Abstract
Until recently, sphingolipid physiology was primarily the domain of oncologists and immunologists. However, mounting evidence implicates ceramides and their derivatives in various aspects of metabolism via directly impacting the insulin receptor as well as modulating critical target cell survival and proliferation.
More recent observations suggest a strong link between a number of adipokines and ceramide catabolism. Here, we aim to briefly review the available data on the established metabolic effects of sphingolipids in various cell types and will discuss how adipokines exert a critical influence on the steady state levels of these lipid mediators.
Keywords: Ceramides, Sphingolipids, Insulin Sensitivity, Plasma ceramide levels, Ceramide target tissues
Introduction
Individuals with a history of diabetes present with an elevated risk for a number of complications, including hypertension, micro- as well as macrovascular complications and deteriorating kidney function. A major mediator of diabetes, with its associated dysregulation in carbohydrate and lipid metabolism, is the resistance to the actions of the hormone insulin in tissues such as liver, muscle and adipose tissue, with a concomitant unopposed action of glucagon. Recent advances in the field have implicated elevated intracellular ceramide levels as key mediators of insulin resistance [1]. This esoteric and rather heterogeneous class of lipids is composed of a sphingosine backbone conjugated to a fatty acid derivative. A number of physiological effects have been attributed to this diverse class of sphingolipids ubiquitously present in all cell types. The cellular sphingolipid biosynthetic and degradative machineries are quite active and subject to multiple levels of regulation by cell type- and nutritional status-specific events, altogether providing a pathway that experiences a high flux through its intermediates. This makes it challenging to predict what intermediate metabolites will actually accumulate in a specific cell or in plasma upon genetic or pharmacological inhibition of the key regulatory components.
Key lipid mediators for insulin resistance and metabolic dysfunction: Diacylglycerols (DAGs) versus Ceramides
The involvement of lipids in insulin resistance has been widely observed and is an accepted mechanism. Whether plasma circulating lipids or the accumulation of lipids within insulin-responsive cell types should be considered as the main driver for this phenomenon is still under investigation. A study by Kuhlmann and colleagues in 2003 showed that in normal weight, non-diabetic adults, intramyocellular triglyceride content is a strong predictor of muscle insulin resistance [2]. Although this study correlated intramyocellular triglyceride to insulin resistance, there have been studies suggesting other intracellular lipids, mainly diacyglycerol and ceramides that play a more major role in mediating insulin resistance [3, 4]. A recent study by Holland et al showed that rats that were infused with either lard oil, which is high in saturated fatty acids, or soy oil, which is primarily unsaturated fatty acids, had increased skeletal muscle diacyglycerol content along with reduced skeletal muscle glucose uptake, and reduced Akt phosphorylation, a mediator of insulin intracellular action [5]. However, only infusion of lard oil increased intramuscular ceramide content. Interestingly, when ceramide accumulation was blocked by treatment with myriocin, an inhibitor of de novo ceramide synthesis, glucose uptake and Akt phosphorylation were normalized in the lard-infused rats, but not in the soy-infused mice. This study not only showed that saturated and unsaturated fats have differential mechanisms of promoting insulin resistance, but also demonstrated that intracellular accumulation of ceramides can indeed result in insulin resistance in insulin responsive tissues. Thus, the discrepancies between studies that correlate insulin resistance to ceramide accumulation post-lipid infusion and those that do not may be attributed to the ratio of saturated to unsaturated fatty acids in the lipid infusion. Furthermore, since plasma free fatty acids in humans are a mixture of both unsaturated and saturated fatty acids, both mediators probably play a role in mediating peripheral insulin resistance. Foods such as butter, cream, and red meats—all rich in saturated fat but not unsaturated fat—have become a common staple in our Western diet [6]. Ceramides are therefore likely to play a more important and clinically relevant role in individuals developing diet-induced obesity in developed nations than previously thought.
Production of Ceramides and Sphingosine 1-phosphate
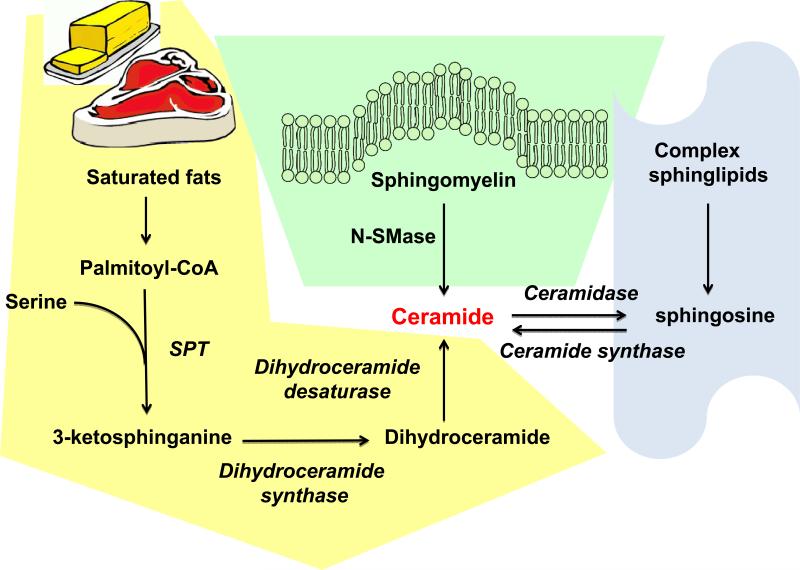
Ceramides are typically generated via 3 different pathways (Fig. 1). De novo synthesis occurs via the addition of a serine moiety to a palmitoyl-CoA. This reaction is catalyzed by the enzymes serine palmitoyl transferase-1 or -2 and results in the production of 3-ketosphinganine. Via the addition of another fatty acyl-CoA and a desaturation reaction by dihydroceramide desaturase, the final product, ceramide is produced. Depending on the fatty-acyl CoA moieties used, these lipids can have a diverse range of sizes, though commonly C16 through C24 ceramides are the most biologically relevant. The alternative pathway is the direct generation of ceramide via the cleavage of sphingomyelin by sphingomyelinase. From here, ceramides can be phosphorylated by ceramide kinase, or degraded via ceramidase activity to sphingosine. Sphingosine kinase is then able to phosphorylate this molecule to generate sphingosine-1-phosphate (S1P). The degradation of S1P is subsequently controlled by S1P-lyase, which irreversibly cleaves and destroys it. Finally, the third pathway, the so-called “salvage pathway”, is able to produce ceramides from the direct breakdown of sphingolipids to sphingosine, which can be converted to ceramides by the enzyme ceramide synthase. Alterations in the enzymatic activity in any of these steps can drastically alter the intracellular levels of these lipid moieties; a process which can be favorable or deadly for a cell, depending on the physiological conditions [7]. Given that three separate pathways can be active in parallel, inhibition of any one of them may have profound consequences for cellular physiology, or only a marginal effect, depending on the specific cell type, developmental stage and nutritional setting.
Fig. 1.
Ceramides can be synthesized through three different pathways: de novo biosynthesis, sphingomyelinase pathway, and the salvage pathway. The de novo pathway of ceramide generation is regulated by its rate-limiting enzyme, serine palmitoyl-CoA transferase (SPT). Furthermore, SPT has high specificity for its substrate, palmitoyl-CoA, a saturated fatty acid that is required for the formation of the sphingoid backbone of ceramides. Distinct from the de novo pathway, ceramides can also be generated by neutral sphingomyelinase (N-SMase)-catalyzed hydrolysis of sphingomyelin in cell membranes. Finally, ceramides can also be synthesized through the “salvage pathway” by breakdown of complex sphingolipids mediated by ceramide synthase.
Sphingolipid Synthesis and Metabolism
Ceramides are important members of the sphingolipid family and are essential building blocks for the structure of the phospholipid bilayer that constitutes the cell membrane. Other than structural roles, ceramides also play a part in cell signaling, inflammation, and apoptosis. In the cell, ceramides are synthesized through the three different pathways that we outlined above. The de novo pathway of ceramide generation occurs in the endoplasmic reticulum and is composed of four sequential enzymatic reactions and regulated by its rate-limiting enzyme, serine palmitoyl-CoA transferase (SPT) [8]. Once generated, ceramides are the common precursor to an array of complex sphingolipids and they can also be glucosylated, deacylated, and phosphorylated to produce a variety of downstream metabolites and signaling molecules.
The de novo pathway can be induced by an increase in dietary serine, oxidative stress, and oxidized LDLs [8]. Furthermore, SPT has a high specificity for its substrate, palmitoyl-CoA, the saturated fatty acid that is required for formation of the sphingoid backbone of ceramides. Thus, a diet that is high in saturated fat can effectively drive de novo ceramide synthesis and promote ceramide accumulation in peripheral tissues [9]. In 2002, Chang et al showed that gram negative bacteria infection (via lipopolysaccharide) and induction of inflammatory cytokines such as TNFα and IL-1 can both increase the mRNA expression and activity of SPT in macrophages and ultimately drive de novo ceramide synthesis [10]. Considering that expanding fat pads secrete a wide variety of inflammatory cytokines, including TNFα and IL-1, dysfunctional and inflamed fat pads are likely to contribute to the accumulation of ceramides in tissues [11].
Independent of the de novo synthesis pathway, ceramides are also generated by neutral sphingomyelinase (N-SMase)-catalyzed hydrolysis of sphingomyelin in cell membranes. Studies have shown that inflammation can induce N-SMase activity and drive ceramide synthesis [12, 13].
Plasma Ceramides and Insulin Resistance
Human and murine plasma is a rich source of ceramides, typically reported to circulate in the low micromolar range (0.5-10μM) [14-16]. The most abundant of the plasma ceramides are those with long chain fatty acyl groups including C24:0, C24:1 and C22:0 [15]. 75% of these ceramides are contained in VLDL and LDL particles, with the rest partitioning into HDL particles, with the distribution of the varying ceramide species remaining consistent between the different lipoprotein particles, with few exceptions [17]. While the association with lipoproteins is well established, we cannot rule out other mechanisms of transport, such as serum albumin or via packaging into exosomes [18]. Due to their stable association with lipoproteins, one could postulate that the primary source of circulating ceramides is hepatic in origin. This hypothesis was supported by isolating hepatocytes from obese mice and confirming that these cells secrete a significantly greater amount of ceramide compared to lean controls [19]. This question as to what plasma ceramide levels really reflect is far from being resolved. For the moment, plasma ceramides primarily serve as useful biomarkers for metabolic dysfunction, and future work will need to establish specific biological functions for individual sphingolipid subspecies.
Many clinical studies have reported elevated circulating ceramides in patients with type 2 diabetes, and further that these levels correlate with the severity of insulin resistance [15, 19, 20]. These correlative studies are supported by in vivo evidence showing that LDL particles containing ceramide were capable of inducing insulin resistance when infused into lean mice [19]. Insulin-stimulated glucose uptake was reduced in L6 myotubes exposed to the same LDL containing ceramides [19]. This data supports a possible role of liver derived ceramides contained in LDL particles as a mediator of systemic insulin resistance.
Other studies have shown that total ceramide levels highly correlate with a number of parameters involved in insulin resistance. Elevated ceramides correlate with both elevated circulating TNFα and interleukin-6 [15, 21]. Furthermore, following gastric bypass surgery, plasma ceramides levels decreased, as did circulating levels of TNFα. These reductions were correlated with a dramatic improvement in insulin sensitivity in these patients [22]. Together these studies help to link circulating ceramides, inflammation and the subsequent insulin resistance in a number of different states of obesity and type 2 diabetes.
Role of Sphingolipids in Macrophages
Ceramides
Macrophages are primarily known for their role in the innate immune system, highly important for controlling infections and repairing damaged tissue. Macrophage infiltration into adipose tissue during states of obesity plays a primary driving force for the insulin resistant state of this overly expanded adipose tissue[23-26]. As obesity progresses, macrophages turn from an alternatively activated anti-inflammatory phenotype (M2), to a more classically activated pro-inflammatory phenotype (M1) [27]. M1 macrophages produce a number of pro-inflammatory cytokines, including TNFα, a factor that can increase ceramide levels in several tissues. As Ceramide and its degradation product sphinosine-1-phosphate (S1P), have been implicated in altering sensitivity to insulin as well as mediating processes such as apoptosis, it is of great importance to determine the significance of these lipids on the macrophage population. The role and function of ceramides in macrophages has therefore been explored in great detail over the last decade. Primary reports on experiments with macrophages showed that cultured macrophages (the “Raw264” cell line) are able to ramp up ceramide production upon exposure to lipopolysaccharide [28]. However, this claim has recently been challenged by a group who showed that LPS alone was unable to induce this increase in ceramide production in bone marrow derived macrophages (BMDMs). Rather, LPS could act in concert with palmitate exposure to create a synergistic increase in C16 ceramides [29]. Both groups are in agreement that LPS -mediated increases in ceramide content are dependent on TLR-4 receptor and mediated by de novo synthesis pathways. However, differences between the two groups were manifest in that the former group shows that TLR-4 mediated activation of NF-κB leads to increased SPT1 (serine palmitoyl transferase) production, while the latter group refutes this claim. They show that upon LPS stimulation of bone marrow-derived macrophages, expression of SPT1 does not change at the RNA level (based on RT-QPCR), while SPT2 is elevated, though only slightly. They further substantiate this claim by demonstrating that the de novo synthesis pathway is the predominant mediator of ceramide production. Additional evidence for this mechanism relates to the fact that a dramatic rise in 3-ketosphinganine, the immediate downstream product of the condensation reaction performed by SPT1/2, is present [29]. Differences in the findings between these two groups may be based on cell-intrinsic properties, while the former used the cultured myoblast cell line C2C12 and cultured macrophages, the latter used primary macrophages. If the SPT pathway is already saturated in the primary isolates, the expression may be rendered less responsive to treatment with LPS. Notwithstanding the details, a fairly large body of literature implicates ceramides as mediators of a number of key physiological processes within the macrophage [30].
Recent work has shown that ceramides may be critically important in the activation of PI3-kinase by TLR-4 in macrophages [31, 32]. A short-term challenge of macrophages with LPS results in a burst of ceramide production occurring via the sphingomyelinase degradation pathway. This burst of ceramide production occurs within 30 seconds, but is normalized within 30 minutes following the initial stimulation. The PI3-kinase pathway is of critical importance for signaling during macrophage activation through signal transduction components, including but not limited to Akt, mTOR and NFκB. These observations highlight the pivotal role of LPS-mediated ceramide production for LPS mediated PI3-kinase activation [31, 32]. Ceramide generation by LPS stimulation is highly apoptotic under conditions in which the PI3-kinase pathway is inhibited with the small molecule inhibitor LY294002. However, many groups have shown that in other cell types, ceramides inhibit the activity of PI3-kinase. Therefore, the authors suggest that this may be a macrophage, or even alveolar macrophage-specific finding.
Ceramide-1-phosphate in macrophages
Another important component in this signaling cascade is the phosphorylation of ceramide. Ceramide-1 phosphate is generated via the phosphorylation of ceramide by ceramide kinase. Ceramide-1-phosphate (C-1-P) is capable of stimulating cell division in bone marrow-derived macrophages in mice [33, 34]. Withdrawal of serum from mouse BMDM cultures results in cell death, and that this process correlates with an elevation in sphingomyelinase activity. This activation is further characterized by a concomitant accumulation of ceramide with a corresponding decrease in C-1-P. These authors go on to show that C-1-P improves the viability of these cells following serum withdrawal by preventing activation of caspase-9. More importantly however, they show that C-1-P can inhibit acid sphingomyelinase directly, and hypothesize that this may be the direct mechanism for the prevention of the rise in ceramides and improved cell viability [33].
C-1-P is further able to activate PI3 kinase, and that this results in downstream activation of the MAPK/JNK pathway, though p38 MAPK was not involved in this process [34]. This activity leads to activation of NFκB. All of these findings were underscored by the observation that C-1-P levels rise in macrophages following macrophage colony-stimulating factor (mCSF) treatment, which ultimately results in cell division [34]. This provides compelling evidence that C-1-P can activate the PI3-kinase pathway, leading to cell cycle progression, though no mechanism for this activation has been offered thus far. This remains a question of great interest in the context of the observation that C-1-P and ceramide both activate the PI3-kinase pathway [34, 35]. A strong possibility is that these components may feed into the sphingosine-1-phosphate pathway, S-1-P representing a lipid mediator which activates specific receptors of the G protein coupled receptor upstream of the PI3-kinase pathway.
Sphingosine-1 Phosphate
Sphingosine-1-phosphate is generated by sphingosine kinase–mediated phosphorylation of sphingosine, which is a lipid metabolite generated from the breakdown of ceramides by ceramidase. S1P can signal through any one of its 5 receptors identified so far, all of which are GPCRs, each signaling through a variety of G proteins including Gi, Gs and Gy. These receptors play a diverse role in cellular homeostasis with knockout animals of the individual receptors showing a wide variety of phenotypes. S1P receptor 1 knockout mice are not viable as a result of an extreme vascular instability phenotype [36]. Receptor −2 and −3 knockouts are viable, however S1P receptor-2 knockouts present with seizures and deafness in certain genetic backgrounds, while S1P receptor-3 knockouts present with reduced viability though with an enhanced sepsis outcome [36]. The S1PR4 and S1PR5 receptor knockout mice have no obvious phenotype that has been described so far. Of interest clinically is the pharmacologic agonist FTY720, an immunosuppressive drug used in the treatment of multiple sclerosis and transplant rejection. This drug binds with high affinity to all of the S1P receptors and is proposed to cause receptor down-modulation and internalization upon binding. So even though it acts as a strong agonist initially, the associated down-regulation of the S1P receptors is functionally equivalent to antagonist action. Systemically, this results in the sequestration of lymphocytes within lymph organs [37].
S1P and the macrophage
Clearly, S1P is an important regulator of immune function. Macrophage treatment with LPS skews the macrophage phenotype toward a pro-inflammatory state, characterized by expression of iNOS and increased secretion of TNFα and macrophage chemoattractant protein 1 (MCP-1). Various groups have shown that co-treatment of macrophages with LPS and S1P inhibits this phenotypic switch and clamps macrophages in a more anti-inflammatory state characterized by expression of arginase 1 [38]. Furthermore, treatment with a highly specific S1P receptor 1 agonist (SEW2871) resulted in a similar anti-inflammatory response, while the specific antagonist VPC44116 was capable of inhibiting this phenotypic switch [38]. S1P receptor 2 deficient macrophages showed a blunted response to LPS, but respond similarly to SEW2871 treatment. These changes were all shown to be dependent on the NFκB, a transcription factor whose activation through LPS increases production of inflammatory mediators in the macrophage such as TNFα. S1P inhibits this pathway, as does SEW2871, and this phenomenon critically depends on receptor 1 [38].
S1P receptor response in atherosclerosis models
S1P levels in the blood positively correlate with HDL levels, and therefore it was suggested that S1P may play an important role in atherosclerotic lesions. The S1P receptor 3 deficient, whole body knockout mouse displays changes in the atherosclerotic plaque formation on the ApoE−/− background [39]. These lesions were not smaller in volume, yet contained significantly fewer macrophages than the wild type lesions [39]. Bone marrow transplantation to an ApoE-deficient mouse from a S1P-R3 knockout mouse again demonstrated reduced macrophage infiltration of the plaques; however this effect diminished compared to the whole body knockout, leading various groups to hypothesize that S1PR3 may play in important role in the endothelial response during atherosclerotic development [39]. Another paper, though later retracted for different reasons, showed similar findings in the S1P-R2 deficient mouse on an ApoE−/− background [40]. Surprisingly, no findings have been reported on S1PR1's role in the atherosclerotic progression; however FTY720, the agonist which down-modulates receptors, has been shown to decrease atherosclerotic lesion development in ApoE-deficient mice [41].
Excessive signaling of S1P
Sphingosine-1-phosphate is degraded by sphingosine lyase (SGPL). The SGPL knockout mouse presents with profound lymphopenia and neutrophilia and typically dies around week 4. In this mouse, serum S1P levels are highly elevated, as are the levels of circulating ceramide [42]. Furthermore, these mice are acutely inflamed, presenting with elevated C-reactive protein (CRP), TNFα and IL-6. Furthermore, an injection of LPS into these mice was lethal to the knockouts, while this was not the case in wildtype controls. Intraperitoneal injection of thioglycolate shows neutrophil and macrophage trafficking defects, and that there is a lack of L-selectin on the neutrophils in the knockouts [42]. A bone marrow transplant from SGPL mice into wild type mice recapitulates the findings from the knockout mouse. In the S1P-R4 knockout background, SGPL deficient mice normalize their neutrophil and cytokine levels and aberrant S1P-R4 signaling may be a primary defect resulting in this extreme phenotype of the mice. Finally, they show that G-CSF and IL-17 are elevated in the SGPL null mice, and that this causes increased neutrophil production. This results from a defect in neutrophil egress from the blood, an event which regulates the secretion of Granulocyte colony-stimulating factor (G-CSF) and down regulates further production of these cytokines. Therefore, it is the lack of L-selectin on neutrophils which results in an inability of macrophages to egress from the blood, causing apparently uncontrolled expression of G-CSF, thereby driving the neutrophilia phenotype. Interestingly the findings presented in this SGPL mouse are reminiscent of the findings in the treatment of wildtype mice with FTY720 which also results in severe lymphopenia.
FTY720 and mucosal immunity
As describe above, FTY720 is an immunosuppressant and works as an agonist of all the S1P receptors. Its true mechanism of action is due to its extremely high affinity for its receptors, which are effectively internalized and not recycled [37]. Clinically, this drug's resulting immunosuppressive effects are attributed to lymphocyte sequestration in lymphoid organs, thereby achieving excellent anti-inflammatory effects. However similar to many other anti-inflammatory and immune-suppressive drugs, a significant increase in number of infections per patient has been reported upon long-term treatment with FTY720 [37]. To investigate the role this compound has on gut mucosal immunity, a fluorescent enteric pathogen known as Citrobacter Rodetium was used. C. Rodentium is easily cleared by wild type, drug-naive mice within 14 days. However, FTY720 exposure significantly impaired the ability to clear the infection. 14 days post infection, the treated mice still displayed significant colonization of their colons, ceca and spleens, whereas in the untreated group, no pathogen colonization could be detected. Further, mucosal thickening and epithelial hyperplasia was apparent in the colons of treated mice. This was associated with a significant increase in number of neutrophils present in and around these tissues. IL6 and TNFα were elevated compared to vehicle on day 14 post infection, indicating a systemic inflammatory response despite anti-inflammatory treatment. These results indicate that FTY720 works effectively in the clinic for its intended purpose. Nevertheless, excessive S1P receptor signaling and down modulation of the receptors may have significant negative side effects on individuals and the treatment response must therefore be monitored carefully.
Role of Sphingolipids in Hepatocytes
Concomitant with the rise in obesity, the prevalence of non-alcoholic fatty liver disease (NAFLD), a newly emerging obesity-related disorder, has also been rising steadily [43]. NAFLD is a chronic liver disease that ranges histologically from simple steatosis in its mildest form to nonalcoholic steatohepatitis (NASH) in the more severe form, which is characterized by hepatocyte inflammation and fibrosis. Furthermore, NASH can potentially develop into cirrhosis and hepatocellular carcinoma in extreme cases [43]. Recent data from the National Health and Nutrition Examination Survey has estimated that NAFLD may affect up to 30% of the general population and 80% of obese and diabetic individuals, making it the most common liver dysfunction in the United States [44, 45].
Due to its increasing prevalence and detrimental health consequences the need to identify the mechanisms that mediate the pathogenesis and progression of NAFLD has become increasingly important. In 1998, Day et al proposed the “two-hit” hypothesis for the pathogenesis of NAFLD. According to these authors, hepatic lipid accumulation or steatosis represents the “first hit,” which makes the liver vulnerable to various additional insults that can ultimately result in the inflammation, fibrosis, and cell death observed in NASH, and this concept was further elaborated on by others [46, 47]. Concurrently, Unger et al. introduced the notion of lipotoxicity, which demonstrated that accumulation of lipids, especially saturated fatty acids, in non-adipose tissue leads to cell dysfunction and death [48]. These two concepts were further elaborated on in 2005 when Yamazaki et al identified that the deposition of lipids in the liver, especially triacylglycerol, as an essential step on the path to the development of NAFLD [49], though a much more nuanced understanding of the relationship of hepatic lipid accumulation, NAFLD and insulin resistance has emerged [50]. In addition, additional evidence implicates sphingolipids and their derivatives as important players in NAFLD and its progression to NASH [51].
Ceramides and the liver
Even though ceramide synthesis is ubiquitous in all tissues in the body, many recent in vitro and in vivo studies suggest that the liver is an important site for ceramide production. Nikolova et al showed that treatment of hepatocytes with pro-inflammatory cytokines including TNFα and IL-1β, and strong pro-inflammatory compounds such as lipopolysaccharide (LPS) can all trigger rapid turnover of sphingomyelin and intracellular ceramides [52]. The rapid turnover of sphingomyelin and ceramides is attributed to the inflammation-mediated induction of N-SMase. However, inflammation induced activation of the de novo ceramide synthesis should not be ruled out as a contributor. As early as 1998, Memon et al confirmed this in vivo by administering LPS in Syrian hamsters, which resulted in a 2-fold increase of hepatic SPT mRNA and activity within 16 hours. Hepatic sphingomyelin and ceramides also showed a 2.5-fold increase under these conditions [53]. These studies suggest that infection and the resulting inflammation regulate hepatic ceramide accumulation at the transcriptional level.
LPS induces inflammation by activating Toll-like receptors (TLRs), specifically TLR4. These receptors are essential for mounting inflammatory responses in innate immunity. In addition, studies have shown that saturated fatty acids can serve as agonists for TLR4 [54-56]. Furthermore, studies exposing H4IIE rat liver hepatoma cells to saturated fatty acids (such as palmitate) resulted in significant accumulation of ceramides in the cells [57]. Holland et al showed that TLR4 is an upstream signaling component required for saturated fatty acid-induced ceramide biosynthesis in liver by both in vitro and in vivo studies [58]. This further affirmed the notion that TLR4 is a major player in hepatic ceramide production. In addition, this study also revealed that TLR4 activation by saturated fatty acids can induce insulin resistance. Thus, these findings not only established a plausible mechanism of how saturated fatty acids induce ceramide synthesis but also linked dysregulation of fatty acid metabolism to hepatic ceramide accumulation.
Ceramides and hepatic dysfunction
Liver dysfunction prompts ceramide levels in both liver and plasma to increase correspondingly. Ichi and colleagues induced hepatic necrosis by administering carbon tetrachloride to mice, a potent pro-fibrotic agent and detected a significant increase in both hepatic and plasma ceramide concentrations [59]. Obese mice with hepatic steatosis also have increased hepatic ceramide content [60].
A major contributor in the development of hepatic steatosis is the upregulation of peripheral tissue lipolysis, primarily in adipose tissue, leading to an increased delivery of free fatty acids (FFAs) to the liver [46, 61]. Once in the liver, the FFAs are converted into triglycerides, diacyglycerol, and ceramides or oxidized in the mitochondria to ultimately maintain proper systemic carbohydrate homeostasis [62]. Triglycerides are exported from the liver as VLDL. When the net influx of FFAs is greater than the secretion of triglycerides or oxidation, lipids such as triglyceride and ceramides accumulate in the liver resulting in steatosis [46]. Obesity-associated insulin resistance prompts an increased rate of FFA influx into liver is increased, thereby resulting in NAFLD.
Additional evidence for the importance of ceramides in mediating the development of hepatic steatosis has recently emerged. In 2007, Holland et al showed that blocking de novo ceramide synthesis using myriocin, an SPT inhibitor, reduced macrovesicular hepatic steatosis, hepatic triglyceride content, and SOCS-3 expression, a cytokine mediated gene that is generally seen upregulated in NAFLD [5]. In parallel, mice in this study also showed improved insulin sensitivity as judged by restored Akt signaling, and this was seen in liver and muscle [5]. Studies like these lend support to the notion that ceramides promote insulin resistance and they are indeed directly involved in the development of NAFLD. Nevertheless, it is still not clear whether ceramide accumulation directly mediates hepatic steatosis or rather is the result of ceramide-induced insulin resistance.
Another aspect of the role of ceramides in the pathogenesis of NAFLD arises from studies involving adiponectin and its role in NAFLD. Adiponectin is an adipocyte-derived secretory protein that has been widely studied for its insulin sensitizing, anti-inflammatory, and anti-apoptotic effects on a number of cell types [63, 64]. In 2002, a study by Yamauchi et al found that adiponectin exerts a hepatoprotective role by decreasing FFA influx, de novo lipogenesis, and increasing hepatic FFA β-oxidation [65]. Additional studies showed that adiponectin improved insulin sensitivity, thereby leading to reduced hepatic gluconeogenesis [66, 67]. The mechanisms underlying these effects exerted by adiponectin have been a topic of ongoing research. In 2011, we found that the adiponectin receptors (AdipoR1 & R2) potently mediate ceramidase activity, thereby leading to an enhanced break-down of ceramides, in an adiponectin ligand-dependent manner [68]. Data from this study showed that adiponectin injections can potently reduce hepatic ceramides in vivo. Furthermore, mice with hepatic overexpression of AdipoR1 and R2 through adenoviral infection have significantly improved insulin sensitivity and reduced hepatocyte apoptosis when challenged with a high fat diet [68]. Similarly, clinical studies have shown that patients with advanced steatosis have reduced expression of hepatic AdipoR2 [69]. Considering that the liver is a major target organ of adiponectin action [70], the beneficial metabolic effects of adiponectin have been linked to the lowering of intracellular ceramides. Furthermore, these studies not only confirm that hepatic ceramides are important in the development of NAFLD, but also suggests that they play a more important role as causative factors for the induction of insulin resistance and inflammation that ultimately results in the progression to NASH.
Ceramides and progression to NASH
Insulin resistance and inflammation are known contributors to NAFLD's progression to NASH. Ceramides contribute to systemic insulin resistance by impairing insulin sensitivity in skeletal muscle and liver [5], and are likely to exert similar effects in other tissues as well. At the molecular level, ceramides disrupt the insulin-signaling cascade at the level of Akt activation [71, 72]. The resulting insulin resistance promotes lipolysis and increased FFA delivery to liver, thus increasing hepatic lipid accumulation [62]. Ceramides also interact with TNFα signaling in the liver to increase mitochondrial generation of reactive oxygen species (ROS), resulting in apoptosis and recruitment of inflammatory cells leading to worsening of hepatic inflammation [73]. In addition, the pro-inflammatory cytokine IL-1 is increased in NASH and also induces ceramide synthesis [1]. Ceramides have also been shown to induce the pro-inflammatory transcription factor nuclear factor kappa light chain enhancer of activated B cells (NF-κB). Therefore, once its production is induced by cytokines, ceramides can sustain and amplify inflammation through this positive feedback system [74]. Considering its role in both insulin resistance and inflammation, it seems like ceramide accumulation may provide the important “second hit” in the development towards full-blown NASH.
Role of Sphingolipids in Adipocytes
Once viewed simply as a passive reservoir for triglycerides, adipose tissue is now recognized as an active endocrine organ that plays a central role in the pathogenesis of obesity associated insulin resistance [75]. Although skeletal muscle is the major tissue responsible for peripheral glucose uptake, adipose tissue also expresses the insulin receptor and is responsible for clearing a portion of plasma glucose [76]. Early work on insulin resistance in adipose tissue showed that the cytokine TNFα, which is upregulated in adipose tissue during obesity, is able to induce insulin resistance by attenuating insulin signaling at the level of insulin receptor and by suppressing the expression of the insulin responsive glucose transporter GLUT4 [77, 78]. The mechanism by which TNFα induces these effects is not clear. In 1996, Long et al. demonstrated that TNFα mediates ceramide biosynthesis through the sphingomyelin pathway in 3T3-L1 adipocytes. The resulting rise in intracellular ceramide levels is concomitant with a 60% decrease of GLUT4 mRNA content. Furthermore, treatment of 3T3-L1 adipocytes with C8-ceramide, a membrane-permeable short chain analogue of ceramide, also decreased GLUT4 mRNA content, suggesting that a ceramide initiated signal transduction pathway exist in the adipocyte and plays a role in facilitating TNFα's control on GLUT4 expression [79]. Studies in 3T3-L1 adipocytes have shown that TNF-alpha can also cause insulin resistance by increasing the sphingolipid ganglioside GM3, which can attenuate insulin signaling at the level of the insulin receptor [80]. Clinical studies in humans have linked sphingolipid content in adipose tissue to systemic metabolic health. In 2007, Kolak et al showed that obese individuals with liver steatosis had increased inflammation, ceramide content, and sphingomyelinase activity in adipose tissue compared to their counterparts without liver steatosis [81]. Thus, it is evident that a strong link exists between adipose sphingolipids, inflammation, and insulin resistance, and adipose tissue plays an integral role in this process.
Role of Sphingolipids in β cells
Early indications that ceramides played a significant role came from studies of ZDF rats, a rodent model of β cell failure. These animals present early in life with β cell hyperplasia followed by subsequent failure. Elevated circulating triglycerides in these rats leads to inappropriate deposition of lipids in β cells, and this accumulation causes elevated ceramide content in islets [48]. These ceramides lead to β cell death, and inhibition of the ceramide synthase with fumonisin B rescues cultured ZDF islets from this lipid mediated apoptosis [48]. Follow up studies revealed that these ceramides are generated via the de novo synthesis pathway, and that inhibition of the rate limiting enzyme in this pathway is sufficient to reduce ceramide accumulation and apoptosis of the islets in vivo [82]. Consistent with a role of ceramides in β cell death are additional recent observations from our laboratory. These results show that the adipokine adiponectin is can rescue β cells from apoptosis in vivo via its ability to decrease intracellular ceramides [68].
In light of the compelling evidence arguing that ceramides are toxic to the β cells, a mechanism for this action has been sought in recent years. A common theme emerging from these studies is that ceramides are capable of causing endoplasmic reticulum stress, and that this is partly responsible for the death of β cells. Consistent with this theme is that reduction of ceramides in an insulin secreting β cell line results in decreased ER stress while improving cell survival [83]. Another aspect of ceramide action relates to the fact that they can alter mitochondrial function, and β cells have a very limited tolerance with respect to altered mitochondrial function. This is further highlighted by the treatment of INS-1 cells with a cell permeable ceramide. This markedly reduces the mitochondrial membrane potential, leading to a concomitant increase in cytosolic cytochrome c [84, 85]. While the direct effects of ceramides on mitochondrial membrane integrity are still subject to discussions, this remains a plausible mechanism which has been better defined in other organs, such as the heart.
Role of sphingolipids in the heart
The mammalian heart is a highly aerobic organ deriving approximately 60% of its energy from the direct utilization of lipids via oxidative phosphorylation. Although the heart is well equipped to handle lipids, states of excess lipid deposition occur under conditions of insulin resistance and type 2 diabetes and are linked to diastolic dysfunction in both human and rodent studies [86, 87]. Key mediators of the dysfunction in the heart under these conditions are thought to be ceramides. In vitro studies have shown that short and long chain ceramides can inhibit mitochondrial respiration [88]. This effect is produced via a membrane dependent inhibition of mitochondrial complexes I and III [88]. Similar to the situation in β cells, short chain cell permeable ceramides are directly toxic to isolated rat cardiac myocytes via induction of apoptotic cell death [89]. From these results, we can see that ceramides are directly toxic to cardiomyocytes, in large part via inhibition of mitochondrial respiration. However, most studies were performed on isolated cells and mitochondria. The role in vivo role of ceramides in whole animal cardiac physiology remains less well defined.
Lipoprotein lipase is an enzyme on the surface of cells which mediates the cleavage of fatty acyl groups from circulating lipoprotein particles, thereby stimulating uptake of these lipids into proximal tissues. Overexpression of lipoprotein lipase in the heart results in lipid accumulation and cardiac dysfunction as characterized by left ventricular dilation (LVD) and decreased contractility [90]. Treatment with myriocin, an inhibitor of the de novo ceramide synthesis pathway, corrected both LVD and the loss of contractility, further highlighting the importance of ceramides in diastolic dysfunction during lipid overload in the heart [90].
With respect to acute cell injury, ceramides are greatly elevated during ischemic cardiac cell death in vivo [89]. Recent evidence implicates adiponectin as a potent cardioprotective factor through its activation of cellular ceramidases [68]. Overexpression of adiponectin prevents cardiac cell death upon induction of apoptosis in vivo, while loss of adiponectin enhances cardiac damage and increases mortality [68]. These studies build upon previously published experiments that highlight adiponectin knockout mice as more susceptible to cardiac ischemic death, though a ceramide-dependent mechanism was not tested [91]. Altogether, these studies put ceramides at the center of cardiac health and fitness and emphasize the importance of lipid homeostasis in the mammalian heart.
How do leptin, adiponectin and FGF21 alter sphingolipids
Adipose tissue secretes a wide array of unique factors, adipokines, which have been widely discussed in the literature with respect to their function as regulators of whole body metabolism and insulin sensitivity [75]. Among these adipokines, leptin is one of the most active regulators of body weight and food intake. Dysregulation of leptin usually results in obesity and eventually insulin resistance and type 2 diabetes [92-95]. Rats treated with leptin display systemic improvements in insulin sensitivity and suppress de novo ceramide synthesis [48, 96]. More recently, Bonzon-Kulichenko et al. have demonstrated that central leptin infusion in the hypothalamus in mice reduces total ceramide content in WAT via action through the autonomic nervous system. Central leptin represses serine palmitoyl transferase (SPT), the rate-limiting enzyme of ceramide production in WAT by 30%. Furthermore, the reduction of ceramides in WAT coincides with improved systemic insulin sensitivity; suggesting that reduction of WAT ceramides contributes to improvements in systemic insulin sensitivity [97]. As discussed in the various sections above on adiponectin action in specific target tissues, adiponectin exerts its beneficial metabolic effects by lowering cellular ceramide levels. Furthermore, adiponectin is potently anti-apoptotic in the context of cardiomyocytes and pancreatic β cells in vivo through the lowering of cellular ceramides. The adiponectin receptors, AdipR1 and AdipoR2 exhibit ceramidase activity in an adiponectin dependent manner [68]. Whether the receptors themselves contain the ceramidase activity or they sequester or induce a ceramidase upon activation is still unclear. FGF21 has been the target of much interest [98]. Expressed primarily in the liver upon prolonged fasting, it can also be synthesized in other tissues. In a similar fashion as adiponectin, FGF21 mediates potent beneficial effects on carbohydrate and lipid metabolism [99] and effectively lowers ceramides [100]. These effects critically rely on FGF21's ability to stimulate adiponectin release from the adipocyte. Thiazolidinediones (TZDs), agonists for the nuclear receptor PPARγ, are potent inducers of FGF21 and adiponectin, and in turn lower plasma ceramide levels as well. Based on our own work and that of others, we propose a model in which there is a linear relationship between TZD exposure, leading to the induction of FGF21, which in turn triggers increased release of adiponectin from adipocytes, that prompts a systemic lowering of ceramides [100, 101].
These observations highlight the dual role of the adipocyte, both as a source of lipotoxic fatty acids as well as a source for potently anti-lipotoxic adipokines that include, but may not be limited to, leptin, adiponectin and FGF21 that exert protective roles for susceptible cell types, both during times of maximal FFA release from the adipocyte during fasting as well in the postprandial state.
“Healthy” adipose tissue rescues systemic ceramide accumulation
In the past, we have generated several mouse models that display the ability to massively expand their subcutaneous adipose tissue. We define “healthy” tissue as a tissue that can undergo a very high level of expansion without the usual side effects of obesity, i.e. adipose tissue displaying increased levels of adipogenesis, effective recruitment of precursor cells, proper angiogenesis, lack of hypoxia and fibrosis, with limited infiltration of immune cells and subsequent inflammation [102]. We have achieved that by either local overexpression of adiponectin [103] or overexpression of the mitochondrial protein mitoNEET [104]. In both cases, systemic insulin sensitivity is fully preserved, and minimal ectopic lipids accumulate under those conditions. This highlights the powerful effects that adipose tissue can exert as a “metabolic sink” for excess lipids, that can effectively be neutralized in subcutaneous adipose tissue rather than being deposited ectopically in other tissues, where they give rise to ceramides.
Concluding remarks
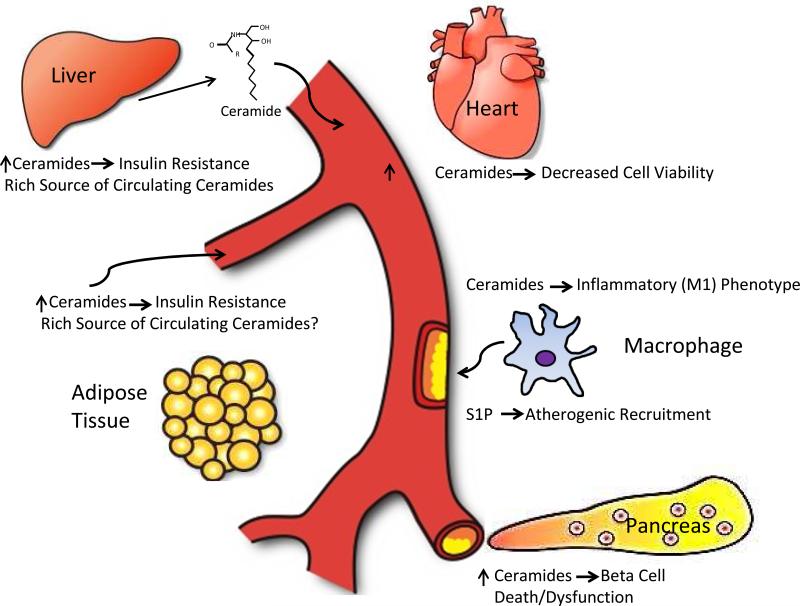
Lipids are a highly heterogeneous class of bioactive molecules that play diverse functions within the cell. Here, we have outlined the important functions of metabolites of the ceramide and sphingosine pathways with respect to cellular and systemic metabolic function (Fig. 2). The involvement of ceramides in the development of diabetes and metabolic syndrome is an emerging field in metabolism research. While the liver is an important site of ceramide synthesis, it is clear that the manipulation of the sphingolipid pathways in other tissues, particularly the adipocyte, may also potently affect the systemic sphingolipid pool. The accumulation of ceramides in the liver can potentially promote hepatic steatosis and further its development into steatohepatitis. This finding suggests that the “cross-talk” between adipose tissue and liver may be important in the development of liver dysfunction and other metabolic impairments. Sphingolipid metabolism is a potent upstream mediator of the insulin sensitizing and anti-inflammatory effects of the key known adipokines, such as leptin, adiponectin and FGF21. One of the key challenges in the field in the future remains to build a better understanding of the actions of these adipokines on their key target tissues and how that translates into measurements of individual sphingolipid subspecies in plasma. This also entails a better understanding of what distinct physiological role the many different subspecies play at the level of the individual target cells, which - though currently a daunting task - will unquestionably contribute in major ways towards an improved holistic understanding of metabolic dysfunction.
Fig. 2.
Overview of the key target tissues and cell types discussed here in the context of metabolic effects of sphingolipids. One of the major unresolved questions remains what the major sources for circulating sphingolipids are and whether there are differences in the tissue origins of different ceramide subspecies circulating in plasma.
Highlights.
Ceramides are key mediators of insulin resistance and apoptosis
Key tissues affected are the liver, adipose tissue, immune cells
Additional tissues affected include the heart, pancreatic β cells and muscle
Pharmacological or genetic disruption of ceramide synthesis improves metabolism
Plasma ceramides serve as markers for the Metabolic Syndrome
Acknowledgements
The authors were supported by US National Institutes of Health Grants R01-DK55758, R01-DK099110 and P01-DK088761 as well as Juvenile Diabetes Research Foundation Grant JDRF 17-2012-36 (P.E.S.). We would like to thank Nancy Heard for help with the figure design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhlmann J, Neumann-Haefelin C, Belz U, Kalisch J, Juretschke HP, Stein M, Kleinschmidt E, Kramer W, Herling AW. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes. 2003;52:138–144. doi: 10.2337/diabetes.52.1.138. [DOI] [PubMed] [Google Scholar]
- 3.Petersen KF, Shulman GI. Etiology of insulin resistance. Am. J. Med. 2006;119:S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Jaworowska A, Blackham T, Davies IG, Stevenson L. Nutritional challenges and health implications of takeaway and fast food. Nutr. Rev. 2013;71:310–318. doi: 10.1111/nure.12031. [DOI] [PubMed] [Google Scholar]
- 7.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr. Rev. 2007;65:S39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 8.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151:4187–4196. doi: 10.1210/en.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YT, Choi J, Ding S, Prieschl EE, Baumruker T, Lee JM, Chung SK, Schultz PG. The synthesis and biological characterization of a ceramide library. Journal of the American Chemical Society. 2002;124:1856–1857. doi: 10.1021/ja017576o. [DOI] [PubMed] [Google Scholar]
- 11.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 12.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 13.Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim. Biophys. Acta. 2006;1758:1893–1901. doi: 10.1016/j.bbamem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Kirwan JP. Plasma ceramides target skeletal muscle in type 2 diabetes. Diabetes. 2013;62:352–354. doi: 10.2337/db12-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brozinick JT, Hawkins E, Hoang Bui H, Kuo MS, Tan B, Kievit P, Grove K. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond) 2012 doi: 10.1038/ijo.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J. Biol. Chem. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocr. Met. : JPEM. 2013:1–4. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 21.de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppanen-Laakso T, Oresic M, Pulkkinen L, Uusitupa M, Erkkila AT. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. doi: 10.1007/s00125-009-1482-9. [DOI] [PubMed] [Google Scholar]
- 22.Huang CK, Goel R, Chang PC, Lo CH, Shabbir A. Single-incision transumbilical (SITU) surgery after SITU laparoscopic Roux-en-Y gastric bypass. J. Laparoendosc. Adv. Surg. Tech. Part A. 2012;22:764–767. doi: 10.1089/lap.2011.0434. [DOI] [PubMed] [Google Scholar]
- 23.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 26.Asterholm IW, McDonald J, Blanchard PG, Sinha M, Xiao Q, Mistry J, Rutkowski JM, Deshaies Y, Brekken RA, Scherer PE. Lack of “immunological fitness” during fasting in metabolically challenged animals. J. Lipid. Res. 2012;53:1254–1267. doi: 10.1194/jlr.M021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 28.Chang ZQ, Lee SY, Kim HJ, Kim JR, Kim SJ, Hong IK, Oh BC, Choi CS, Goldberg IJ, Park TS. Endotoxin activates de novo sphingolipid biosynthesis via nuclear factor kappa B-mediated upregulation of Sptlc2. Prostaglandins Other Lipid Mediat. 2011;94:44–52. doi: 10.1016/j.prostaglandins.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 2013;288:2923–2932. doi: 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levi M, Meijler MM, Gomez-Munoz A, Zor T. Distinct receptor-mediated activities in macrophages for natural ceramide-1-phosphate (C1P) and for phospho-ceramide analogue-1 (PCERA-1) Mol. Cell. Endocrinol. 2010;314:248–255. doi: 10.1016/j.mce.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur. J. Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 32.Fallah MP, Chelvarajan RL, Garvy BA, Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech. Ageing Dev. 2011;132:274–286. doi: 10.1016/j.mad.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20:726–736. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Monick MM, Mallampalli RK, Carter AB, Flaherty DM, McCoy D, Robeff PK, Peterson MW, Hunninghake GW. Ceramide regulates lipopolysaccharide-induced phosphatidylinositol 3-kinase and Akt activity in human alveolar macrophages. J. Immunol. 2001;167:5977–5985. doi: 10.4049/jimmunol.167.10.5977. [DOI] [PubMed] [Google Scholar]
- 36.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 37.Murphy CT, Hall LJ, Hurley G, Quinlan A, MacSharry J, Shanahan F, Nally K, Melgar S. The sphingosine-1-phosphate analogue FTY720 impairs mucosal immunity and clearance of the enteric pathogen Citrobacter rodentium. Infect. Immun. 2012;80:2712–2723. doi: 10.1128/IAI.06319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, Takuwa N, Gonda K, Yamamoto Y, Ohkawa R, Nishiuchi T, Sugimoto N, Yatomi Y, Mitsumori K, Asano M, Kinoshita M, Takuwa Y. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J. Clin. Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 42.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve. Clin. J. Med. 2008;75:721–728. doi: 10.3949/ccjm.75.10.721. [DOI] [PubMed] [Google Scholar]
- 44.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 45.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006;40(Suppl 1):S17–29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 46.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 48.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J. Biol. Chem. 2005;280:21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- 50.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell. Biochem. 2008;49:469–486. doi: 10.1007/978-1-4020-8831-5_18. [DOI] [PubMed] [Google Scholar]
- 53.Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler. Thromb. Vasc. Biol. 1998;18:1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 54.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J. Biol. Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 56.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J. Physiol. Endocrinol. Metab. 2006;291:E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 58.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichi I, Nakahara K, Fujii K, Iida C, Miyashita Y, Kojo S. Increase of ceramide in the liver and plasma after carbon tetrachloride intoxication in the rat. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:53–56. doi: 10.3177/jnsv.53.53. [DOI] [PubMed] [Google Scholar]
- 60.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- 63.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 64.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 66.Berg AH, Combs T, Du X, Brownlee M, Scherer PE. The Adipocyte-Secreted Protein Acrp30 Enhances Hepatic Insulin Action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 67.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous Glucose Production is Inhibited by the Adipose-Derived Protein Acrp30. J. Clin. Inv. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma H, Gomez V, Lu L, Yang X, Wu X, Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2009;24:233–237. doi: 10.1111/j.1440-1746.2008.05548.x. [DOI] [PubMed] [Google Scholar]
- 70.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic Fate of the Adipocyte-Derived Factor Adiponectin. Diabetes. 2009 doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 72.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G583–589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 74.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 76.Laviola L, Perrini S, Cignarelli A, Giorgino F. Insulin signalling in human adipose tissue. Arch. Physiol. Biochem. 2006;112:82–88. doi: 10.1080/13813450600736174. [DOI] [PubMed] [Google Scholar]
- 77.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNFα. Cell. 1993;73:625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- 78.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 79.Long SD, Pekala PH. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem. J. 1996;319(Pt 1):179–184. doi: 10.1042/bj3190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 81.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 82.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 83.Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, Fuller M, Laybutt DR, Meikle PJ, Biden TJ. A lipidomic screen of palmitate-treated MIN6 beta-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem. J. 2011;435:267–276. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 84.Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10:841–850. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- 85.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 86.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 87.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 88.Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 89.Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, Tomei LD, Hannun YA, Umansky SR. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 1997;151:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 90.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 93.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 94.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob. Nat. Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 95.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 96.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 97.Bonzon-Kulichenko E, Schwudke D, Gallardo N, Molto E, Fernandez-Agullo T, Shevchenko A, Andres A. Central leptin regulates total ceramide content and sterol regulatory element binding protein-1C proteolytic maturation in rat white adipose tissue. Endocrinology. 2009;150:169–178. doi: 10.1210/en.2008-0505. [DOI] [PubMed] [Google Scholar]
- 98.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao Y, Dunbar JD, Kharitonenkov A. FGF21 as a therapeutic reagent. Adv. Exp. Med. Biol. 2012;728:214–228. doi: 10.1007/978-1-4614-0887-1_14. [DOI] [PubMed] [Google Scholar]
- 100.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]