributed to the initiation of the HIV partnership and to the development of analytic tools; B.K. and L.S. contributed to the recruitment of patients and provided HIV-1 samples for research.
The research was supported by the Agence Nationale de Recherche pour le Sida et hepatites virales through the ANRS HIV Vaccine Network programme via a Collaborative Research Agreement (CRA 2007-291), the parental anti-CD40 mAb was developed with support from grants from the National Institutes of Health (U-19 AI-57234) and the generosity of the Baylor Healthcare System through the auspices of the Baylor Research Institute.
Footnotes
Conflicts of interest A.L.F., M.M., J.B., Y.L. and G.Z. are inventors on a patent application related to the work described in this study. The application is held by the Baylor Research Institute, a nonprofit research arm of the Baylor Heath Care System, and by INSERM/ANRS.
References
- 1.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 4.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen OJ, Demarest JF, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 5.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41:1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 11.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, et al. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J Virol. 2010;84:4998–5006. doi: 10.1128/JVI.00138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 18.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 19.Levy Y, Gahery-Segard H, Durier C, Lascaux AS, Goujard C, Meiffredy V, et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. AIDS. 2005;19:279–286. [PubMed] [Google Scholar]
- 20.Levy Y, Durier C, Lascaux AS, Meiffredy V, Gahery-Segard H, Goujard C, et al. Sustained control of viremia following therapeutic immunization in chronically HIV-1-infected individuals. AIDS. 2006;20:405–413. doi: 10.1097/01.aids.0000206504.09159.d3. [DOI] [PubMed] [Google Scholar]
- 21.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 24.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, et al. Intensified and protective CD4+ T cell immunity in mice with antidendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klinguer C, David D, Kouach M, Wieruszeski JM, Tartar A, Marzin D, et al. Characterization of a multilipopeptides mixture used as an HIV-1 vaccine candidate. Vaccine. 1999;18:259–267. doi: 10.1016/s0264-410x(99)00196-6. [DOI] [PubMed] [Google Scholar]
- 28.Durier C, Launay O, Meiffredy V, Saidi Y, Salmon D, Levy Y, et al. Clinical safety of HIV lipopeptides used as vaccines in healthy volunteers and HIV-infected adults. AIDS. 2006;20:1039–1049. doi: 10.1097/01.aids.0000222077.68243.22. [DOI] [PubMed] [Google Scholar]
- 29.Gahery-Segard H, Pialoux G, Charmeteau B, Sermet S, Poncelet H, Raux M, et al. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine. J Virol. 2000;74:1694–1703. doi: 10.1128/jvi.74.4.1694-1703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pialoux G, Gahery-Segard H, Sermet S, Poncelet H, Fournier S, Gerard L, et al. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS. 2001;15:1239–1249. doi: 10.1097/00002030-200107060-00005. [DOI] [PubMed] [Google Scholar]
- 31.Gahery H, Daniel N, Charmeteau B, Ourth L, Jackson A, Andrieu M, et al. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res Hum Retroviruses. 2006;22:684–694. doi: 10.1089/aid.2006.22.684. [DOI] [PubMed] [Google Scholar]
- 32.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Zhang S, Li M, Chen C, Yao Q. Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhances SHIV-VLP-induced dendritic cell activation and boosts immune responses against HIV. Vaccine. 2010;28:5114–5127. doi: 10.1016/j.vaccine.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr TA, McCormick AL, Carlring J, Heath AW. A potent adjuvant effect of CD40 antibody attached to antigen. Immunology. 2003;109:87–92. doi: 10.1046/j.1365-2567.2003.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hangalapura BN, Oosterhoff D, Aggarwal S, Wijnands PG, van de Ven R, Santegoets SJ, et al. Selective transduction of dendritic cells in human lymph nodes and superior induction of high-avidity melanoma-reactive cytotoxic T cells by a CD40-targeted adenovirus. J Immunother. 2010;33:706–715. doi: 10.1097/CJI.0b013e3181eccbd4. [DOI] [PubMed] [Google Scholar]
- 36.Schjetne KW, Fredriksen AB, Bogen B. Delivery of antigen to CD40 induces protective immune responses against tumors. J Immunol. 2007;178:4169–4176. doi: 10.4049/jimmunol.178.7.4169. [DOI] [PubMed] [Google Scholar]
- 37.Flamar AL, Zurawski S, Scholz F, Gayet I, Ni L, Li XH, et al. Noncovalent assembly of antidendritic cell antibodies and antigens for evoking immune responses in vitro and in vivo. J Immunol. 2012;189:2645–2655. doi: 10.4049/jimmunol.1102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, et al. Targeting self- and foreign antigens to dendritic cells via DCASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honey-borne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saez-Cirion A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gagspecific CD8 T cell responses. J Immunol. 2009;182:7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 42.Gahery-Segard H, Pialoux G, Figueiredo S, Igea C, Surenaud M, Gaston J, et al. Long-term specific immune responses induced in humans by a human immunodeficiency virus type 1 lipopep-tide vaccine: characterization of CD8+-T-cell epitopes recognized. J Virol. 2003;77:11220–11231. doi: 10.1128/JVI.77.20.11220-11231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karina Yusim DB, Koup Richard, Korber Bette T. M., de Boer Rob, Moore John P., Brander Christian, et al. Los Alamos National Laboratory, Theoretical Biology and Biophysics. Los Alamos, New Mexico: 2011. HIV Molecular Immunology 2011; pp. 12–10074. LA-UR. http://www.hiv.lanl.gov/content/immunology/pdf/2012/immuno2012.pdf. [Google Scholar]
- 44.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 45.Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human antihuman DEC205 monoclonal antibody. Blood. 2010;116:3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 48.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobb A, Roberts LK, Palucka AK, Mead H, Montes M, Ranganathan R, et al. Development of a frozen therapeutic dendritic cell vaccine pulsed with HIV-1-antigen derived lipopeptides. J Immunol Methods. 2011;365:27–37. doi: 10.1016/j.jim.2010.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.