Abstract
Mitochondrial dysfunction plays a pivotal role in necroapoptotic cell death and in the development of acute kidney injury (AKI). Evidence suggests that glycogen synthase kinase (GSK) 3β resides at the nexus of multiple signaling pathways implicated in the regulation of mitochondrial permeability transition (MPT). In cultured renal tubular epithelial cells, a discrete pool of GSK3β was detected in mitochondria. Co-immunoprecipitation assay confirmed that GSK3β physically interacts with cyclophilin F and voltage-dependent anion channel (VDAC), key MPT regulators that possess multiple GSK3β phosphorylation consensus motifs, suggesting that GSK3β has a direct control of MPT. Upon a strong burst of reactive oxygen species elicited by the pro-oxidant herbicide paraquat, the activity of the redox-sensitive GSK3β was drastically enhanced. This was accompanied by augmented phosphorylation of cyclophilin F and VDAC, associated with MPT and cell death. Inhibition of GSK3β by either the selective inhibitor 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione(TDZD-8) or forced expression of a kinase-dead mutant obliterated paraquat-induced phosphorylation of cyclophilin F and VDAC, prevented MPT and improved cellular viability. Conversely, ectopic expression of a constitutively active GSK3β amplified the effect of paraquat on cyclophilin F and VDAC phosphorylation and sensitized cells to paraquat induced MPT and death. In vivo, paraquat injection elicited marked oxidant stress in the kidney and resulted in acute kidney dysfunction and massive tubular apoptosis and necrosis. Consistent with in vitro findings, the activity of GSK3β was augmented in the kidney following paraquat injury, associated with increased phosphorylation of cyclophilin F and VDAC and sensitized MPT. TDZD-8 blocked GSK3β activity in the kidney, intercepted cyclophilin F and VDAC phosphorylation, prevented MPT, attenuated tubular cell death and ameliorated paraquat-induced AKI. Our data suggests that the redox-sensitive GSK3β regulates renal tubular injury in AKI via controlling the activity of MPT regulators.
Keywords: acute kidney injury, cyclophilin F, voltage-dependent anion channel, glycogen synthase kinase 3β, mitochondrial permeability transition, paraquat
Acute kidney injury (AKI) is a common catastrophic complication of critical illness related to trauma, major surgery, sepsis, and acute intoxication. At a lower incidence, it also occurs in general populations or due to iatrogenic administration of nephrotoxic drugs. AKI manifests with an abrupt or rapid decline in renal filtration function and is often under-recognized and associated with severe consequences that result in substantial morbidity and mortality [1-4]. So far, no specific intervention that either prevents or treats kidney injury or improves survival has been clinically applied. There exists a desperate need for developing a novel and effective therapy for AKI.
Mitochondrial dysfunction is central to necroapoptotic cell death and plays an important role in the pathogenesis of AKI [5-7]. Accumulation of cytotoxic reactive oxygen species (ROS), mostly in and around mitochondria, can be induced in tubular epithelial cells by a lot of injurious stimuli including ischemia reperfusion, nephrotoxic substances, pathogens and so forth [8-10]. ROS can trigger the opening of the end-effector mitochondrial permeability transition (MPT) pore, which is accompanied by the immediate dissipation of the mitochondrial membrane potential, and results in permeability of the mitochondrial inner membrane to allow solutes with a molecular weight less than 1,500 Daltons to leak from the mitochondria [11, 12]. The subsequent breaking of mitochondrial outer membrane will lead to the release of proteins in the inter-membrane space such as cytochrome c and other factors that play a critical role in apoptosis or necrosis depending on the availability of ATP[13]. The opening threshold of the MPT pore and the sensitivity of MPT to ROS are determined by the activation status of the mitochondrial membrane proteins like cyclophilin F (also known as mitochondrial cyclophilin D) and voltage-dependent anion channel (VDAC) [14, 15], which are regulated by a myriad of signaling pathways[16-19]. Of many of these pathways, glycogen synthase kinase (GSK) 3β has emerged as the integration point and plays a crucial role in transferring regulatory signals downstream to modify susceptibility to MPT in excitable cells including neurons and cardiomyocytes[20]. Whereas, the role of GSK3β in controlling MPT in non-excitable cells like kidney cells remains largely unknown and merits further investigation.
GSK3β is a well-conserved, ubiquitously expressed serine/threonine protein kinase originally characterized as one that phosphorylates glycogen synthase and regulates glucose metabolism[21]. GSK3β is constitutively active in quiescent cells and its activity is negatively regulated by the Wnt signaling pathway or by protein kinase B/Akt via inhibitory phosphorylation of the N-terminal serine-9[22]. Interest in GSK3β expanded greatly with the realization that it is a key regulator of multiple pivotal cell processes extending well beyond glycogen metabolism to signal transduction, insulin action, gene transcription, translation, cytoskeletal organization and cell cycle progression, cell death and survival[21, 23, 24]. More recent evidence suggests that GSK3β plays dirty in acute kidney injury[25]. Inhibition of GSK3β by small-molecule inhibitors or genetic knockout strikingly prevented acute renal histological injury induced by ischemia reperfusion, nephrotoxic substances including NSAID and mercury [26-28]. However, the exact mechanisms of action underlying this kidney protective effect remain obscure. This study explored the role of GSK3β in oxidative stress induced MPT, mitochondria dysfunction and the ensuing necroapoptotic cell death in renal tubular cells acutely injured by the pro-oxidant herbicide paraquat. The effect of GSK3β inhibition on mitochondrial dysfunction was also examined in a murine model of paraquat induced AKI.
MATERIALS AND METHODS
Cell culture
Immortalized murine proximal tubule epithelial (TKPT) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 5% FBS (Life Technologies, Grand Island, NY). Cells were plated at approximately 60% confluence for 24 h and then subjected to serum starvation for another 24 h. TDZD-8 (Sigma, St. Louis, MO, USA) or paraquat (Sigma) was added with fresh serum-free medium to the cultures at indicated concentration respectively. Cells were harvested at the indicated time points.
Cellular viability assay
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was used to assess cell viability. MTT (Sigma) was added (final concentration of 0.5 mg/ml) to individual cultures one hour before harvest, and tetrazolium released by dimethyl sulfoxide. Optical density was determined with a spectrophotometer (570 nm), and data were normalized to solvent-treated cultures.
Transient transfection
The expression vectors encoding the constitutively active GSK3β mutant (S9A-GSK3β-HA/pcDNA3), and kinase-dead GSK-3β mutant (KD-GSK3β-HA/pcDNA3) were applied as previous reported [26, 29]. Transient transfection of TKPT cells was carried out by using the Lipofectamine 2000 according to the instructions specified by the manufacturer (Invitrogen, Carlsbad, CA). After transfection with equal amounts of expression plasmid or empty vector (EV), immunofluorescent staining revealed that >75% of the cells expressed the hemagglutinin-tagged constructs 24 h after transfection. Cells were then subjected to different treatments as indicated.
Fluorescent immunocytochemistry
Cells were fixed with 4% paraformaldehyde. Following serum blocking for 30 min, cells were incubated with the primary antibody to GSK3β, cyclophilin F, VDAC (Santa Cruz, CA) or preimmune IgG and then the Alexa fluorophore-conjugated secondary antibody (Invitrogen). For mitochondria staining, live cells were incubated with Mito Tracker Green (Invitrogen) for 30 min before fixation. Finally, all cells were counterstained with 4′,6-Diamidino-2-phenylindole (DAPI) and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and visualized using a fluorescence microscope.
Animal experimental design
Animal experimental studies were approved by the institution’s Animal Care and Use Committee and they conform to the United States Department of Agriculture regulations and the National Institutes of Health guidelines for humane care and use of laboratory animals. Male C57BL/6 mice at 8 weeks old were randomly divided into four groups of six animals each as follows: Group Control (Ctrl), mice were injected intraperitoneally with normal saline; Group T, mice received intraperitoneal injection of TDZD-8 (10mg/kg, dissolved in 10% dimethyl sulfoxide); Group PQ, mice received intraperitoneal injection of vehicle 1 h before intraperitoneal injection of paraquat (30 mg/kg, dissolved in saline); Group T+PQ, mice received intraperitoneal injection of TDZD-8 (10mg/kg) 1 h before intraperitoneal injection of paraquat (30 mg/kg). All mice were killed 72 h after paraquat injury. Before sacrifice, serum and urine samples were collected and kidneys and livers harvested for further investigation.
Serum and urine assays
Serum creatinine was measured with Creatinine Assay Kit (Biovision, Milpitas, CA, USA). Blood urea nitrogen (BUN) levels were determined by a BUN assay kit (BioAssay Systems, Hayward, CA, USA). Urine neutrophil gelatinase - associated lipocalin (NGAL) was analyzed by mouse NGAL ELISA kit (BioPorto, Denmark).
Histological studies
Formalin-fixed kidneys were embedded in paraffin and prepared in 3μm thick sections. Sections were stained with hematoxylin eosin to estimate gross histological kidney injury. One observer performed semi-quantitative morphometric analysis in a blinded manner. Histological changes due to tubular injury score were evaluated in the stripe of the outer medulla and the cortex, and were quantified by counting the percentage of tubules that displayed cell necrosis, loss of brush border, and cytoplasm vacuolation as follows: 0 = none, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76%. At least 10 fields (×400) were reviewed for each slide. TUNEL staining (Roche Applied Science, Indianapolis, IN) and DAPI (4′, 6-diamidino-2-phenylindole) staining were performed on methanol/acetone-fixed (1:1) frozen cryostat sections or cell cultures according to the manufacturer’s instructions.
Detection of ROS generation by fluorescence
Paraquat is a prototypic toxin known to exert injurious effects through oxidative stress [30]. Changes in ROS production were measured by use of 2′, 7′-dichlorofluorescein-diacetate (DCF-DA; Sigma) [31]. Briefly, cells were loaded with 20 μM DCF-DA and incubated at 37°C for 30 minutes. Then, fresh serum-free media was added, and a baseline fluorescence reading was measured before treatment. Fluorescence was determined by fluorometric analysis of cell lysates at different time points following treatments. Fresh kidney cryostat sections were incubated with 10μM DCF in a light protected humidified chamber at 37°C for 30 minutes, subsequently washed two times with PBS for 5 min, mounted with Mounting Medium (Vector Laboratories) and visualized with a fluorescence microscope and fluorescence quantified by morphometric analysis.
MPT assay
Mitochondria are a major source of paraquat-induced ROS production [30]. In turn, ROS overproduction will lead to MPT, ultimately causing cell death [11, 12]. To measure MPT, mitochondria were isolated from kidney tissues or cultured cells as previously described [32-34]. The protein concentration was determined with bovine serum albumin as the standard. Mitochondrial swelling was estimated on the basis of the decrease in the absorbance of mitochondria (1.0 mg protein) at 540nm in 1ml of a medium containing 125 mmol/l sucrose, 65 mM KCl, 5 mM succinate, 5 μM rotenone, 20 μM CaCl2, and 10 mM Hepes-KOH, pH 7.2, at 30°C.
Western immunoblot analysis and immunoprecipitation
Cultured tubular epithelial cells were lysed and mice kidneys/livers homogenized in RIPA buffer supplemented with protease inhibitors. Mitochondrial fractions were prepared from cultured cells or tissues by using the mitochondria isolation kit (Pierce, Rockford, IL USA). Samples with equal amounts of total protein (50 mg/ml) were processed for immunoblot as described previously [35]. NGAL has been widely recognized as a biomarker for subclinical or overt renal tubular injury [36]. The antibodies against GSK3β, p-GSK3β, caspase-3, cyclophilin F, and VDAC were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). To determine the paraquat-induced cellular toxicity in the kidney, kidney homogenates were processed for immunoblot analysis of NGAL. The antibody against NGAL was purchased from R & D Systems (Minneapolis, MN, USA). For detection of phosphorylated cyclophilin F or VDAC and to examine potential physical interactions between GSK3β and cyclophilin F or VDAC, cyclophilin F or VDAC antibody (Santa Cruz Biotechnology) was used as the immunoprecipitation antibody and the antibody against phosphorylated serine or phosphorylated threonine or GSK3β (Santa Cruz Biotechnology) was used to probe the immunoprecipitates by immunoblot analysis.
GSK3β activity assay
Activity of GSK3β in the cytosol or mitochondria was measured using GENMED GSK3β activity assay kit (Genmed Scientifics, Arlington, MA, USA) according to manufacturer’s instructions. Mitochondria isolation kit for cultured cells (Pierce) was used to separate mitochondria fractions from cytosol fractions of cell lysates. GSK3β activity located in the cytosolic or mitochondrial fractions were estimated separately for transfected cells.
Statistical analyses
All quantitative data are expressed as mean±SD, the qualitative data were presented as percentages. Statistical analysis of the data from multiple groups was performed by one-way analysis of variance followed by the Student-Newman-Kuels test. Data from two groups were compared using t-test. A value of P < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS v.18.0 (IBM, Armonk, NY, USA).
RESULTS
Inhibition of GSK3β improves mitochondrial dysfunction following oxidant injury by paraquat in tubular cells
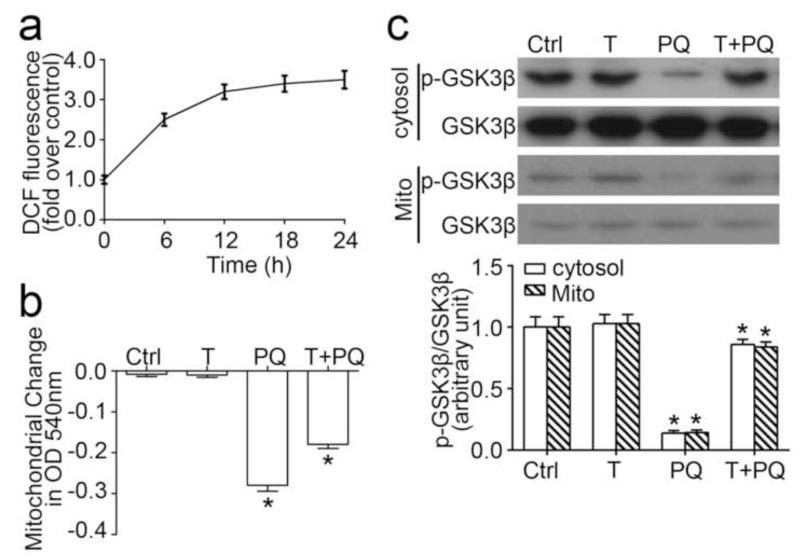
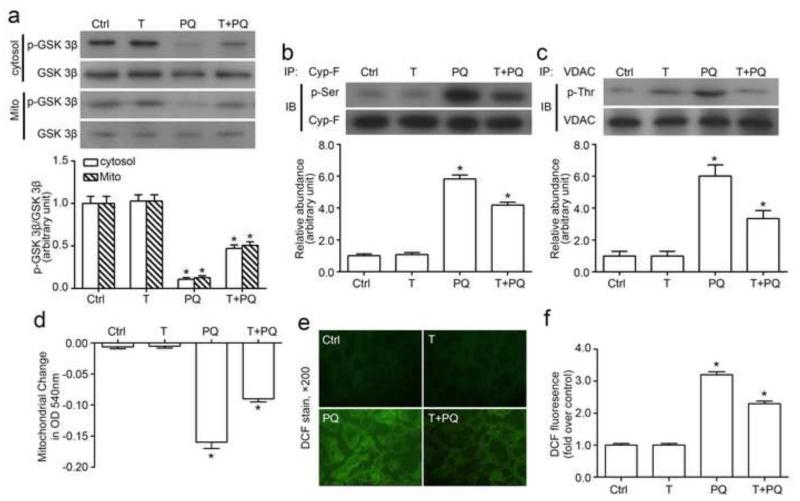
Paraquat is a pro-oxidant herbicide that causes cell damages via inducing oxidative stress. Probed by the ROS marker, DCF-DA, stimulation with paraquat elicited an immediate ROS burst in cultured tubular epithelial cells (Figure 1a). ROS is a major inducer of MPT, and vice verse. To assess the effect of paraquat on MPT, mitochondria were purified from cultured cells, and calcium-induced mitochondrial swelling was measured as a decrease in spectrophotometric absorbance at 540 nm, which correlates with the susceptibility to the MPT. Mitochondria isolated from paraquat-stimulated cells displayed a greater decrease in absorbance at 540nm upon calcium overload (Figure 1b), denoting an accelerated rate of mitochondrial swelling and marked mitochondrial dysfunction. TDZD-8 treatment significantly counteracted this effect. Accumulating evidence suggests that ROS regulates the activity of numerous redox-sensitive cell signaling transducers, including GSK3β [37-40]. In the TKPT cells, a constitutive expression of GSK3β with considerable inhibitory phosphorylation at serine 9 residue was detected in both cytosol and mitochondria of the cultured tubular epithelial cells under basal conditions (Figure 1c). TDZD-8 treatment alone barely altered this phosphorylation. By contrast, paraquat stimulation for 24 h significantly abrogated GSK3β phosporylation in both cytosol and mitochondria, denoting an enhanced GSK3β kinase activity. This effect was markedly overridden by TDZD-8 co-treatment. Moreover, no significant difference in the levels of total GSK3β in either cytosol or mitochondria was observed between different groups.
Figure 1. GSK3β inhibition improves mitochondrial dysfunction following oxidative injury by paraquat in tubular cells.
(a) The amount of reactive oxygen species as measured by the fluorescent DCF in cells treated with parquet (PQ, 0.25mM) for different periods of time (n = 6). (b) TKPT cells were exposed toTDZD-8 (10μM) and/or paraquat (0.25mM) for 24hours before the mitochondria were isolated. Mitochondria permeability transition was assessed by the decrease in spectrophotometric absorbance of calcium-challenged mitochondria at 540 nm. (c) TKPT cells were treated with TDZD-8 (10μM) and/or paraquat (0.25mM) for 24hours, and then the expression levels of GSK3β (in total cell lysates or mitochondrial fraction) and p-GSK3β (Ser9) (in total cell lysates) were determined by western blotting. Arbitrary units of p-GSK3β/GSK3β ratios expressed as immunoblot densitometric ratios of the molecules as folds of the control group. Abbreviation: Ctrl, Control; Mito, mitochondria. *P<0.05 versus other groups; (n=6).
GSK3β is a mitochondrial molecule and phosphorylates cyclophilin F and VDAC, key regulators of MPT
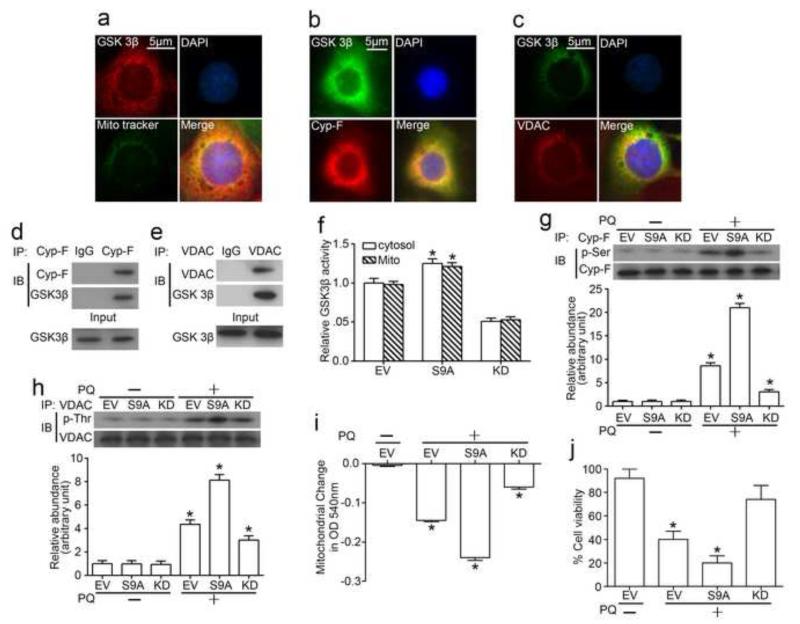
To understand the role of GSK3β involved in mitochondria dysfunction, fluorescent immunocytochemistry staining of GSK3β was carried out in cultured tubular epithelial cells. As shown in Figure 2a, an abundant and discrete expression of GSK3β was observed in cytoplasm with a considerable pool colocalizing with mitochondria which was stained with Mito Traker Green, a green-fluorescent mitochondrial stain, suggesting a discrete pool of GSK3β distributes in mitochondria. To further explore the localization of GSK3β in the mitochondria, GSK3β was co-stained with multiple mitochondrial molecules and was found to co-localize with adenine nucleotide translocator (data not shown), cyclophilin F (Figure 2b) and VDAC (Figure 2c), key mitochondrial membrane proteins that regulate MPT. Next, we opted to validate this finding and examine if GSK3β physically interacts with these molecules. Cyclophilin F and VDAC was immunoprecipitated from total cell lysates, and samples were probed for GSK3β. Shown in Figure 2d and 2e, cyclophilin F and VDAC evidently coprecipitated with GSK3β in cell lysates, suggesting GSK3β physically interacts with cyclophilin F and VDAC in tubular cells. To further understand whether this physical interaction between GSK3β and cyclophilin F or VDAC affects the activity of cyclophilin F or VDAC, the aminoacid sequences of cyclophilin F and VDAC were analyzed. Computational phosphorylation site prediction indicated that residues Ser118 and Ser190 of cyclophilin F as well as residue Thr51 of VDAC1 situates in a GSK3β consensus motif, signifying cyclophilin F and VDAC as putative substrates for GSK3β. To verify this result, the activity of GSK3β was selectively manipulated in cells by forced expression of the kinase dead mutant GSK3β (KD) or the constitutively active mutant GSK3β (S9A). GSK3β activity assay was carried out and confirmed that the activity of GSK3β in both cytosol and mitochondria were enhanced in cells expressing S9A while blunted in cells expressing KD (Figure 2f), and no significant difference was observed between mitochondira and cytosol in terms of the change in GSK3β activity. As shown in Figure 2g and 2h, under basal conditions, expression of S9A or KD mutant did not significantly change the phosphorylation of cyclophilin F and VDAC in mitochondria of tubular cells. Upon paraquat stimulation, phosphorylation of cyclophilin F and VDAC was drastically enhanced in cells ectopically expressing S9A, accompanied with aggravated mitochondria dysfunction (Figure 2i) and worsened cellular viability (Figure 2j). By contrast, phosphorylation of cyclophilin F and VDAC upon paraquat injury was significantly repressed in cells expressing KD (Figure 2g, 2h). And this was associated with desensitized MPT (Figure 2i), improved mitochondria dysfunction and cellular viability (Figure 2j). Collectively, these findings suggest that GSK3β physically interacts with and phosphorylates cyclophilin F and VDAC, regulates the sensitivity of MPT and thereby determines cellular viability.
Figure 2. GSK3β is a mitochondrial molecule and phosphorylates cyclophilin F and VDAC, key regulators of MPT.
(a) Immunofluorescence staining of GSK3β and mitochondria (Mito) (by Mito Tracker Green) in TKPT cells. (b) Immunofluorescence staining of GSK3β and cyclophilin F (Cyp-F) in TKPT cells. (c) Immunofluorescence staining of GSK3β and VDAC in TKPT cells. (d) Lysates of TKPT cells were subjected to immunoprecipitation by an anti-cyclophilin F antibody, and immunoprecipitates were probed for cyclophilin F and GSK3β. (e) Lysates of TKPT cells were subjected to immunoprecipitation by an anti-VDAC antibody, and immunoprecipitates were probed for VDAC and GSK3β. (f) TKPT cells were transfected with indicated vectors. Relative kinase activity of GSK3β located in the cytosolic and mitochondrial fractions extracted from the transfected cells were estimated separately following treatment with paraquat (0.25mM) or vehicle for 24h. (g, h) Mitochondrial lysates were subjected to immunoprecipitation by an anti-cyclophilin F antibody or an anti-VDAC antibody, and immunoprecipitates were probed respectively for either cyclophilin F and p-serine (p-Ser), or VDAC and p-threonine (p-Thr). (i) Mitochondria permeability transition was assessed by the decrease in spectrophotometric absorbance of calcium-challenged mitochondria at 540 nm. (j) Cell viability of transfected cells was estimated by MTT assay. Abbreviation: DAPI, 4′, 6-diamidino-2-phenylindole; EV, empty vector; S9A, vectors encoding the constitutively active mutant GSK3β (S9A-GSK3β-HA/pcDNA3); KD, vectors encoding kinase-dead mutant GSK3β (GSK-3β-KD/pcDNA3). *P<0.05 vs other groups.
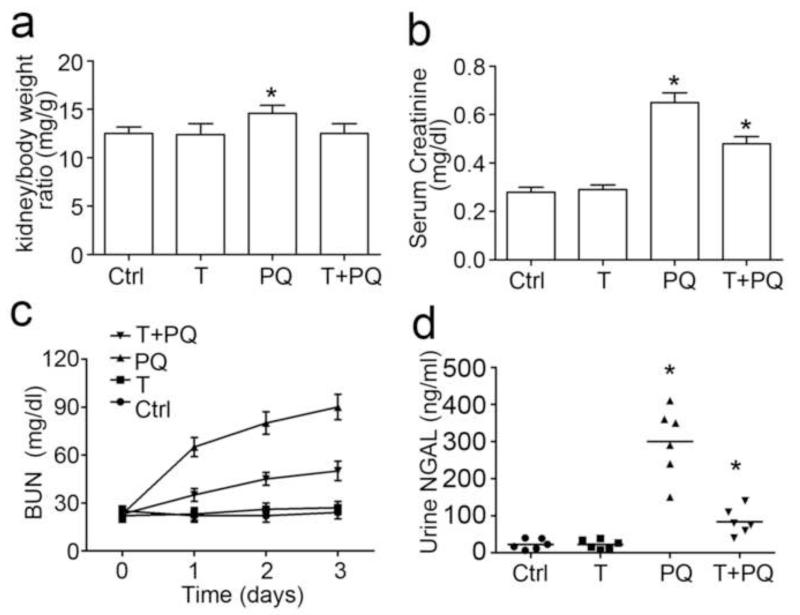
Inhibition of GSK3β improves general conditions and acute kidney dysfunction in paraquat-injured mice
In a murine model of the pro-oxidant paraquat induced AKI, mice were pretreated with TDZD-8 or vehicle 1 h before a single intraperitoneal injection of paraquat (30 mg/kg). Twelve hours later, lethargy and weakness were very evident in the animals that were treated with paraquat and vehicle. Significant increases in both kidney to body weight ratios (Figure 3a), serum creatinine levels (Figure 3b), and BUN (Figure 3c) were observed on day 3 after paraquat injury. By contrast, treatment with TDZD-8 remarkably recovered animal activities and food intake, preserved kidney to body weight ratio, and corrected kidney dysfunction in paraquat-injured mice. Urine NGAL assay by ELISA demonstrated that NGAL, a well established biomarker of AKI [29], was barely detectable in urine samples from control or TDZD-8 alone treated mice, but was precipitously induced within 72 h of paraquat injury (Figure 3d). TDZD-8 treatment considerably diminished the urine levels of NGAL in paraquat-injured mice, implying a protective effect on acute tubular injury.
Figure 3. GSK3β inhibition improves general conditions and acute kidney dysfunction in paraquat-injured mice.
Mice were subjected to paraquat (30 mg/kg) and/or TDZD-8 (10mg/kg) treatments. All mice were sacrificed 72 h after paraquat injury. (a) The kidney-to-body weight ratio was measured as the weight of two kidneys per body weight (mg/g). (b) Mice treated with TDZD-8 significantly reduced serum creatinine levels elicited by parauqat injury.(c) Blood urea nitrogen (BUN) was measured in mice on day 0, day 1, day 2 and day 3. TDZD-8 significantly reduced BUN levels elicited by parauqat injury. (d) Urine NGAL was measured by ELISA on urine samples collected from mice on day 3. *P<0.05 versus other groups (n=6).
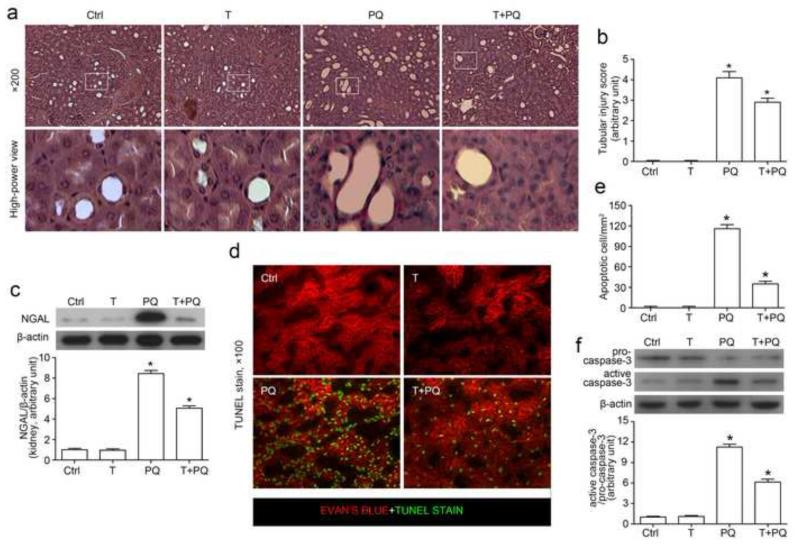
Inhibition of GSK3β ameliorates kidney injury and prevents tubular cell death in paraquat injured mice
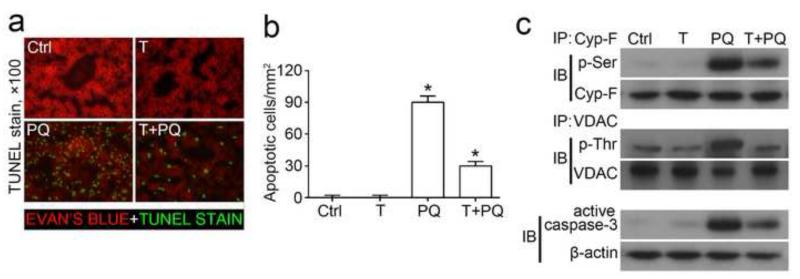
Shown in Figure 4a, TDZD-8 alone treated mice presented a normal tubulointerstitial and glomerular histology as observed in the control kidney. Paraquat exposure induced a typical pattern of acute tubular necrosis in both proximal and distal tubules, characterized by isometric vacuolization of the tubular epithelium, luminal ectasia, sloughing of cells into the lumen, loss of brush border, nuclear enlargement and pleomorphism, nuclear pyknosis and karyolysis. This acute renal histological change was evidently ameliorated by TDZD-8. Semi-quantitative morphometric analysis indicated that TDZD-8 treatment markedly attenuated the paraquat induced tubular injury score (Figure 4b). To confirm the morphologic findings, expression of the AKI biomarker NGAL in the kidney was examined by immunoblot analysis of kidney homogenates. Shown in Figure 4c, NGAL expression was scarcely detected in kidney homogenates from control or TDZD-8 alone treated mice, but was dramatically increased after paraquat injury. The paraquat elicited NGAL expression in the kidney was markedly prevented by TDZD-8 treatment. To examine whether cellular apoptosis is attributable to the paraquat-induced kidney injury, apoptotic cells were labeled by TUNEL staining in kidney specimens (Figure 4d, 4e). TUNEL staining was barely observed in kidneys from control or TDZD-8 alone treated animals. In contrast, a prominent amount of TUNEL-positive cells were detected and mainly located to the proximal tubules in the juxtamedullary cortex in paraquat injured kidneys. TDZD-8 substantially diminished the number of TUNEL positive apoptotic cells in the kidney (Figure 4e). Activation of the caspase-3 directed signaling cascade is an indispensable mechanism for apoptosis [41-43]. Immunoblot analysis on kidney homogenates revealed that paraquat prominently triggered the conversion of pro-caspase 3 to activated caspase 3 and increased the ratio of active/proform caspase 3 (Figure 4f). This effect was largely prevented by concomitant treatment with TDZD-8. Collectively, these findings in combination with the histological data suggest that inhibition of GSK3β by TDZD-8 potently protect the kidney from paraquat induced acute renal histological injury and apoptosis.
Figure 4. Inhibition of GSK3β ameliorates histological injury and prevents tubular cell death in paraquat injured mice.
Mice were subjected to paraquat (30 mg/kg) and/or TDZD-8 (10mg/kg) treatments. All mice were sacrificed 72h after paraquat administration. (a) Representative micrographs of hematoxylin eosin staining (× 200 and hgih-power view). (b) Histological changes were semi-quantitatively scored. (c) Western immunoblot analysis of NGAL expression in kidney homogenates. (d) Representative micrographs of TUNEL staining (counterstained with Evan’s blue; × 100). (e) TUNEL positive cells were counted and expressed as cells per mm2. (f) Western immunoblot analysis of kidney homogenates for pro-caspase-3 and active caspase-3 in mice. β-actin served as a loading control. Arbitrary units of active caspase-3/ pro-caspase-3 ratios expressed as immunoblot densitometric ratios of the molecules as folds of the control group. *P<0.05 versus other groups (n=6).
Inhibition of GSK3β inhibits phosphorylation of cyclophilin F and VDAC, desensitizes MPT and reduces oxidative stress in paraquat injured kidney
The mechanisms underlying the protective effect of TDZD-8 against paraquat induced AKI in mice was explored next. Consistent with in vitro findings, paraquat injury markedly enhanced the activity of GSK3β in both cytosols and mitochondria extracted from kidney tissues, marked by reduced GSK3β phosphorylation as probed by immunoblot analysis (Figure 5a). TDZD-8 blocked GSK3β activity and significantly reinstated the inhibitory phosphorylation of GSK3β. No significant difference in the abundance of total GSK3β in renal cytosols or mitochondria was observed between different animal groups. In agreement with the notion that cyclophilin F and VDAC serve as a putative substrate of GSK3β, the alternations in GSK3β activity in kidney tissues were associated with the changes in phosphorylation of cyclophilin F (Figure 5b) and VDAC (Figure 5c) in mice. Moreover, mitochondria isolated from paraquat-injured kidneys exhibited a greater reduction in absorbance at 540nm upon calcium overload (Figure 5d) as compared to mitochondria isolated from control kidneys, denoting a sensitized MPT and marked mitochondrial dysfunction. This effect was largely abrogated in mitochondria prepared from TDZD-8 pretreated and paraquat-injured kidneys, denoting a mitochondrial protective effect by TDZD-8. In line with the effect on MPT, paraquat injured kidneys exhibited substantial oxidative stress, probed by the ROS marker, DCF (Figure 5e, 5f). TDZD-8 treatment evidently reduced DCF staining in the kidney tissues and diminished DCF fluorescence intensity in the kidney homogenates (Figure 5f), suggesting a reduced ROS production ensuing mitochondrial protection.
Figure 5. Inhibition of GSK3β obliterates phosphorylation of cyclophilin F and VDAC, desensitizes MPT and reduces oxidative stress in paraquat injured kidney.
(a) Western immunoblot analysis of renal cytosol and mitochondria for p-GSK3β and GSK3β. (b) Kidney homogenates were subjected to immunoprecipitation by an anti-cyclophilin F antibody, and immunoprecipitates were probed for cyclophilin F or p-serine. (c) Kidney homogenates were subjected to immunoprecipitation by an anti-VDAC antibody, and immunoprecipitates were probed for VDAC or p-threonine. (d) Mitochondria were isolated from kidneys. Mitochondria permeability transition was assessed by the decrease in spectrophotometric absorbance of calcium-challenged mitochondria at 540 nm. (e) Representative micrographs of DCF staining of fresh kidney cryostat sections; (f) ROS generation was evaluated as the fluorescence intensity of DCF in the kidney tissues expressed as fold induction over the control group. *P<0.05 versus other groups (n=6).
Inhibition of GSK3β ameliorates liver injury in paraquat injured mice
To determine whether the mitochondrial protection mediated by GSK3β inhibition also confers benefits in other organ systems, liver specimens from all animal groups were analyzed. Apoptotic cells, labeled by TUNEL staining, were barely detected in livers from control or TDZD-8 alone treated animals (Figure 6a, 6b). In contrast, paraquat injury resulted in a prominent amount of TUNEL-positive cells in the liver. TDZD-8 treatment evidently diminished the number of TUNEL positive apoptotic cells in paraquat-injured livers. Immunoblot analysis of liver homogenates revealed that caspase-3 activation was elicited by the paraquat injury and this was substantially abrogated by TDZD-8 treatment, associated with a remarkable reduction of the paraquat triggered phosphorylation of cyclophilin F and VDAC (Figure 6c).
Figure 6. Inhibition of GSK3β ameliorates liver injury in paraquat injured mice.
Mice were subjected to paraquat (30 mg/kg) and/or TDZD-8 (10mg/kg) treatments. All mice were sacrificed 72h after paraquat injury. (a) Representative micrographs of TUNEL staining (counterstained with Evan’s blue; × 100). (b) TUNEL positive cells were counted and expressed as cells per mm2. (c) Western immunoblot analysis of active caspase-3 expression in kidney homogenates, β-actin was used as a loading control. Liver homogenates were subjected to immunoprecipitation by an anti-cyclophilin F or an anti-VDAC antibody, and immunoprecipitates were probed for either cyclophilin F and p-serine, or VDAC and p-threonine. *P<0.05 versus other groups (n=6).
DISCUSSION
GSK3β, initially identified as a regulator of glycogen biosynthesis, is now known to be a key transducer involved in a large number of cellular signaling pathways [21, 24, 44]. Recent evidence from this and other groups suggest that GSK3β plays dirty in the pathogenesis of AKI and inhibition of GSK3β by either chemical inhibitors or genetic knockout conveys a kidney protective effect in AKI[25-27]. In a rat model of AKI induced by ischemia reperfusion injury, GSK3β inhibition by TDZD-8 improved kidney dysfunction and ameliorated acute renal histological injury[28]. In addition, in mice exposed to mercuric chloride, renal proximal tubule-specific knockout of GSK3β gene improved general survival and kidney function[27]. Moreover, GSK3β inhibition by TDZD-8, a highly selective small-molecule inhibitor of GSK3β, prevented tubular necrosis and apoptosis in the kidney and corrected kidney dysfunction in mice exposed to high dose diclofenac[26]. In our current study, paraquat injection induced florid tubular necrosis and apoptosis and resulted in acute kidney insufficiency, and this could be dramatically attenuated by TDZD-8. Although more and more evidence proves that inhibition of GSK3β is beneficial for AKI, the mechanism of action remains largely elusive. Our study indicates that a proportion of cytoplasmic pool of GSK3β situates in the mitochondria and physically interacts with and phosphorylates cyclophilin F, a structural component of the MPT pore complex that is located at contact sites between the mitochondrial outer and inner membranes, and is known to promote development of the MPT pore[18, 45]. Besides, GSK3β also colocalized and interacted with VDAC, an outer mitochondrial membrane protein that plays a pivotal role in regulating MPT[14, 15]. By controlling the activity of cyclophilin F and VDAC, GSK3β modified the sensitivity of MPT thus determined the fate of renal tubular cells upon injury.
Paraquat (1,1′-Dimethyl-4,4′-bipyridinium dichloride), one of the most widely used herbicides, is a prototypical pro-oxidant toxin and has caused many deaths worldwide either accidentally or by suicides[46, 47]. The high mortality (>50%) is due to inherent toxicity and lack of effective treatments [48-50]. Though paraquat is rapidly distributed to various tissues after ingestion, concentrations accumulating within the kidneys are the highest [51]. As the kidney is the main excretory route, it produces early and severe nephrotoxicity and AKI. Acute kidney dysfunction leads to paraquat accumulation in the body and contributes to toxic injury in other organs [52, 53]. Paraquat can cause mitochondrial cytopathy by triggering the MPT pore opening in multiple organ systems, including brain, liver and kidney [54-57]. As the renal proximal tubule is a major site for the excretion of xenobiotics and is highly dependent on oxidative mitochondrial metabolism for ATP production, it is particularly vulnerable to deleterious effects of toxins targeting mitochondria [18, 58]. However, until now, it is still largely unknown whether the MPT pore opening can be modified in paraquat-injured kidney. Paraquat is a prototypic pro-oxidant toxin known to cause enormous oxidative stress [30, 59]. Indeed, paraquat stimulation generates a burst of ROS in cultured tubular cells and induced massive oxidative stress in renal tubules in vivo. The ROS has been implicated as important effectors of tubule cell injury in most forms of AKI including ischaemic reperfusion injury and nephrotoxic acute tubular necrosis [60, 61]. In response to oxidative stress, the activity of numerous redox-sensitive cell-signaling transducers, including GSK3β, could be altered[37]. In our hand, associated with paraquat induced ROS production, phosphorylation of GSK3β was reduced, denoting an enhanced kinase activity of GSK3β. Enhanced GSK3β activity could promote the phsporylation of its substrates. Of note, both computational phosphorylation site prediction and co-immunoprecipitation assay suggested that cyclophilin F and VDAC are putative substrates for GSK3β. And this was validated by detecting an association between the changes in the phosphorylation of cyclophilin F and VDAC and the artificially manipulated GSK3β activity. Thus paraquat induces ROS production, enhances the activity of redox-sensitive GSK3β and subsequently augments phosphorylation of cyclophilin F and VDAC, cognate substrates of GSK3β and key MPT regulators. Phosphorylation of cyclophilin F and VDAC has been associated with sensitized MPT. In our study, enhanced phosphorylation of cyclophilin F and VDAC correlated with an elevated sensitivity of MPT as reflected by a greater decrease in absorbance at 540nm upon calcium challenge measured by spectrophotometry of the extracted mitochondria. Subsequent to MPT, cytochrome c and second mitochondria-derived activator of caspases (Smac) will be released from mitochondria to the cytosol, where it activates the caspase family of proteases and initiates the signaling machinery driving cell death[13, 62-66]. In addition, MPT with ensuing mitochondira depolarization is indispensable for the ROS induced ROS release and thus exacerbates oxidative injury [67-69]. In our study, increased expression of active (cleaved) caspase-3 and massive apoptosis marked by TUNEL staining as well as substantial oxidative stress probed by the ROS marker DCF were noted in the paraquat-injured kidney. Blockade of GSK3β by TDZD-8 overrode the phosphorylation of cyclophilin F and VDAC induced by paraquat, desensitized MPT and reduced oxidative stress and tubular injury in the kidney. Moreover, this MPT inhibitory effect achieved by GSK3βb inhibition seems to confer a universal cellular protection, because the paraquat induced injuries in other organ systems like the liver was also markedly ameliorated after TDZD-8 treatment.
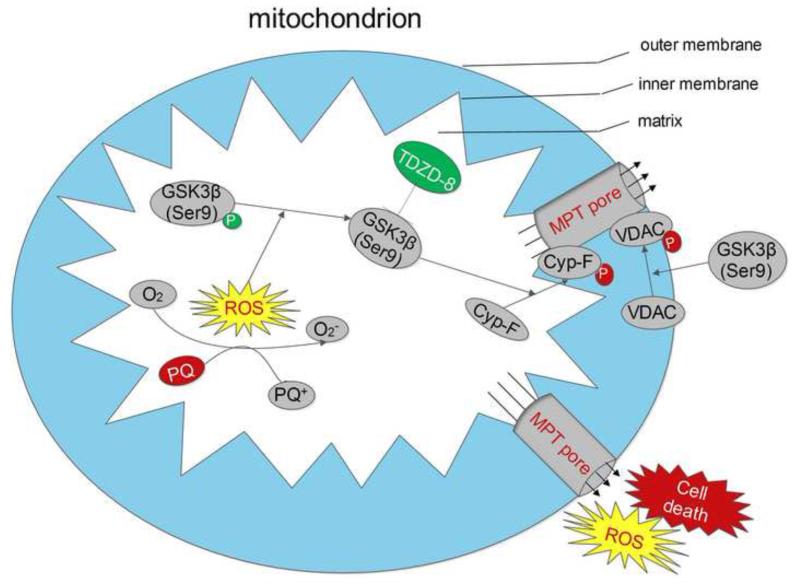
In summary, the pro-oxidant paraquat induced ROS overproduction in tubular cells and augmented the activity of the redox-sensitive GSK3β in the mitochondria, which subsequently phosphorylated cyclophilin F and VDAC in the mitochondria, sensitized MPT and eventually resulted in tubular injury and AKI. TDZD-8, a highly selective small-molecule inhibitor of GSK3β, blocked GSK3β activity, reduced phosphorylation of cyclophilin F and VDAC, desensitized MPT and prevented paraquat induced acute tubular cell death and AKI (Figure 7). Our findings suggest that inhibition of GSK3β by TDZD-8 or by existing FDA approved agents with GSK3β inhibitory activities, including lithium and sodium valproate, might represent a novel therapeutic strategy to desensitize MPT and to treat AKI induced by mitochondrial toxins like paraquat.
Figure 7. Schematic diagram depicts the mechanisms of action of the GSK3β controlled MPT and the ensuing oxidative injury and cell death induced by paraquat.
Paraquat induces reactive oxygen species (ROS) overproduction in renal tubular cells, followed by enhanced activity of GSK3β. Cyclophilin F and VDAC, key regulators of MPT, physically interact with GSK3β and are cognate substrates for GSK3β. Enhanced GSK3β activity promotes cyclophilin F and VDAC phosphorylation. Subsequently, increased activities of cyclophilin F and VDAC potentiate MPT pore opening upon ROS challenge, and this eventually aggravates necroapoptotic cell death, amplifies ROS release and results in acute kidney injury. TDZD-8, a highly selective small molecule inhibitor of GSK3β, blocked GSK3βactivity, diminishes cyclophilin F and VDAC phosphorylation, desensitizes MPT and thereby reduces oxidative injury and ameliorates necroapoptotic cell death and acute kidney injury. Abbreviation:ROS, reactive oxygen species; Cyp-F, cyclophilin F.
Highlights.
Paraquat injury enhances the activity of redox-sensitive GSK3β in renal tubular cells.
Cyclophilin F and VDAC, regulators of MPT, are putative substrates for GSK3β.
GSK3β regulates the MPT sensitivity via controlling the activity of Cyclophilin F and VDAC.
Inhibition of GSK3β desensitizes MPT and confers cellular protection against paraquat injury.
ACKNOWLEDGEMENTS
Drs. Zhen Wang and Hui Bao are International Society of Nephrology (ISN) Fellows and recipients of the ISN fellowship. This work was made possible in part by the funding from the Chinese 973 fund 2012CB517600 the Natural Science Foundation of China (Program No. 81101414/H1503, 81171792/H1503, 81270136/H0111), the ISN Sister Renal Center Trio Program, and the US National Institutes of Health grant R01DK092485.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury, N. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11 doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toth R, Breuer T, Cserep Z, Lex D, Fazekas L, Sapi E, Szatmari A, Gal J, Szekely A. Acute Kidney Injury Is Associated With Higher Morbidity and Resource Utilization in Pediatric Patients Undergoing Heart Surgery. Annals of Thoracic Surgery. 2012;93:1984–1991. doi: 10.1016/j.athoracsur.2011.10.046. [DOI] [PubMed] [Google Scholar]
- [3].Valette X, Parienti J-J, Plaud B, Lehoux P, Samba D, Hanouz J-L. Incidence, morbidity, and mortality of contrast-induced acute kidney injury in a surgical intensive care unit: a prospective cohort study. Journal of critical care. 2012;27:322.e321–325. doi: 10.1016/j.jcrc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- [4].Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- [5].Mergner WJ, Trump BF, Valigors Jm, Garbus J, Dees JH. STRUCTURAL AND FUNCTIONAL CHANGES IN HUMAN KIDNEY AND LIVER-MITOCHONDRIA IN ACUTE CELL INJURY AFTER SHOCK AND TRAUMA. Am. J. Pathol. 1972;66:A36. [Google Scholar]
- [6].Hall AM. Pores for Thought: New Strategies to Re-energize Stressed Mitochondria in Acute Kidney Injury. Journal of the American Society of Nephrology. 2011;22:986–989. doi: 10.1681/ASN.2011030309. [DOI] [PubMed] [Google Scholar]
- [7].Jankauskas S, Plotnikov E, Pevzner I, Chupyrkina A, Kirpatovsky V, Zorov D. Mitochondria-targeted antioxidant SkQR1 prevents acute kidney injury after ischemia/reperfusion, rhabdomyolyisis and gentamicin toxicity. Febs J. 2012;279:205–205. [Google Scholar]
- [8].Bakeeva LE, Barskov IV, Egorov MV, Isaev NK, Kapelko VI, Kazachenko AV, Kirpatovsky VI, Kozlovsky SV, Lakomkin VL, Levina SB, Pisarenko OI, Plotnikov EY, Saprunova VB, Serebryakova LI, Skulachev MV, Stelmashook EV, Studneva IM, Tskitishvili OV, Vasilyeva AK, Victorov IV, Zorov DB, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2.Treatment of some ROS- and Age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke) Biochemistry-Moscow. 2008;73:1288–1299. doi: 10.1134/s000629790812002x. [DOI] [PubMed] [Google Scholar]
- [9].Caro P, Gomez J, Sanchez I, Naudi A, Ayala V, Lopez-Torres M, Pamplona R, Barja G. Forty Percent Methionine Restriction Decreases Mitochondrial Oxygen Radical Production and Leak at Complex I During Forward Electron Flow and Lowers Oxidative Damage to Proteins and Mitochondrial DNA in Rat Kidney and Brain Mitochondria. Rejuvenation Research. 2009;12:421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- [10].Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010;198:49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- [11].Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, Hayashi H. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology. 2009;296:H1125–H1132. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- [12].Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin M-A, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. Journal of Clinical Investigation. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nature Cell Biology. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- [14].Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- [15].Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. Journal of Biological Chemistry. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim. Biophys. Acta-Bioenerg. 2010;1797:1113–1118. doi: 10.1016/j.bbabio.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. American journal of physiology. Renal physiology. 2011;301:F134–150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chinopoulos C, Adam-Vizi V. Modulation of the mitochondrial permeability transition by cyclophilin D: Moving closer to F-0-F-1 ATP synthase? Mitochondrion. 2012;12:41–45. doi: 10.1016/j.mito.2011.04.007. [DOI] [PubMed] [Google Scholar]
- [20].Miura T, Miki T. GSK-3 beta, a Therapeutic Target for Cardiomyocyte Protection. Circ. J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- [21].Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kang UG, Seo MS, Roh M-S, Kim Y, Yoon SC, Kim YS. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS letters. 2004;560:115–119. doi: 10.1016/S0014-5793(04)00082-1. [DOI] [PubMed] [Google Scholar]
- [23].Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Medicinal Research Reviews. 2002;22:373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- [24].Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nelson PJ, Cantley L. GSK3β Plays Dirty in Acute Kidney Injury. Journal of the American Society of Nephrology. 2010;21:199–200. doi: 10.1681/ASN.2009121214. [DOI] [PubMed] [Google Scholar]
- [26].Bao H, Ge Y, Zhuang S, Dworkin LD, Liu Z, Gong R. Inhibition of glycogen synthase kinase-3beta prevents NSAID-induced acute kidney injury. Kidney international. 2012;81:662–673. doi: 10.1038/ki.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Howard C, Tao S, Yang H-C, Fogo AB, Woodgett JR, Harris RC, Rao R. Specific deletion of glycogen synthase kinase-3beta in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney international. 2012;82:1000–1009. doi: 10.1038/ki.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC. GSK3β promotes apoptosis after renal ischemic injury. Journal of the American Society of Nephrology. 2010;21:284–294. doi: 10.1681/ASN.2009080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gong R, Rifai A, Ge Y, Chen S, Dworkin LD. Hepatocyte growth factor suppresses proinflammatory NF kappa B activation through GSK3β beta inactivation in renal tubular epithelial cells. Journal of Biological Chemistry. 2008;283:7401–7410. doi: 10.1074/jbc.M710396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. Journal of Biological Chemistry. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhu C, Huang S, Yuan Y, Ding G, Chen R, Liu B, Yang T, Zhang A. Mitochondrial Dysfunction Mediates Aldosterone-Induced Podocyte Damage A Therapeutic Target of PPAR gamma. Am. J. Pathol. 2011;178:2020–2031. doi: 10.1016/j.ajpath.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Enrique Guerrero-Beltran C, Calderon-Oliver M, Martinez-Abundis E, Tapia E, Zarco-Marquez G, Zazueta C, Pedraza-Chaverri J. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: Quinone oxidoreductase 1 and gamma glutamyl cysteine ligase: Studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol. Lett. 2010;199:80–92. doi: 10.1016/j.toxlet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [33].Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. Journal of the American Society of Nephrology. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Silva AM, Oliveira PJ. Evaluation of respiration with clark type electrode in isolated mitochondria and permeabilized animal cells. Methods in molecular biology (Clifton, N.J.) 2012;810:7–24. doi: 10.1007/978-1-61779-382-0_2. [DOI] [PubMed] [Google Scholar]
- [35].Gong R, Rifai A, Dworkin LD. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney international. 2006;69:1166–1174. doi: 10.1038/sj.ki.5000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Johnson AC, Becker K, Zager RA. Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. American journal of physiology. Renal physiology. 2010;299:F426–435. doi: 10.1152/ajprenal.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chiara F, Gambalunga A, Sciacovelli M, Nicolli A, Ronconi L, Fregona D, Bernardi P, Rasola A, Trevisan A. Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3alpha/beta and Bax, leading to permeability transition pore opening and tumor cell death. Cell death & disease. 2012;3:e444–e444. doi: 10.1038/cddis.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aw TY. Tissue oxidative stress revisited: Significance of ROS and redox signaling. Semin. Cell Dev. Biol. 2012;23:721–721. doi: 10.1016/j.semcdb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- [39].Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell and Environment. 2012;35:259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- [40].Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends in Neurosciences. 2012;35:700–709. doi: 10.1016/j.tins.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [42].Niquet J, Allen SG, Baldwin RA, Wasterlain CG. Evidence of caspase-3 activation in hyposmotic stress-induced necrosis. Neuroscience Letters. 2004;356:225–227. doi: 10.1016/j.neulet.2003.11.063. [DOI] [PubMed] [Google Scholar]
- [43].Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell death and differentiation. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- [44].Ge Y, Si J, Tian L, Zhuang S, Dworkin LD, Gong R. Conditional ablation of glycogen synthase kinase 3 beta in postnatal mouse kidney. Laboratory Investigation. 2011;91:85–96. doi: 10.1038/labinvest.2010.142. [DOI] [PubMed] [Google Scholar]
- [45].Crompton M. The mitochondrial permeability transition pore and its role in cell death. The Biochemical journal. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- [46].Koo J-R, Kim J-C, Yoon J-W, Kim G-H, Jeon R-W, Kim H-J, Chae D-W, Noh J-W. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. American Journal of Kidney Diseases. 2002;39:55–59. doi: 10.1053/ajkd.2002.29880. [DOI] [PubMed] [Google Scholar]
- [47].Bullivant C. Accidental poisoning by paraquat: Report of two cases in man. British Medical Journal. 1966;1:1272. doi: 10.1136/bmj.1.5498.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br. J. Clin. Pharmacol. 2011;72:745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Copland GM, Kolin A, Shulman HS. Fatal pulmonary intra-alveolar fibrosis after paraquat ingestion. New England Journal of Medicine. 1974;291:290–292. doi: 10.1056/NEJM197408082910607. [DOI] [PubMed] [Google Scholar]
- [50].Rodrigues Lacerda AC, Rodrigues-Machado M. d. G., Mendes PL, Novaes RD, Cavalcante Carvalho GM, Zin WA, Gripp F, Coimbra CC. Paraquat (PQ)-induced pulmonary fibrosis increases exercise metabolic cost, reducing aerobic performance in rats. Journal of Toxicological Sciences. 2009;34:671–679. doi: 10.2131/jts.34.671. [DOI] [PubMed] [Google Scholar]
- [51].Rose MS, Lock EA, Smith LL, Wyatt I. Paraquat accumulation: Tissue and species specificity. Biochemical Pharmacology. 1976;25:419–423. doi: 10.1016/0006-2952(76)90344-0. [DOI] [PubMed] [Google Scholar]
- [52].Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol. Dial. Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- [53].Lee EY, Kwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicology and Industrial Health. 2002;18:201–206. doi: 10.1191/0748233702th141oa. [DOI] [PubMed] [Google Scholar]
- [54].Yang W, Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: Involvement of p53 and mitochondria. J. Toxicol. Env. Health Part A. 2008;71:289–299. doi: 10.1080/15287390701738467. [DOI] [PubMed] [Google Scholar]
- [55].Pardo C, Jimenez-Del-Rio M, Lores-Arnaiz S, Bustamante J. Protective Effects of the Synthetic Cannabinoids CP55,940 and JWH-015 on Rat Brain Mitochondria upon Paraquat Exposure. Neurochem. Res. 2010;35:1323–1332. doi: 10.1007/s11064-010-0188-1. [DOI] [PubMed] [Google Scholar]
- [56].Costantini P, Petronilli V, Colonna R, Bernardi P. On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitric oxide. Toxicology. 1995;99:77–88. doi: 10.1016/0300-483x(94)02997-9. [DOI] [PubMed] [Google Scholar]
- [57].Huang CL, Lee YC, Yang YC, Kuo TY, Huang NK. Minocycline prevents paraquat-induced cell death through attenuating endoplasmic reticulum stress and mitochondrial dysfunction. Toxicol. Lett. 2012;209:203–210. doi: 10.1016/j.toxlet.2011.12.021. [DOI] [PubMed] [Google Scholar]
- [58].Hall AM, Unwin RJ, Hanna MG, Duchen MR. Renal function and mitochondrial cytopathy (MC): more questions than answers. Qjm-an International Journal of Medicine. 2008;101:755–766. doi: 10.1093/qjmed/hcn060. [DOI] [PubMed] [Google Scholar]
- [59].Kielar F, Helsel ME, Wang Q, Franz KJ. Prochelator BHAPI protects cells against paraquat-induced damage by ROS-triggered iron chelation. Metallomics. 2012 doi: 10.1039/c2mt20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aksu U, Demirci C, Ince C. The Pathogenesis of Acute Kidney Injury and the Toxic Triangle of Oxygen, Reactive Oxygen Species and Nitric Oxide. In: Kellum JA, Ronco C, Vincent JL, editors. Controversies in Acute Kidney Injury. 2011. pp. 119–128. [DOI] [PubMed] [Google Scholar]
- [61].Sedaghat Z, Kadkhodaee M, Seifi B, Salehi E, Najafi A, Dargahi L. Remote per-conditioning reduces oxidative stress, down-regulates cyclooxygenase-2 expression and attenuates ischaemia/reperfusion-induced acute kidney injury. Clinical and Experimental Pharmacology and Physiology. 2013;40:97–103. doi: 10.1111/1440-1681.12044. [DOI] [PubMed] [Google Scholar]
- [62].Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiological reviews. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- [63].Borutaite V. Mitochondria as Decision-Makers in Cell Death. Environ. Mol. Mutagen. 2010;51:406–416. doi: 10.1002/em.20564. [DOI] [PubMed] [Google Scholar]
- [64].Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochimica et biophysica acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- [65].Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- [66].Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annual Review of Physiology. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- [67].Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. The Journal of experimental medicine. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et biophysica acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- [69].Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxidants & redox signaling. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]