Abstract
1H, 13C, and 15N chemical shift assignments are presented for the isolated four-helical bundle membrane localization domain from the domain of unknown function 5 (DUF5) effector (MLDVvDUF5) of the MARTX toxin from Vibrio vulnificus in its solution state. We have assigned 97% of all backbone and side-chain carbon atoms, including 96% of all backbone residues. Secondary chemical shift analysis using TALOS+ demonstrates four helices that align with those predicted by structure homology modeling using the MLDs of Pasteurella multocida toxin (PMT) and the clostridial TcdB and TcsL toxins as templates. Future studies will be towards solving the structure and determining the dynamics in the solution state.
Keywords: Membrane localization domain, four helical bundle, MARTX toxins, Pasteurella multocida toxin
Biological Context
The multifunctional autoprocessing repeats-in-toxins (MARTXs) are a class of toxins produced by an array of known bacterial pathogens, including Vibrio cholerae and Vibrio vulnificus. The hallmark of all MARTX toxins is the presence of a cysteine protease domain that, following translocation into host cells and binding to inositol hexakisphosphate, cleaves the full-length toxin to release effector domains from the central portion of the toxin.
The MARTX toxin encoded by Vibrio vulnificus (MARTXVv) is a 5206 amino acid protein toxin that causes destruction of the intestinal epithelium during infection (Jeong and Satchell, 2012). The toxin carries 5 distinct toxic effector domains that confer a variety of activities to V. vulnificus including cytolysis, actin depolymerization, RhoGTPase-inactivation, caspase 3/7-dependent apoptosis, and induction of reactive oxygen species (Satchell 2011). Two of these domains, the Rho-inactivation domain (RID) and domain of unknown function 5 (DUF5) have N-terminal plasma membrane localization domains (MLD), although only the MLD associated with DUF5 (MLDVvDUF5) is a 8.8 kDa, 79 residue domain that retains full functionality during in vivo membrane localization and binding to anionic lipids (Geissler et al. 2010, 2011). Substitution of two basic residues for alanine (K15A or R66A) or a key phenylalanine to asparagine in the L1 region (F16N) significantly reduces phospholipid binding and plasma membrane localization, implicating these as important in membrane association for MLDVvDUF5 (Geissler et al. 2011). Importantly, anionic phospholipids are found in high abundance in the late endosome and in the inner leaflet of the plasma membrane, where DUF5 is presumably exerting its biological function (van Meer et al., 2008).
The conserved region of the MLD is 79 residues; homology models of MLDVvDUF5 have been generated on the basis of crystal structures of related MLDs from Pasteurella multocida toxin (2EBF) and the clostridial glucoslyating toxins TcdA (3SS1), TcdB (2BVL), and TcsL (2VL8). However, the relatively low sequence identity among the MLDs (often < 50%) would indicate that future studies of MLDVvDUF5 would benefit from experimental data to determine the structures and verify the implied homology. Moreover, NMR studies will help to elucidate the significance of conserved residues, including basic residues such as K15 and R66 as well as hydrophobic residues and helices, including Y25.
Here, we report the resonance assignments of the backbone and sidechain atoms (95 %) of MLDVvDUF5 in solution. Secondary chemical shift analysis using TALOS+ demonstrates that MLDVvDUF5 has similarity to that predicted by homology modeling (Geissler et al. 2010). Resonance assignments will enable studies to determine the mechanism of MLD-membrane association.
Methods and Experiments
Recombinant protein expression and purification
Uniformly labeled 13C, 15N MLDVvDUF5 was expressed in E. coli BL21(DE3) cells (Promega, Fitchburg, WI) from the pYCpet plasmid encoding the MLDVvDUF5 gene using previously published conditions (Geissler et al. 2011). Briefly, a 4-mL starter culture in LB was grown at 37°C to an OD600 = 2, from which 1 mL of the culture was harvested and resuspended in 1 mL of M9 salt buffer (pH 7.4, 64 mM phosphate, 20 mM KCl, 200 mM NaCl), and then inoculated into a flask containing 200 mL of the labeling medium with 13C, 15N-BioExpress, U-13C-glucose, and 15N-NH4Cl (Cambridge Isotopes Laboratories, Inc., Andover, MA) (Studier 2005). Specifically, 13C, 15N-BioExpress (10X stock solution) was diluted to 0.1× in M9 minimal media at pH 7.5 containing 2 g/L U-13C-glucose and 2 g/L U-15N NH4Cl. The culture was grown at 37 °C to an OD600 ~0.8. Expression of MLDVvDUF5 was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside for 4 h. The resulting labeled protein was isolated and purified as previously published (Geissler et al. 2011). Briefly, the cells were lysed by using sonication in 50 mM Tris-HCl, pH 7.4, containing 500 mM NaCl, and the cell debris pelleted. The supernatant was purified by Ni2+ affinity chromatography (HiPrep 16/160, GE Biosciences, Piscataway, NJ). Protein-containing fractions were concentrated using an Amicon Ultra-15 centrifugal filter unit with MWCO of 3K (Millipore, Billerica, MA), and then separated by Sephadex-S100 size exclusion chromatography using 50 mM Tris-HCl, pH 7.4, with 500 mM NaCl as elution buffer (GE Biosciences, Piscataway, NJ). The protein was then re-concentrated using an Amicon Ultra-2 centrifugal filter with MWCO of 3K (Millipore, Billerica, MA). This resulted in >99% purity based on SDS-PAGE analysis with a yield of 40–50 mg/L of 1 mM U-13C, 15N-labeled MLDVvDUF5 in 50 mM Tris-HCl, pH 7.4, with 500 mM NaCl, 10% D2O (v/v) and 0.01% 4,4-dimethyl-4-silapentane-1-sulfonic acid.
NMR spectroscopy
Solution NMR spectra were acquired at the School of Chemical Sciences NMR Facility (University of Illinois at Urbana-Champaign) on a Varian INOVA 600 spectrometer equipped with a 5 mm, triple resonance (1H-13C-15N) triaxial gradient probe, using VNMRJ version 2.3 with the BioPack suite of pulse programs. Spectra of the U-13C, 15N MLDVvDUF5 samples were acquired at 30°C. 2D 1H-15N HSQC spectra were collected before and after the spectra used for assignments, consisting of the standard suite of gradient-based heteronuclear triple resonance 3D spectra (HNCO, HN(CA)CO, HNCACB, CBCA(CO)NH) (Clubb et al. 1992; Kay et al. 1990; Grzesiek and Bax 1992b; Grzesiek and Bax 1992a). 13C-HCCH-TOCSY, CC(CO)NH, and 13C-NOESY-HSQC (Bax et al. 1990; Grzesiek et al. 1993; Marion et al. 1989) were acquired and analyzed to obtain sidechain resonance assignments. Spectra were processed using NMRPipe (Delaglio et al. 1995) and analyzed in Sparky (Goddard and Kneller 2006).
Extent of assignments and data deposition
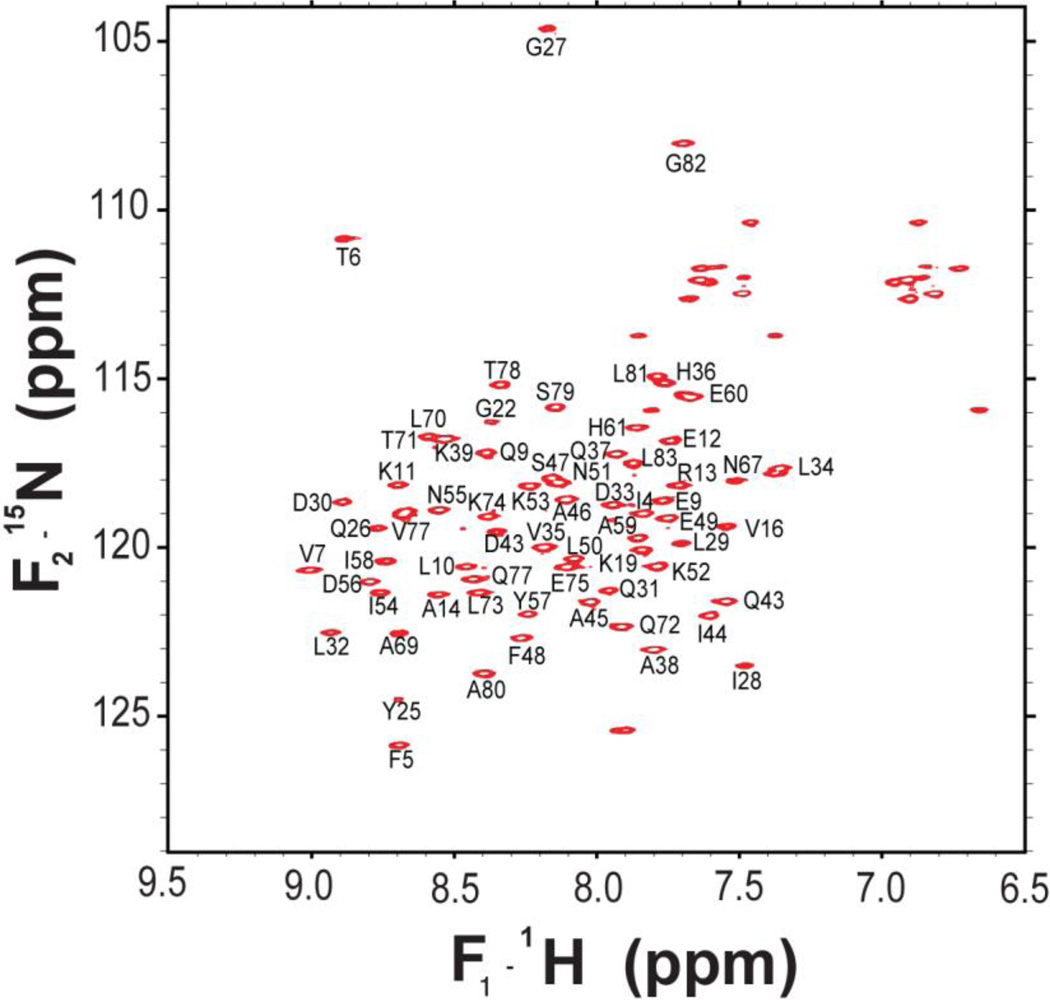
The data sets enabled assignments of ~95% of the HN, N, Cα, Cβ, C′ and carbon side-chain assignments of MLDVvDUF5, including 96% of the backbone resonances. We have used the same naming convention as the crystal structure (PDB ID 4ERR). The assignments are deposited in the BMRB under accession number 18562. The 1H-15N gradient HSQC spectrum (Fig. 1) demonstrates good resolution and dispersion, consistent with a uniform, folded structure. We do not observe amide 1H-15N correlations in the HSQC for the following residues: Q3, F17, R41, T63, G65, R66, and Q68. Of the backbone amides signals observed, all but one were assigned; the missing assignment likely arises from the C-terminal hexahistidine-tag. In most cases, we were able to assign the CA, CB, and C’ of the residues missing amide frequencies in the HSQC using CBCA(CO)NH and HNCO experiments. C(CO)NH and 13C-edited HSQC-TOCSY experiments enabled us to assign the 13C side-chain resonances. In these experiments, we observe significant degeneracy in many of the aliphatic residues for the CD of leucines 29, 32, 34, 50, 70, 73, 81, and 83 as well as the CG in V16.
Figure 1.
1H -15N 2D HSQC spectrum of MLDVvDUF5 in 50 mM Tris-HCl, pH 7.4, containing 500 mM NaCl, acquired at 30°C and 600 MHz (1H frequency).
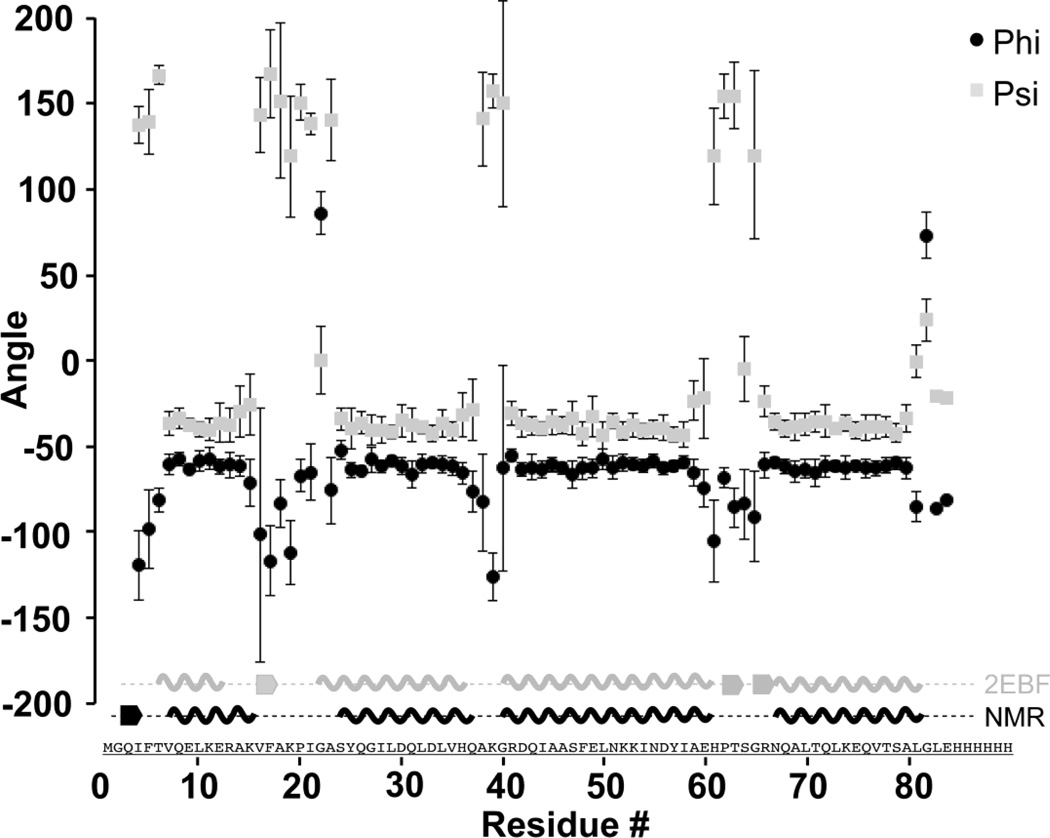
TALOS+ (Shen et al. 2009) analysis demonstrates four helices, consistent with those observed in the homology model of MLDVvDUF5, based on structure prediction from the crystal structure of PMT (Fig. 2). The helices consist of residues 7–15 (H1), 23–36 (H2), 40–61 (H3), and 67–81 (H4), with intervening loop residues 16–22 (L1), 37–39 (L2), and 62–66 (L3). The missing assignments of non-proline amide resonances belong to the N-terminal methionine, the C-terminal hexahistidine-tag, a single residue in the first turn of H3 (R41), and residues in L1 (F17) and L3 (T63, G65) or the first turn of H4 (R66, Q68). This suggests amide exchange in these regions, which indicates an exposure to solution, as would be expected in loop regions or solvent exposed helices.
Figure 2.
Secondary structure elements of MLDVvDUF5 identified by TALOS+ analysis (Shen et al. 2009). Plotted are the Phi (Φ) (black circles) and Psi (Ψ) (gray squares) backbone torsion angles as predicted by TALOS+ analysis (Shen et al. 2009) with error bars. Along the x-axis is a cartoon representation of the secondary structure based on the crystal structure of PMT [PDB 2EBF (grey) (Kitadokoro et al. 2007)] and the secondary structure predicted by TALOS+(black).
According to structure prediction, loops L1 and L3 are adjacent to each other at the same end of the 4HBM. Chemical exchange broadening is observed throughout these regions, which may be key for membrane association (Geissler et al. 2010; Geissler et al. 2011). Resonance assignments provide a basis for solving the structure of the isolated MLDVvDUF5 and understanding the conformation and dynamics of membrane association.
Acknowledgments
This work was supported by NIH award AI051490 (K.J.F.S.), Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund (K.J.F.S.), NIH/NIAID grant AI038396 (B.A.W.), University of Illinois (C.M.R.), NIH/CBITG (T32 GM070421), and the Department of Homeland Security Fellowship (to M.C.B). The authors thank Dr. Lingyang Zhu from the University of Illinois School of Chemical Sciences NMR Facility for technical assistance.
Abbreviations
- DUF
Domain of unknown function
- MARTX
Multifunctional autoprocessing repeats-in-toxins
- MLD
Membrane localization domain
- MLDPMT
Membrane localization domain from Pasteurella multocida toxin
- MLDVvDUF5
Membrane localization domain from MARTX toxin from Vibrio vulnificus
- PMT
Pasteurella multocida toxin
References
- Bax A, Clore GM, Gronenborn AM. 1H-1H Correlation Via Isotropic Mixing of 13C Magnetization, a New 3-Dimensional Approach for Assigning 1H and 13C Spectra of 13C-Enriched Proteins. J Magn Reson. 1990;88(2):425–431. [Google Scholar]
- Clubb RT, Thanabal V, Wagner G. A Constant-Time 3-Dimensional Triple-Resonance Pulse Scheme to Correlate Intraresidue 1H(N), 15N, and 13C (') Chemical-Shifts in 15N-13C-Labeled Proteins. J Magn Reson. 1992;97(1):213–217. [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe - a Multidimensional Spectral Processing System Based On Unix Pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Geissler B, Ahrens S, Satchell KJF. Plasma membrane association of three classes of bacterial toxins is mediated by a basic-hydrophobic motif. Cell Microbiol. 2011;14(2):286–298. doi: 10.1111/j.1462-5822.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Tungekar R, Satchell KJF. Identification of a conserved membrane localization domain within numerous large bacterial protein toxins. P Natl Acad Sci USA. 2010;107(12):5581–5586. doi: 10.1073/pnas.0908700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Kneller DG. Sparky 3. 3.106 edn. San Francisco: University of California; 2006. [Google Scholar]
- Grzesiek S, Anglister J, Bax A. Correlation of Backbone Amide and Aliphatic Side-Chain Resonances in 13C/N-15-Enriched Proteins by Isotropic Mixing of 13C Magnetization. J Magn Reson Ser B. 1993;101(1):114–119. [Google Scholar]
- Grzesiek S, Bax A. Correlating Backbone Amide and Side-Chain Resonances in Larger Proteins by Multiple Relayed Triple Resonance NMR. J Am Chem Soc. 1992a;114(16):6291–6293. [Google Scholar]
- Grzesiek S, Bax A. An Efficient Experiment for Sequential Backbone Assignment of Medium-Sized Isotopically Enriched Proteins. J Magn Reson. 1992b;99(1):201–207. [Google Scholar]
- Kamitani S, Kitadokoro K, Miyazawa M, Toshima H, Fukui A, Abe H, Miyake M, Horiguchi Y. Characterization of the Membrane-targeting C1 Domain in Pasteurella multocida Toxin. J Biol Chem. 2010;285(33):25467–25475. doi: 10.1074/jbc.M110.102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LE, Ikura M, Tschudin R, Bax A. 3-Dimensional Triple-Resonance NMRSpectroscopy of Isotopically Enriched Proteins. J Magn Reson. 1990;89(3):496–514. doi: 10.1016/j.jmr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Kitadokoro K, Kamitani S, Miyazawa M, Hanajima-Ozawa M, Fukui A, Miyake M, Horiguchi Y. Crystal structures reveal a thiol protease-like catalytic triad in the C-terminal region of Pasteurella multocida toxin. P Natl Acad Sci USA. 2007;104(12):5139–5144. doi: 10.1073/pnas.0608197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D, Driscoll PC, Kay LE, Wingfield PT, Bax A, Gronenborn AM, Clore GM. Overcoming the Overlap Problem in the Assignment of 1H-NMR Spectra of Larger Proteins by Use of 3-Dimensional Heteronuclear 1H-15N Hartmann-Hahn Multiple Quantum Coherence and Nuclear Overhauser Multiple Quantum Coherence Spectroscopy - Application to Interleukin-1-Beta. Biochemistry. 1989;28(15):6150–6156. doi: 10.1021/bi00441a004. [DOI] [PubMed] [Google Scholar]
- Satchell KJF. Structure and Function of MARTX Toxins and Other Large Repetitive RTX Proteins. Annu Rev Microbiol. 2011;65:71–90. doi: 10.1146/annurev-micro-090110-102943. [DOI] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expres Purif. 2005;41(1):207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Ho MF. Cellular and molecular action of the mitogenic protein-deamidating toxin from Pasteurella multocida. FEBS J. 2011;278(23):4616–4632. doi: 10.1111/j.1742-4658.2011.08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Bio. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]