Abstract
Context
Diagnostic imaging use increased significantly within fee-for-service models of care. Little is known about patterns of imaging among members of integrated health care systems.
Objective
To estimate trends in imaging utilization and associated radiation exposure among members of integrated healthcare systems.
Design, Setting, and Participants
Retrospective analysis of electronic records of members of six large integrated health systems from diverse regions of the country. Review of medical records allowed direct estimation of radiation exposure from selected tests. Between 1–2 million member-patients were included each year from 1996 to 2010.
Main Outcome Measure
Advanced diagnostic imaging rates, and cumulative annual radiation exposure from medical imaging.
Results
During the 15 year study period, enrollees underwent a total of 30.9 million imaging examinations (over 25.9 million person-years), reflecting 1.18 (95% CI 1.17–1.19) tests per person per year, of which 35% were for advanced diagnostic imaging (CT, MRI, nuclear medicine, and ultrasound). Use of advanced diagnostic imaging increased from 1996 to 2010; computed tomography examinations increased from 52/1000 enrollees in 1996 to 149/1000 in 2010, 7.8% annual growth (95% CI 5.8%, 9.8%); MRI increased from 17/1000 to 65/1000, 10% annual growth (95% CI 3.3%, 16.5%); and ultrasound increased from 134/1000 to 230/1000, 3.9% annual growth (95% CI 3.0% to 4.9%). While nuclear medicine decreased from 32/1000 to 21/1000, 3% annual decline (95% CI 7.7% decline to 1.3% increase), after 2004, PET imaging rates increased from 2.2/10 000 to 23.5/10 000, 15.2% annual growth. While imaging increased within all health systems, the adoption of different modalities for anatomic area assessment varied. Increased use of CT resulted in increased radiation exposure for enrollees, with a doubling in the mean per capita effective dose and in the proportion of enrollees who received high (>20–50 mSv) or very high (>50mSv) annual radiation exposure. By 2010, nearly 7% of enrollees who underwent imaging received a high annual radiation exposure (>20–50 mSv) and 4% received a very high annual exposure (>50 mSv).
Conclusion
Within integrated health care systems there was a large increase in the rate of advanced diagnostic imaging and associated radiation exposure between 1996 and 2010.
INTRODUCTION
The use of diagnostic imaging in the Medicare population has increased significantly over the last two decades, particularly using expensive new technologies such as computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine positron emission technology (PET).1,2 The development and improvement in these advanced diagnostic imaging technologies is widely credited with leading to earlier and more accurate diagnoses of disease using non-invasive techniques. However, utilization and costs of advanced diagnostic imaging in the United States are high and rapidly growing,3,4 and payments to physicians for diagnostic imaging have had the highest rate of growth among all physician services over the last decade.3,4 CT and nuclear medicine examinations deliver much higher doses of ionizing radiation than conventional radiographs, and extensive epidemiological evidence has linked exposure to radiation levels in this range with the development of radiation-induced cancers.5,6 It is estimated that 2% of future cancers will result from current imaging use, if imaging continues at current rates.7,8
Most studies that have evaluated patterns of diagnostic imaging have assessed insurance claims for fee-for-service insured populations1,9–11 where financial incentives encourage imaging.12,13 No large, multisite studies have assessed imaging trends in integrated healthcare delivery systems that are clinically and fiscally accountable for the outcomes and health status of the population served.13,14 Understanding imaging utilization, and associated radiation exposure in these settings could help inform us about how much of the increase in imaging may be independent of direct financial incentives.
We conducted a population-based study of diagnostic imaging trends between 1996 and 2010 among members of six geographically diverse integrated health care delivery systems that have both care delivery and insurance relationships with their member-patients. The availability of administrative and electronic medical record data on all health care received—including diagnostic imaging—allowed us to assess patterns of imaging over time as they varied by health system and patient demographics.
METHODS
Data Sources And Study Population
The study population includes members enrolled in one of six health care systems, each of which participates in the HMO Research Network,15 including Group Health Cooperative in Washington State; Kaiser Permanente in Colorado, Georgia, Hawaii, and Oregon/Washington; and Marshfield Clinic/Security Health Plan in Wisconsin. We included all members enrolled in group- and staff-model plans, and in addition to commercial plans, the health system members include enrollees with prepaid Medicaid contracts, state-subsidized prepaid plans and Medicare Advantage plans. We excluded data for health plan members who purchased fee-for-service (network) plans and patients treated at health plan facilities without being enrolled in the HMO plans because of the high likelihood of incomplete capture of imaging for these patients. Enrollees were included in the study for each year in which they were continuously enrolled. A data resource utility called the Virtual Data Warehouse (VDW) that includes comprehensive diagnostic imaging data was used to capture standardized data from the electronic medical and administrative records.16 A waiver of informed consent was received for each participating health care system.
Imaging Utilization
All diagnostic imaging tests were included regardless of where they were ordered, performed, or interpreted. Imaging procedures done in conjunction with radiation treatment for cancer were not included. Individual health systems contributed data for at least 11 and up to 15 years, depending on participation in their local VDW. Imaging procedures were coded using standardized ICD-9-CM, CPT-4, and HCPCS codes.17 There were 1467 unique imaging codes across all years, and each was mapped to an anatomic area (ie, abdomen/pelvis [abdomen], brain [central nervous system, abbreviated as CNS], breast, cardiovascular, chest, extremity, obstetric, spine, and other/unknown) and where they could not differentiated, extremity and spine were combined as musculoskeletal. Exams were also characterized by modality (ie, angiography/fluoroscopy; CT; MRI; nuclear medicine [PET, a subset of nuclear medicine categorized separately because of its high cost]; Radiography; ultrasound; and other/unknown17). Multiple examinations with the same procedure code performed on the same patient on the same day were counted only once to reduce the likelihood of over-counting.
Radiation Dose
Of the 1467 imaging codes, 1068 were associated with the delivery of ionizing radiation, including angiography/fluoroscopy, CT, nuclear medicine, and radiography. Ultrasound and MRI do not useionizing radiation and thus were not included. Because CT and nuclear medicine exams contribute such a large proportion of the radiation exposure from imaging, we newly abstracted additional patient-level data to allow accurate estimation of dose. For CT, we randomly selected patients based on age and sex in each year from across the participating sites who underwent the most frequent CT examination types (N=4188 examinations) and abstracted the primary determinants of dose from each examination (ie, body region imaged, scan length, kilovolt peak [kVp], milliampere [mA] or mA seconds [mAs], rotation time, pitch, and CT manufacturer and model). These technical parameters were abstracted using automated computer programs that extracted these data from the Digital Imaging and Communications in Medicine (DICOM) tags stored in the Picture Archiving and Communications System (PACS). For examinations prior to when each health plan adopted PACS, these parameters were manually abstracted from the stored or printed CT images. Using these parameters, we estimated the effective dose for each CT examination. Effective dose is a metric that takes into account both the amount of radiation to which a person is exposed and the biological effect of that radiation on the exposed organs. It is defined as a dose equivalent to the dose (and subsequent harm) that would have been received had the full body been irradiated by a single source. Thus a large dose to a single organ might have a similar effective dose, and thus estimated to have a similar future cancer risk, as a smaller dose to the entire body. In general, effective dose is estimated as a weighted average of organ doses, with weights corresponding to the sensitivity of each organ to developing cancer after radiation exposure. We used a new method for estimating organ-specific dose based on improved gender- and age-specific phantom anatomy using mathematical hybrid phantoms.18,19 Organ-equivalent doses were weighted to generate effective dose by the tissue weighting factors provided in the International Commission on Radiological Protection (ICRP) Publication 103.20 For nuclear medicine examinations, effective dose varies by the administered radiopharmaceutical and activity. At a single facility, we collected data on the type, and injected volume, of radiopharmaceutical for a consecutive sample of 5502 nuclear medicine examinations. Using these data and standard calculations for estimating effective dose for each examination with methods described by the Medical Internal Radiation Dose (MIRD) committee,21 we estimated the dose for each examination type. For mammography, we obtained unpublished patient-level data from the American College of Radiology Imaging Network digital mammography imaging screening trial (DMIST) to estimate dose and variation in dose.22 For the remaining imaging study types (ie, less common CT types, fluoroscopy, angiography/fluoroscopy, and radiography), we completed a detailed review of the published literature to estimate dose and measure of variability in dose for each study.
The dosage data provided detailed information on the variability in effective radiation dose within procedure type. We used this information to create a dose estimate that accounts for the variability of dose. A truncated lognormal distribution was a good fit to dose data, with the truncation occurring at three standard deviations on the log scale. For each exam each patient underwent during the study period, we randomly imputed a dose value from the log-normal distribution. This technique allowed each person to be assigned a dose value that reflected the true underlying distribution in dose, rather than assuming each person received the mean dose associated with a particular exam type.
We used the data describing the number of imaging tests and associated radiation dose per test to calculate the total dose of radiation each member received each year of the study, and to calculate the collective effective dose to the entire population. To estimate cumulative annual radiation exposure for each patient, we summed the doses for each examination within each year. If patients underwent more than one examination using the same modality on the same anatomic area on the same day, we only included the examination with the highest radiation exposure to calculate dose so as not to overestimate doses received.
Statistical Analysis
We calculated the number of imaging examinations per 1000 enrollees per year (rates) by modality, anatomic area, and health system (overall and stratified by site and age). The calculations of rates over time were adjusted for site, age, and sex. This standardization was performed in Stata® by fitting log-linear regression models with Gaussian errors, including site, age, gender, and year as covariates. Based on the fitted model, we estimated average rates using marginal standardization, treating each site with equal weight and adjusting to the observed age and gender distribution.23 For modality-specific analyses, we calculated the per capita dose in mSv per person per year by dividing the collective effective dose by the number of individuals (whether exposed to radiation from diagnostic imaging or not). For analyses that ignored modality, we estimated the individual radiation dose per year and calculated descriptive statistics in annual radiation exposure, including the percentage of enrollees who underwent a high annual radiation exposure (defined as 20–50 mSv)11,20 or a very high radiation exposure (>50 mSv).11,20 We calculated the relative contribution (mean dose and percentage of dose) from the different imaging modalities over time. When showing imaging rates (Table 1), we show data from 2008, the most recent year where all sites contributed data. We used SAS version 9.2 and Stata versions 11.1 and 12.0 for statistical analysis. All statistical tests were two sided and significance was set at p < .05.
Table 1.
Number of exams (N) and percent of exams per 1000 enrollees per year, by modality, anatomic area, and health system, shown for 2008, the most recent year where each health plan is included. All rates adjusted to a standardized age and sex distribution. Each column represents one health plan, and within each column, the percents sum to 100%.
| Average | A | B | C | D | E | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Angiography/Fluroscopy | 64 | (4.5%) | 66 | (4.1%) | 62 | (4.6%) | 23 | (1.9%) | 103 | (6.1%) | 54 | (3.8%) | 73 | (5.8%) |
| Abdomen | 14 | (1.0%) | 14 | (0.9%) | 16 | (1.2%) | 11 | (0.9%) | 18 | (1.0%) | 14 | (1.0%) | 10 | (0.8%) |
| Cardiovascular | 43 | (3.1%) | 48 | (3.0%) | 35 | (2.6%) | 7 | (0.6%) | 79 | (4.7%) | 33 | (2.3%) | 58 | (4.7%) |
| Other | 7 | (0.5%) | 4 | (0.3%) | 12 | (0.9%) | 5 | (0.4%) | 6 | (0.4%) | 7 | (0.5%) | 5 | (0.4%) |
| Computed Tomography | 177 | (12.5%) | 195 | (12.3%) | 176 | (13.0%) | 160 | (13.1%) | 199 | (11.8%) | 200 | (13.9%) | 136 | (10.8%) |
| Abdomen | 89 | (6.3%) | 97 | (6.1%) | 87 | (6.4%) | 84 | (6.9%) | 94 | (5.6%) | 100 | (7.0%) | 71 | (5.6%) |
| Cardiovascular | 9 | (0.6%) | 9 | (0.6%) | 11 | (0.8%) | 1 | (0.1%) | 11 | (0.6%) | 16 | (1.1%) | 5 | (0.4%) |
| Chest | 23 | (1.6%) | 26 | (1.6%) | 21 | (1.6%) | 20 | (1.6%) | 27 | (1.6%) | 24 | (1.7%) | 23 | (1.8%) |
| CNS | 43 | (3.0%) | 45 | (2.9%) | 43 | (3.2%) | 45 | (3.7%) | 54 | (3.2%) | 45 | (3.1%) | 27 | (2.2%) |
| Spine | 6 | (0.5%) | 7 | (0.5%) | 8 | (0.6%) | 4 | (0.4%) | 6 | (0.3%) | 8 | (0.6%) | 5 | (0.4%) |
| Other | 6 | (0.5%) | 10 | (0.6%) | 6 | (0.4%) | 5 | (0.4%) | 8 | (0.5%) | 6 | (0.4%) | 4 | (0.3%) |
| Magnetic Resonance Imaging | 72 | (5.1%) | 88 | (5.6%) | 73 | (5.4%) | 60 | (4.9%) | 80 | (4.7%) | 75 | (5.3%) | 55 | (4.3%) |
| Cardiovascular | 5 | (0.4%) | 7 | (0.4%) | 5 | (0.3%) | 6 | (0.5%) | 9 | (0.6%) | 3 | (0.2%) | 2 | (0.2%) |
| CNS | 18 | (1.3%) | 23 | (1.4%) | 18 | (1.3%) | 15 | (1.2%) | 21 | (1.3%) | 23 | (1.6%) | 11 | (0.9%) |
| Extremity | 19 | (1.3%) | 26 | (1.7%) | 16 | (1.2%) | 16 | (1.3%) | 18 | (1.0%) | 19 | (1.3%) | 18 | (1.4%) |
| Spine | 23 | (1.6%) | 27 | (1.7%) | 23 | (1.7%) | 19 | (1.6%) | 24 | (1.4%) | 26 | (1.8%) | 17 | (1.4%) |
| Other | 7 | (0.5%) | 5 | (0.3%) | 11 | (0.8%) | 5 | (0.4%) | 8 | (0.5%) | 5 | (0.3%) | 6 | (0.5%) |
| Nuclear Medicine | 53 | (3.7%) | 60 | (3.8%) | 31 | (2.3%) | 49 | (4.0%) | 85 | (5.0%) | 45 | (3.1%) | 50 | (4.0%) |
| Cardiovascular | 41 | (2.9%) | 45 | (2.8%) | 16 | (1.2%) | 41 | (3.3%) | 70 | (4.1%) | 34 | (2.3%) | 40 | (3.2%) |
| Chest | 2 | (0.1%) | 1 | (0.1%) | 1 | (0.1%) | 1 | (0.1%) | 3 | (0.2%) | 2 | (0.1%) | 1 | (0.1%) |
| Musculoskeletal | 5 | (0.4%) | 7 | (0.5%) | 6 | (0.5%) | 4 | (0.3%) | 5 | (0.3%) | 4 | (0.3%) | 6 | (0.5%) |
| Other | 6 | (0.4%) | 7 | (0.4%) | 8 | (0.6%) | 4 | (0.3%) | 6 | (0.4%) | 5 | (0.4%) | 4 | (0.3%) |
| Radiographs | 783 | (55.1%) | 866 | (54.7%) | 771 | (57.0%) | 682 | (56.0%) | 870 | (51.8%) | 826 | (57.6%) | 681 | (54.3%) |
| Abdomen | 28 | (2.0%) | 32 | (2.0%) | 27 | (2.0%) | 22 | (1.8%) | 37 | (2.2%) | 25 | (1.7%) | 24 | (1.9%) |
| Breast * | 330 | (23.2%) | 375 | (23.7%) | 317 | (23.5%) | 278 | (22.9%) | 371 | (22.1%) | 351 | (24.5%) | 287 | (22.9%) |
| Chest | 195 | (13.7%) | 197 | (12.5%) | 196 | (14.5%) | 207 | (17.0%) | 220 | (13.1%) | 185 | (12.9%) | 164 | (13.0%) |
| Musculoskeletal | 357 | (25.2%) | 406 | (25.6%) | 356 | (26.4%) | 290 | (23.8%) | 379 | (22.6%) | 407 | (28.4%) | 305 | (24.3%) |
| Other | 26 | (1.9%) | 30 | (1.9%) | 22 | (1.6%) | 14 | (1.2%) | 36 | (2.1%) | 21 | (1.5%) | 34 | (2.7%) |
| Ultrasound | 271 | (19.1%) | 308 | (19.5%) | 238 | (17.6%) | 244 | (20.0%) | 344 | (20.5%) | 235 | (16.4%) | 260 | (20.7%) |
| Abdomen | 80 | (5.7%) | 87 | (5.5%) | 90 | (6.7%) | 67 | (5.5%) | 81 | (4.8%) | 96 | (6.7%) | 60 | (4.8%) |
| Breast * | 33 | (2.3%) | 34 | (2.2%) | 23 | (1.7%) | 35 | (2.9%) | 50 | (3.0%) | 27 | (1.9%) | 26 | (2.1%) |
| Cardiovascular | 111 | (7.8%) | 128 | (8.1%) | 73 | (5.4%) | 107 | (8.8%) | 168 | (10.0%) | 63 | (4.4%) | 127 | (10.1%) |
| Obstetric * | 71 | (5.0%) | 80 | (5.1%) | 81 | (6.0%) | 58 | (4.7%) | 83 | (4.9%) | 82 | (5.7%) | 46 | (3.6%) |
| Other | 24 | (1.7%) | 31 | (2.0%) | 20 | (1.5%) | 20 | (1.7%) | 23 | (1.4%) | 17 | (1.2%) | 35 | (2.8%) |
| Total | 1420 | (100%) | 1582 | (100%) | 1351 | (100%) | 1218 | (100%) | 1680 | (100%) | 1435 | (100%) | 1254 | (100%) |
Breast and obstetrical rates calculated among women.
RESULTS
Between 933 897 and 1 998 650 enrollees were included during each year of the study. The age distribution of health plan members roughly paralleled that of the states in which the members were enrolled, and 52.5% of enrollees were female (see online table). Enrollees underwent a total of 30.9 million imaging examinations during the 15-year study (25.8 million person-years), reflecting an average of 1.18 tests per person per year (95% CI 1.17 to 1.19), of which 35% were advanced diagnostic imaging (ie, CT, MRI, nuclear medicine, and ultrasound). The rates of imaging examinations per 1000 enrollees in 2008, by modality, site, and anatomic area are provided in Table 1. The total rate of imaging was 1420 examinations per 1000 enrollees per year—the most common being radiography (783/1000, 55.1% of all examinations), followed by ultrasound (271/1000, 19.1%), CT (177/1000, 12.5%), MRI (72/1000, 5.1%), angiography/fluoroscopy (64/1000, 4.5%), and nuclear medicine (53/1000, 3.7%). While the percent of examinations for each modality were relatively similar across health systems (51.8%–57.6% of examinations involved radiography across sites and 10.8%–13.9% of examinations used CT across sites), there were significant differences across health systems in the rates of imaging for each examination type. For example, the utilization of MRI ranged from 55–88 examinations/1000 enrollees (RR between highest and lowest site 1.6, [p < 0.0001]). Angiography/fluoroscopy utilization varied the most across sites (RR 4.4, p < 0.0001) and radiography had the least variation in use across sites (RR 1.3 p<0.0001).
Utilization Over Time
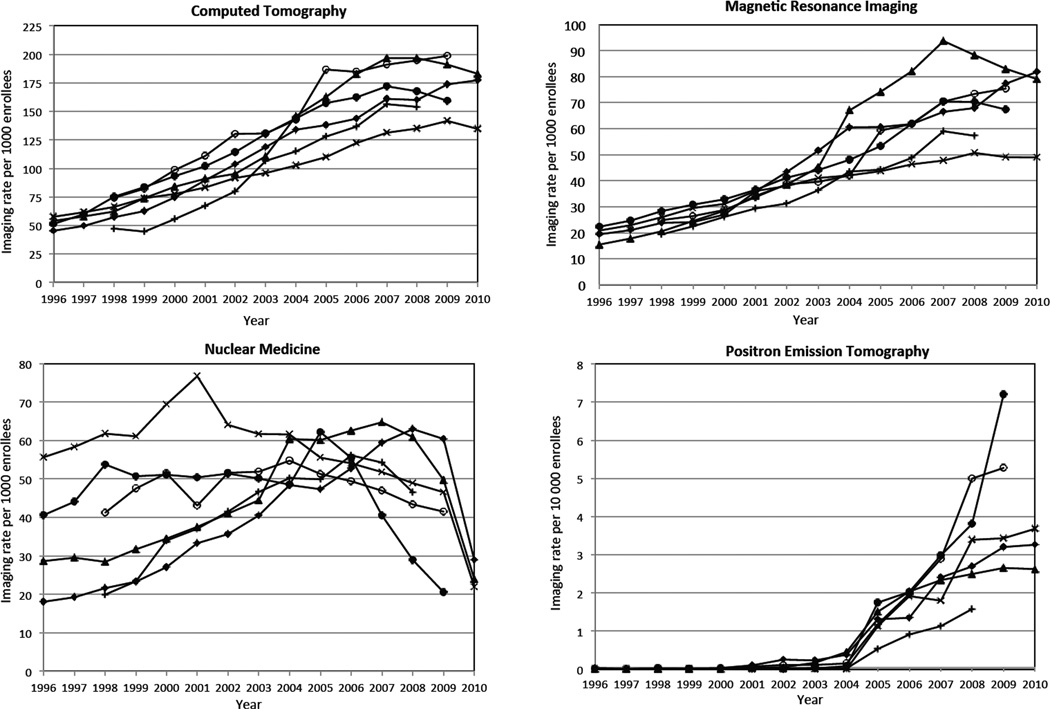
Radiography and angiography/fluoroscopy rates were relatively stable over time: radiography increased 1.2% per year, and angiography/fluoroscopy decreased 1.3% per year. In contrast, the utilization of advanced diagnostic imaging changed markedly (Figure 1). Computed tomography examinations tripled (52/1000 enrollees in 1996 to 149/1000 in 2010, 7.8% annual growth (95% CI 5.8%, 9.8%); MRI quadrupled (17/1000 to 65/1000, 10% annual growth (95% CI 3.3%, 16.5%); ultrasound approximately doubled over the same period (134/1000 to 230 /1000, 3.9% annual growth [95% CI 3.0% to 4.9%]. figure not shown). Nuclear medicine decreased (32/1000 to 21/1000, 3% annual decline (95% CI 7.7% decline to 1.3% increase), although after 2004, PET imaging rates increased from 2.2/10,000 to 23.5/10,000, 15.2% annual growth.
Figure 1.
Imaging examinations per 1000 enrollees, by modality and year, adjusted to a standard age distribution across sites and years. Each line reflects a single health plan.
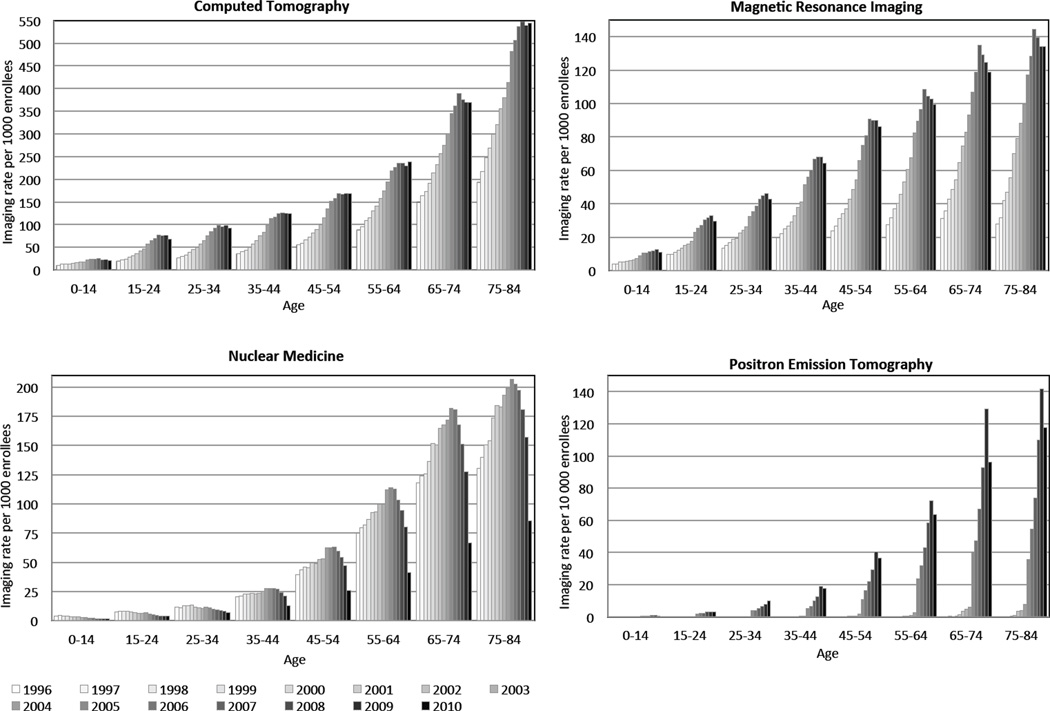
The rise in advanced diagnostic imaging with both age and year is shown in Figure 2. Diagnostic imaging increased with age, and within each age group, advanced diagnostic imaging rates increased rapidly for many years and then flattened or minimally declined in the more recent years. For CT, growth in imaging tended to flatten around 2007. For MRI, rates peaked around 2007, with slight decline in subsequent years. For nuclear medicine, a marked reduction in imaging rates occurred from 2006 onward; however, PET imaging rates increased steadily through 2009.
Figure 2.
Imaging examinations per 1000 enrollees by modality, age and year. All results adjusted to a standard age distribution. Each cluster of lines reflects changing rates of imaging over time within age strata.
Radiation Exposure and Changes Over Time
The increase in the utilization of CT resulted in an increase in enrollee exposure to radiation, with the mean per capita effective dose rising from 1.2 mSv in 1996 to 2.3 mSv in 2010. The percent of enrollees who received high (>20–50 mSv) or very high (>50 mSv) radiation exposure during a given year also approximately doubled across study years. By 2010, 2.5% of enrollees received a high annual dose of >20–50 mSv, and 1.4% received a very high annual dose of >50 mSv (Table 2).
Table 2.
Distribution in enrollees exposure to ionizing radiation by age during 1996 and 2010, and the percent of patients within each age category that received moderate, high, and very high radiation exposures. Results show among all patients and limited to patients who underwent imaging. N reflects the number of enrollees in each category.
| All Patients (Dose in milli Sieverts/Year) |
Patients Who Underwent Imaging (Dose in milli Sieverts/Year) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Low | Moderate | High | Very High | Moderate | High | Very High | |||||||||||
| 0 | > 0 – 3 mSv | > 3 – 20 mSv | > 20 – 50 mSv | > 50 mSv | > 3 – 20 mSv | > 20 – 50 mSv | > 50 mSv | |||||||||||
| Age | N | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| 1996 | ||||||||||||||||||
| 0–14 | 230,181 | 195,542 | (85.0%) | 32,076 | (13.9%) | 2,224 | (1.0%) | 265 | (0.1%) | 72 | (0.0%) | 2,224 | (6.4%) | 265 | (0.8%) | 72 | (0.2%) | |
| 15–44 | 466,078 | 367,852 | (78.9%) | 81,836 | (17.6%) | 12,626 | (2.7%) | 2775 | (0.6%) | 988 | (0.2%) | 12,626 | (12.9%) | 2,775 | (2.8%) | 988 | (1.0%) | |
| 45–64 | 267,162 | 160,025 | (59.9%) | 86,289 | (32.3%) | 14,189 | (5.3%) | 4405 | (1.6%) | 2,255 | (0.8%) | 14,189 | (13.2%) | 4,405 | (4.1%) | 2,255 | (2.1%) | |
| 65+ | 129,712 | 58,164 | (44.8%) | 49,192 | (37.9%) | 14,120 | (10.9%) | 5140 | (4.0%) | 3,095 | (2.4%) | 14,120 | (19.7%) | 5,140 | (7.2%) | 3,095 | (4.3%) | |
| ALL | 1,093,133 | 781,583 | (71.5%) | 249,393 | (22.8%) | 43,159 | (3.9%) | 12,585 | (1.2%) | 6,410 | (0.6%) | 43,159 | (13.9%) | 12,585 | (4.0%) | 6,410 | (2.1%) | |
| 2010 | ||||||||||||||||||
| 0–14 | 152,419 | 125,169 | (82.1%) | 24,878 | (16.3%) | 1,908 | (1.3%) | 358 | (0.2%) | 106 | (0.1%) | 1,908 | (7.0%) | 358 | (1.3%) | 106 | (0.4%) | |
| 15–44 | 350,268 | 263,377 | (75.2%) | 69,278 | (19.8%) | 12,063 | (3.4%) | 4014 | (1.1%) | 1,538 | (0.4%) | 12,063 | (13.9%) | 4,014 | (4.6%) | 1,538 | (1.8%) | |
| 45–64 | 303,414 | 159,381 | (52.5%) | 107,357 | (35.4%) | 20,974 | (6.9%) | 9955 | (3.3%) | 5,748 | (1.9%) | 20,974 | (14.6%) | 9,955 | (6.9%) | 5,748 | (4.0%) | |
| 65+ | 127,796 | 47,825 | (37.4%) | 48,892 | (38.3%) | 16,432 | (12.9%) | 8822 | (6.9%) | 5,824 | (4.6%) | 16,432 | (20.5%) | 8,822 | (11.0%) | 5,824 | (7.3%) | |
| ALL | 933,897 | 595,752 | (63.8%) | 250,405 | (26.8%) | 51,377 | (5.5%) | 23,149 | (2.5%) | 13,216 | (1.4%) | 51,377 | (15.2%) | 23,149 | (6.8%) | 13,216 | (3.9%) | |
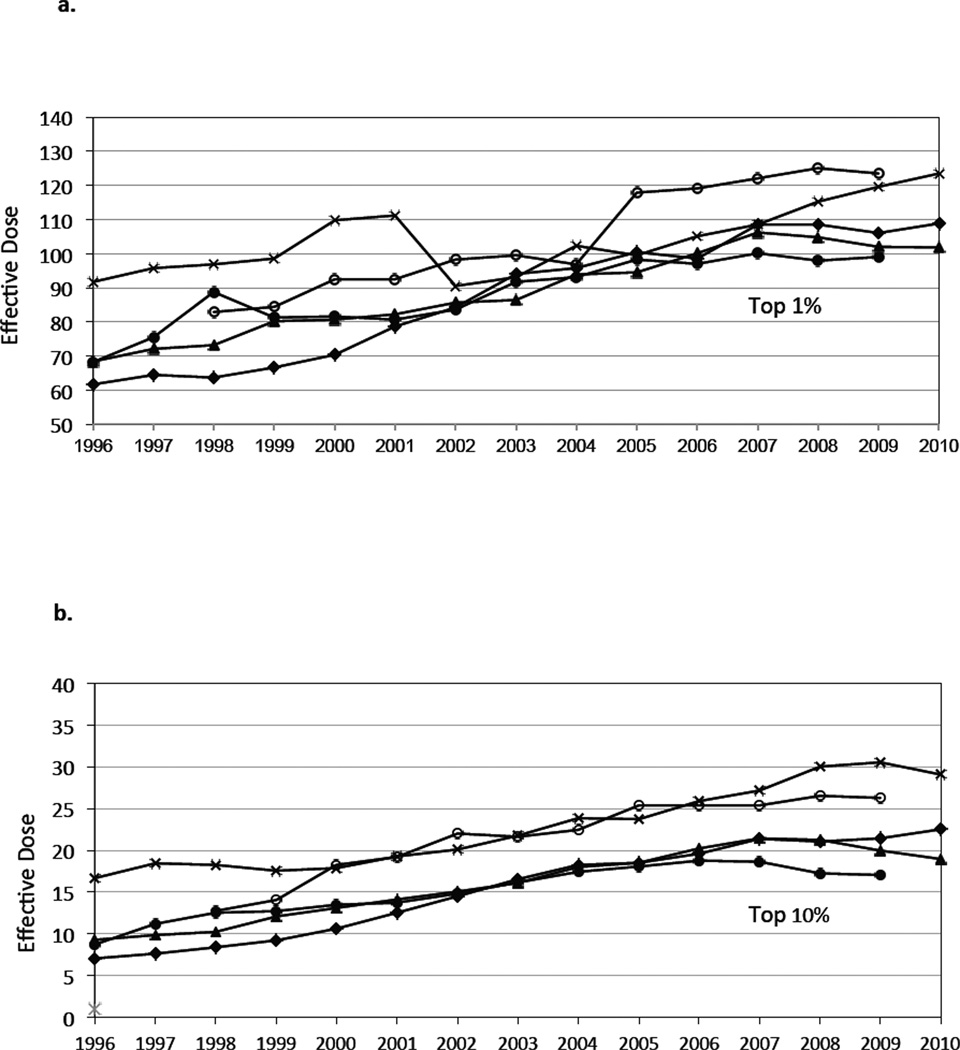
The average effective dose to those individuals who were exposed to any radiation from medical imaging increased from approximately 4.8 mSv in 1996 to 7.8 in 2010 (3.2% annual growth, 95% CI 3.1%, 3.3%). By 2010, 6.8% of patients who underwent imaging received a high dose of >20–50 mSv and 3.9% of patients received a very high dose above >50 mSv during this single year. The distribution in dose over time is shown in Figure 3. There was a gradual rise in the radiation dose received by individuals in the top 1% and 10% of those exposed. By 2010, the highest 1% of exposed individuals received around 100 mSv, and the highest 10% of exposed individuals received around 20 mSv.
Figure 3.
a. Annual average effective dose received by the highest 1% of health plan member-enrollees by health plan and year. Each line reflects a single health plan.
b. Annual average effective dose received by the highest 10% of health plan member-enrollees by health plan and year. Each line reflects a single health plan.
Footnote. The 95% confidence intervals were narrow. We calculated the average effective dose received by the highest 1% of health plan members across all sites, and the 95% confidence interval had a width < 2mSv for Figure 3a and < .1 mSv for Figure 3b.
The rise in CT accounted for the increase in the number of enrollees exposed to high (>20–50 mSv) and very high (>50 mSv) radiation exposures. In 1996, CT accounted for 5.7% of examinations and 30.3% of enrollees’ exposure to ionizing radiation while contributing to a per capita exposure of .38 mSv per enrollee. By 2010, CT accounted for 12.0% of examinations, 67.8% of radiation exposure, and contributed 1.58 mSv per enrollee (a fourfold increase in per capita radiation exposure from CT). Angiography/fluoroscopy accounted for a declining proportion of examinations (reduced from 7.4% to 4.6%) and a reduced contribution to absolute radiation exposure (reduced from .52 to .34 mSv per year), reflecting a reduction from 42.3% to 14.6% of enrollees’ total radiation exposure.
DISCUSSION
We found the growth in imaging among six large integrated HMOs was high over the last 15 years, paralleling the growth reported among fee-for-service insured populations. For example, among the HMO enrollees age 65 and older, imaging with CT grew an average of 10.2% annually between 1998 and 2005, and slowed to 4.2% annual growth from 2005 to 2008, similar to the respective 10.1% and 5.1% growth rates recently reported among Medicare fee-for-service beneficiaries during the same time periods.2,24
MRI also grew rapidly among the HMO enrollees, with 14.5% and 6.5% average annual growth in these two periods—similar to that reported for Medicare fee-for-service beneficiaries (13.5% and 2.2%, respectively).2 The rise in imaging use over this period was likely driven by many factors, including improvements in the technology that have led to expansion of clinical applications, patient and physician-generated demand, defensive medical practices,25 and medical uncertainty26,27—all factors that would be expected to influence utilization across all systems of medical care. Interestingly, strategies that have been adopted by most private commercial payers to control imaging costs, such as use of radiology benefit management firms that require preapproval or prenotification,28,29 and member copayments have not been widely adopted within these settings. Only two of these health plans have recently adopted copayments for advanced imaging (one at $10 and one at $50). While several plans have recently adopted decision support software, it is too early to assess whether greater adherence to appropriateness criteria included with the software products may influence utilization rates.
Although growth in imaging was similar between HMO members and Medicare fee-for-service insured beneficiaries, the rates of imaging seem to be modestly lower among HMO enrollees. For example, in 2006, HMO enrollees age 65 and older underwent 474 CTs and 123 MRIs per 1000 enrollees, whereas Medicare fee-for-service enrolled adults underwent 550 CTs and 192 MRIs, 15% and 35% lower rates, respectively.2 While some of this difference may be due to underlying geographic variation in imaging rates (we included six HMOs where the cited numbers were based on a larger national sample)9,30 and due to possible differences in age and health status among HMO enrollees (who may be healthier than non-HMO enrollees), imaging rates do seem to be lower within the included HMO settings.
We found that imaging rose steeply with age, particularly for CT and nuclear medicine examinations, resulting in high radiation exposures received by the oldest enrollees. Among enrollees age 45 and above who underwent imaging, nearly 20% received high or very high radiation exposure annually. Although cancer risks from radiation are often considered to decline with age, recent models suggest that cancer risks decline with age until middle age, when cancer risks may then increase in a U-shaped distribution.6,31 Thus radiation related cancer risks after exposure in middle and older ages may be higher than previously believed.26 Since the utilization of imaging is higher in older adults, and since the potential harm from these tests may also be higher in these patients, it is particularly important to quantify the benefits of imaging in these patients
We found the per capita exposure to radiation from diagnostic imaging was 2.7 mSv in 2006, similar to the annual per capita exposure reported by the National Council on Radiation Protection (NCRP), describing the entire US population32 (3.0 mSv), and Fazel and colleagues,11 describing a fee-for-service insured population (2.4 mSv). A notable difference is that we found a significantly larger number of patients received very high radiation exposures annually. For example, Fazel reported that 0.2% of insured individuals incurred a very high (>50 mSv) annual radiation dose, as contrasted to our finding that 1.4% of enrollees received such high exposures—a seven-fold increase. As shown in Figure 4, a significant number of future cancers are likely to result from these exposures. There are several possible reasons for this difference, including that we did not assume that every test delivered the same radiation exposure but rather modeled the actual distribution in dose when estimating the proportion of patients with high exposures. Our work adds to the work of the NCRP and Fazel by assessing doses limited to enrollees who underwent imaging, as it is only these individuals who are at risk of radiation-related carcinogenesis. By 2010, 10.8% of enrollees who underwent imaging received an annual exposure greater than 20 mSv. It is notable that even when limited to patients who were imaged during both time periods, the average dose per person nearly doubled, suggesting more intensive medical imaging among those who undergo any imaging.
In order to further help put these typical patient doses into context, 20 mSv is the annual allowable occupational exposure to radiation in Europe,33,34 and 50 mSv is the annual allowable occupational exposure in the United States.35 While it is not appropriate to set exposure limits when radiation is required for health benefit, the number of patients exposed to such levels highlights the need to consider this potential harm when ordering imaging tests, and to track radiation exposures for individual patients so that this information is available to physicians who are ordering tests. The National Academy of Sciences’ National Research Council concluded, after a comprehensive review of the published literature, that patients who receive radiation exposures in the same range as a single CT—10 mSv—may be at increased risk of developing cancer;6,36,37 16.5% of patients who underwent imaging in 2010 received a dose at least this high.
There is growing concern about the geographic variation in health care utilization, noted to be particularly high for diagnostic imaging,30,38 raising concerns that use in some areas may be inappropriate. We found even greater variation in imaging rates among these health systems than reported for Medicare fee-for-service enrollees.30,38 For example, we found over a ten-fold difference in cardiovascular imaging rates across sites, using both conventional angiography and CT. The rate of cardiovascular angiography ranged from 7 to 79 examinations per 1000 enrollees, and the rate of cardiovascular CT ranged from 1 to 16 examinations per 1000 enrollees. In clinical settings where imaging is associated with clear clinical benefit, radiation exposure and its small attendant risk are likely outweighed by the benefit. However, the threshold for using advanced diagnostic imaging has been lowered in part due to nonclinical reasons (ie, financial incentives, defensive test ordering due to malpractice fears, intolerance for uncertainty and strong patient demand), and the profound variation in imaging rates we found is likely driven by many of these factors. In these settings, quantifying the potential risks of imaging, and discussing potential harm of imaging with patients, including the risks associated with radiation, can lead to more appropriate and balanced use of imaging. If such variation occurs among these non-profit organizations without physician financial incentives to order these tests, it becomes even more important to study the benefits and harms of these tests for various indications in order to learn what usage is appropriate.
We did not assess costs for imaging within these integrated settings. Costs for imaging among fee-for-service insured elderly adults have declined since 2005, despite increasing utilization.10,28 As part of the Deficit Reduction Act of 2005, Congress enacted a provision to equalize the reimbursement rate for imaging examinations regardless of where they were performed; among fee-for-service Medicare enrollees, a 12.7% reduction in imaging costs followed enactment.28 Because of bundled payments for imaging within our integrated settings, these types of per-examination reductions in payment would not be expected to have had the same impact on utilization as they have in the fee-for-service environment.13,28
The HMO Research Network that we relied upon provides a unique opportunity to conduct analyses of patterns of imaging because of the complete capture of health care utilization by their members, including all diagnostic testing, standardization of how these data are collected, and storage of detailed imaging records so that actual radiation exposures could be measured. However, there are several limitations of our work. We focused on individuals enrolled in comprehensive health care plans, and excluded data from fee-for-service enrollees due to incompleteness of the available data. For inpatient imaging examinations, only the admission date was available to us, thus collapsing claims could lead to undercounting of multiple exams performed during the same hospital stay. Similarly, for patients who underwent multiple examinations with the same procedure code on a single day were only counted once, and for these patients we have likely under-estimated their exposures. We limited assessment to beneficiaries enrolled throughout a given year, and imaging may differ for patients who dis-enroll. To assess medical radiation dose, we used an estimate of the dose for each patient based on a sampling of high dose studies, but we did not use actual dose information for each individual patient examination, as these data are not routinely stored in an easily accessible format. To account for underlying variations in dose, we included an estimate of the variation in dose when estimating the number of individuals above dose thresholds, and we believe our estimates to be conservative, as they do not allow for any within-person correlation in the random deviation. We only evaluated cumulative exposure within each year and not cumulative exposure over time. We did not study cumulative exposures because of the fluidity of enrollment over time. We used effective dose as the measure to summarize multiple exposures and this measure is imprecise, particularly when trying to sum across different anatomic areas that might be imaged. No alternative measure exists, and this measure will almost certainly capture those patients receiving high exposures.39 We only assessed radiation exposure as a potential harm of testing, but there are several other potential harms associated with imaging, such as false positive test results that may begin a cascade of unnecessary testing, and over-diagnosis of otherwise indolent disease that leads to unnecessary treatment.
The growth in advanced diagnostic imaging has almost certainly contributed to both improved patient care processes and outcomes, but there are remarkably few data to quantify the benefits of imaging. Given the high costs of imaging—estimated at $100 billion annually—and the potential risks of cancer and other harms, these benefits should be quantified and evidence-based guidelines for using imaging should be developed that very clearly balance benefits against financial costs and health risk.
Supplementary Material
Table 3.
Contribution of different modalities to the total number of tests and percent of exams, and contributing to enrollee's annual per capita radiation exposure and percent of exposure (in mSv).
| Number and Percent of Exams per 1000 Enrollees |
Average per Capita Radiation Dose (mSv) and Percent of Dose by Modality |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1996 | 2003 | 2010 | 1996 | 2003 | 2010 | |||||||
| N | (%) | N | (%) | N | (%) | mSv | (%) | mSv | (%) | mSv | (%) | |
| Angiography/Fluroscopy | 68 | (7.4%) | 64 | (5.8%) | 57 | (4.6%) | 0.52 | (42.3%) | 0.49 | (26.5%) | 0.34 | (14.6%) |
| Computed Tomography | 52 | (5.7%) | 108 | (9.7%) | 149 | (12.0%) | 0.38 | (30.3%) | 0.90 | (48.3%) | 1.58 | (67.8%) |
| Magnetic Resonance Imaging | 17 | (1.9%) | 41 | (3.7%) | 65 | (5.2%) | * | * | * | |||
| Nuclear Medicine | 32 | (3.5%) | 45 | (4.0%) | 21 | (1.7%) | 0.17 | (13.3%) | 0.26 | (13.9%) | 0.19 | (8.1%) |
| Radiographs | 610 | (66.8%) | 668 | (60.1%) | 722 | (58.0%) | 0.17 | (14.0%) | 0.21 | (11.4%) | 0.22 | (9.5%) |
| Ultrasound | 134 | (15.0%) | 186 | (16.7%) | 230 | (18.5%) | * | * | * | |||
| Total | 914 | (100%) | 1,112 | (100%) | 1,245 | (100%) | 1.24 | (100%) | 1.86 | (100%) | 2.34 | (100%) |
MRI and ultrasound do not use ionizing radiation.
Acknowledgments
Funding/Support: This study was supported by the National Cancer Institute-funded Cancer Research Network Across Health Care Systems (U19CA79689), NIH R21CA131698 and NIH K24 CA125036. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health. The funding organizations had no role in the design and conduct of the study; collection management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Additional Contributions: We thank the following people for their valuable assistance in gathering radiology data for this study: Julianne Endres and Deborah Seger BA of Group Health Research Institute, Brenda Rush RN and Donna Gleason of Kaiser Permanente Northwest; Autumn Deedon and Paul Hitz BS of Marshfield Clinic Research Foundation; Kimberly Bischoff MSHA from Kaiser Permanente Colorado; Ann Hanson BS of Health Partners Research Foundation; Peter Joski, MSPH, of Kaiser Permanente Georgia; and Aileen Uchida MPH and Mark Schmidt BA of Kaiser Permanente Hawaii. All individuals listed were compensated for their work.
Footnotes
Author Contributions: Dr Smith-Bindman had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Smith-Bindman, Miglioretti, Greenlee, and Solberg.
Acquisition of data: Smith-Bindman, Miglioretti, Johnson, Feigelson, Flynn, Greenlee, Kruger, Lee, Solberg, Vanneman, Weinmann, and Williams.
Analysis and interpretation of data: Smith-Bindman, Miglioretti, Greenlee, Hornbrook, Johnson, Kruger, Lee, Roblin, Solberg, Weinmann, and Williams.
Drafting of the manuscript: Smith-Bindman, Hornbrook, Johnson, Lee, Miglioretti, Roblin
Critical revision of the manuscript for important intellectual content: Smith-Bindman, Miglioretti, Johnson, Feigelson, Flynn, Greenlee, Hornbrook, Kruger, Lee, Miglioretti, Roblin, Solberg, Vanneman, Weinmann, and Williams.
Statistical analysis: Smith-Bindman, Miglioretti, Johnson, Lee, and Hornbrook.
Obtained funding: Smith-Bindman and Miglioretti
Administrative, technical, or material support: Smith-Bindman, Feigelson, Flynn, Kruger, Vanneman, Weinmann, and Williams.
Study supervision: Smith-Bindman, Miglioretti, Roblin, and Vanneman.
Conflict of Interest Disclosure: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Sponsor: None
Online-Only Material: Online Table. Number of enrollees across health plans by age during 2004.
References
- 1.Statement of Glenn M. Hackbarth, Chairman, Medicare Payment Advisory Commission, Testimony Before the Subcommittee on Health of the House Committee on Ways and Means, May 10, 2007. [accessed July 30, 2008]; available online http://waysandmeans.house.gov/hearings.asp?formmode=printfriendly&id=5893.
- 2.Government Accountability Office. Report to Congressional requesters: Medicare Part B imaging services: rapid spending growth and shift to physician offices indicated need for CMS to consider additional management practices. In: Office GA, editor. Vol. Washington, DC: 2008. GAO-08-452. [Google Scholar]
- 3.Winter A, Stensland J. Introduction to expert panel on new research on use of imaging services: presentation to Medicare Payment Advisory COmmission. Washingto, DC: 2008. Spetember. 2008. [Google Scholar]
- 4.Iglehart JK. Health insurers and medical-imaging policy--a work in progress. N Engl J Med. 2009 Mar 5;360(10):1030–1037. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 5.Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, D.C.: The National Academies Press; 2006. Board of Radiation Effects Research Division on Earth and Life Sciences National Research Council of the National Academies. [PubMed] [Google Scholar]
- 6.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007 Jul;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 7.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009 Dec 14;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 9.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology. 2005 Mar;234(3):824–832. doi: 10.1148/radiol.2343031536. [DOI] [PubMed] [Google Scholar]
- 10.Levin DC, Rao VM, Parker L. Physician orders contribute to high-tech imaging slowdown. Health Aff (Millwood) 2010 Jan-Feb;29(1):189–195. doi: 10.1377/hlthaff.2009.0528. [DOI] [PubMed] [Google Scholar]
- 11.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009 Aug 27;361(9):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham S. Imaging is everything. Diagnostic imaging took off a few years ago. Get ready for the next wave of growth. Monday, February 28, 2005, 12:00am EST - Last Modified: Thursday, February 24, 2005, 2:37pm EST. Baltimore Business Journal. 2005 [Google Scholar]
- 13.Hackbarth G, Reischauere R, Mutti A. Collective acountability for medical care-toward bundled Medicare payments. N Engl J Med. 2008;359:3–5. doi: 10.1056/NEJMp0803749. [DOI] [PubMed] [Google Scholar]
- 14.Shortell SM, McCurdy RK. Integrated health systems. Stud Health Technol Inform. 2010;153:369–382. [PubMed] [Google Scholar]
- 15.Lieu TA, Hinrichsen VL, Moreira A, Platt R. Clin Med Res; Collaborations in population-based health research: the 17th annual HMO Research Network Conference; March 23–25, 2011; Boston, Massachusetts, USA. 2011. Nov, pp. 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornbrook MC, Hart G, Ellis JL, et al. Building a virtual cancer research organization. J Natl Cancer Inst Monogr. 2005;(35):12–25. doi: 10.1093/jncimonographs/lgi033. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Bindman R, Miglioretti D, Larson E. Rising Use of Diagnostic Medical Imaging in an Integrated Health Plan. Health Affairs. 2008;26(6):1491–1502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Lodwick D, Hurtado J, Pafundi D, Williams JL, Bolch WE. The UF family of reference hybrid phantoms for computational radiation dosimetry. Phys Med Biol. 2010 Jan 21;55(2):339–363. doi: 10.1088/0031-9155/55/2/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Kim KP, Long D, et al. Organ doses for reference adult male and female undergoing computed tomography estimated by Monte Carlo Simulations. Med Phys. 2011 Mar;38(3):196–206. doi: 10.1118/1.3544658. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protection ICoR. Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP. 2007;37(2–4) doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Murray IPC, Ell PJ, Van der Wall H. Nuclear medicine in clinical diagnosis and treatment. 2nd ed. Edinburgh; New York: Churchill Livingstone; 1998. [Google Scholar]
- 22.Hendrick RE, Pisano ED, Averbukh A, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic imaging screening trial. AJR Am J Roentgenol. 2010 Feb;194(2):362–369. doi: 10.2214/AJR.08.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999 Jun;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 24.Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Bending the curve: the recent marked slowdown in growth of noninvasive diagnostic imaging. AJR Am J Roentgenol. 2011 Jan;196(1):W25–W29. doi: 10.2214/AJR.10.4835. [DOI] [PubMed] [Google Scholar]
- 25.Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005 Jun 1;293(21):2609–2617. doi: 10.1001/jama.293.21.2609. [DOI] [PubMed] [Google Scholar]
- 26.Redberg RF, Walsh J. Pay now, benefits may follow--the case of cardiac computed tomographic angiography. N Engl J Med. 2008 Nov 27;359(22):2309–2311. doi: 10.1056/NEJMp0805920. [DOI] [PubMed] [Google Scholar]
- 27.Douglas PS. Improving imaging: our professional imperative. J Am Coll Cardiol. 2006 Nov 21;48(10):2152–2155. doi: 10.1016/j.jacc.2006.04.107. [DOI] [PubMed] [Google Scholar]
- 28.Government Accounting Office. Medicare: Trends in Fees, Utilization, and Expenditures for Imaging Services before and after Implementation of the Deficit Reduction Act of 2005. 2008 Sep 26; GAO-08-1102R.
- 29.Levin DC, Bree RL, Rao VM, Johnson J. A prior authorization program of a radiology benefits management company and how it has affected utilization of advanced diagnostic imaging. J Am Coll Radiol. 2010 Jan;7(1):33–38. doi: 10.1016/j.jacr.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Parker L, Levin DC, Frangos A, Rao VM. Geographic variation in the utilization of noninvasive diagnostic imaging: national medicare data, 1998–2007. AJR Am J Roentgenol. 2010 Apr;194(4):1034–1039. doi: 10.2214/AJR.09.3528. [DOI] [PubMed] [Google Scholar]
- 31.Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010 Nov 3;102(21):1628–1636. doi: 10.1093/jnci/djq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Council on Radiation Protection and Measurements. NCRP Report No 160, Ionizing Radiation Exposure of the Population of the United States, press release available, full report soon to be released. [Accessed March 5, 2009];2009 http://www.ncrponline.org/. [Google Scholar]
- 33.International Atomic Energy Agency. International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. Vol. 115. IAEA; 1996. ISSN 0074-1892; Safety standards Vienna. [Google Scholar]
- 34.International Committee on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. Oxford, England: Pergamom; 1991. ICRP publication No 60. [Google Scholar]
- 35.United States Nuclear Regulatory Commission. United States Nuclear Regulatory Commission, NRC Regulations, Title 10, Code of Federal Regulations, Part 20 - Standards for Protection Against Risks, Subpart C-Occupational Dose Limits. [Accessed September 27, 2011]; http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-1201.html.
- 36.Charles M. UNSCEAR report 2000: sources and effects of ionizing radiation. United Nations Scientific Comittee on the Effects of Atomic Radiation. J Radiol Prot. 2001 Mar;21(1):83–86. doi: 10.1088/0952-4746/21/1/609. [DOI] [PubMed] [Google Scholar]
- 37.Board of Radiation Effects Research Division on Earth and Life Sciences National Research Council of the National Academies. Committee on the Biological Effects of Ionizing Radiation, Health Effects of Exposure to Low Levels of Ionizing Radiation: BEIR V. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 38.Report to the Congress: Variation and Innovation in Medicine. Medicare Payment Advisory Commission. Washington, DC: 2003. Jun, [Google Scholar]
- 39.Smith-Bindman R, Miglioretti D. CTDIvol, DLP, and effective dose as excellent measures for use in CT quality improvement. Radiology. 2011;261(3):999. doi: 10.1148/radiol.11111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.