Abstract
Rationale
It is important to study age-related differences that may put adolescents at risk for alcohol-related problems. Adolescents seem less sensitive to the aversive effects of ethanol than adults. Less is known of appetitive effects of ethanol and stress-modulation of these effects.
Objectives
To describe effects of acute social or restraint stress on ethanol-precipitated locomotor activity (LMA), in adolescent and adult rats. Effects of activation of the kappa system on ethanol-induced LMA were also evaluated.
Methods
Adolescent or adult rats were restrained for 90 min, exposed to social deprivation stress for 90 or 180 min or administered the kappa agonist U62,066E before being given ethanol and assessed for LMA.
Results
Adolescents were significantly more sensitive to the stimulating, and less sensitive to the sedative, effects of ethanol than adults. Basal locomotion was significantly increased by social deprivation stress in adult, but not in adolescent, rats. U62,066E significantly reduced basal and ethanol-induced locomotion in the adolescents. Corticosterone and progesterone levels were significantly higher in adolescents than in adults.
Conclusions
Adolescents exhibit greater sensitivity to ethanol-induced LMA and reduced sensitivity to ethanol-induced motor sedation than adult rats. Ethanol’s effects on motor activity were not affected by acute stress. Unlike adults, adolescents were insensitive to acute restraint and social deprivation stress, but exhibited motor depression after activation of the endogenous kappa opioid receptor system.
Keywords: adolescent, adult, locomotor activation, rat, stress, kappa opioid system
Introduction
Studies have indicated that adolescent rats are less sensitive than adults to ethanol-induced narcosis (Silveri and Spear 1998) and motor impairment (Little et al. 1996). Likewise, administration of low to moderate ethanol doses induces social facilitation in adolescents, whereas ethanol-induced reductions in social behavior are more prevalent at these doses in adults (Varlinskaya and Spear 2002). This pattern of response to ethanol in adolescents may put them at risk for the development of alcohol-related problems (Doremus-Fitzwater et al., 2010; Spear & Varlinskaya, 2010).
Seeking and intake of ethanol are mainly modulated by the balance between the appetitive, aversive and anxiolytic effects of ethanol (Roma et al. 2008). When compared to adults, adolescents display a lessened sensitivity to the aversive effects of ethanol when indexed via ethanol-induced conditioned taste aversions (CTA) (Anderson et al. 2010). Although stressors and the endogenous kappa opioid receptor system (KOR) are thought to play a role in the aversive effects of ethanol (e.g., Pautassi et al. 2012), kappa opiate receptor manipulations (Anderson et al. 2012) and repeated restraint were not found to differentially affect these age differences in ethanol CTA. Ethanol, however, was found to reverse the anxiogenic effect of restraint stress in adolescent, but not in adult, rats (Varlinskaya and Spear 2010, 2012). Little is known about age-, stressor- and kappa-related influences on the appetitive effects of ethanol.
Ethanol-induced acute locomotor activity (LMA) can be considered a measure, albeit indirect, of ethanol-induced appetitive reinforcement (Pautassi et al. 2009). Studies have shown that infant and adolescent, but not adult, rats exhibit ethanol-induced LMA (Acevedo et al. 2010, 2012; Arias et al. 2008, 2009; Masur et al., 1986) and that these acute motor effects of ethanol are modulated by treatments that affect ethanol-induced motor behavior sensitization in mice. These studies suggest that adolescent rats may be, when compared to adults, more sensitive to ethanol’s motor activating and less sensitive to ethanol’s motor depressing effects. These hypotheses are based on across-study comparisons and have yet to be systematically tested in rats within the same study and experimental paradigm.
Stress exposure is an important factor leading to drug use, abuse and relapse; and some studies indicate age-related differences in sensitivity to stress. For instance, social deprivation radically enhanced play-fighting behavior in adolescents, but only mildly enhanced this behavior in older animals (Doremus-Fitzwater et al. 2009; Varlinskaya and Spear 2006). The mere stress associated with repeated daily injections significantly reduced social investigation in adolescent but not in adult subjects (Varlinskaya and Spear 2010). These and other studies (e.g., Doremus-Fitzwater et al. 2009; Varlinskaya & Spear 2010) suggest that adolescents are more vulnerable than adults to stress under some circumstances. These studies, however, analysed only effects of chronic, repeated stress exposure. Chronic stress studies may reflect proximal effects of the last stress exposure, accumulated effects of stress, adaptations that take place after repeated stress or any combination of these factors. It is therefore important to study acute consequences of stressors, effects that may affect later responsivity to other stressors or to drugs of abuse (Perkins & Grobe, 1992). Response to acute, moderate stress has been recognized as a precipitator of drug-induced relapse (Self & Nestler, 1998) and regulates response to ethanol differentially in adolescent and adult rats (Varlinskaya & Spear, 2012). Moreover, recent experimental work revealed that acute, mild stress affected processing of alcohol-related cues in social drinkers (Ceballos et al., 2012). Effects of acute stress on ethanol’s consequences across age have been, however, scarcely analyzed. Varlinskaya and Spear (2012) found that anxiety induced by acute stress was inhibited by ethanol in adolescent but not adult rats. This result suggests that adolescent rats are more sensitive to the anxiolytic effects of ethanol than adult counterparts. It is unknown if acute stress modulates appetitive effects of ethanol and whether this effect is age-dependent.
The present study aimed at analyzing ethanol-induced LMA in adolescent and adult rats and the modulatory role of social and restraint acute stress on these ethanol effects. Animals were acutely restrained, exposed to social deprivation, or left unmanipulated prior to testing. They were subsequently given varying doses of ethanol and assessed for ethanol-induced LMA (Exp. 1a and 2). The hypotheses were that treatment with ethanol would induce greater motor stimulation but less motor sedation in adolescents than in adults; and that stress would exacerbate ethanol-induced stimulation, perhaps significantly more in adolescents. Blood and brain ethanol levels (BECs and BrECs, respectively) and corticosterone and progesterone levels (CORT and PROG, respectively) were measured in Experiment 1b.
Effects of activation of the kappa system on ethanol-induced LMA were also evaluated (Exp. 3). Kappa agonists substitute for stress (McLaughlin et al. 2006), and reduce motor activity in adults (Ukai and Kameyama 1985) but increase it in infant rats (Duke et al. 1997; Pautassi et at. 2012). The effects of KOR activation on spontaneous and ethanol-induced LMA in adolescence are unknown.
Methods and General Procedures
Subjects
A total of 528 Sprague–Dawley adult and adolescent male rats, representative of 129 litters, were employed (Experiment 1a: 288 animals, 64 litters; Experiment 1b: 22 animals; 24 litters; Experiment 2: 138 animals; 29 litters; Experiment 3: 80 animals; 12 litters). Rats were born and reared in breeding facilities at Binghamton University (Binghamton, NY, USA), as described in Pautassi et al. (2012). Weaned animals were housed with 4–5 littermates until PD 42, when they were housed in pairs. Experimental procedures began on postnatal day (PD28, adolescents) or PD70 (adults).All experiments were approved by the Binghamton University Institutional Review Committee for the Use of Animal Subjects and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Drug preparation and administration procedures
Ethanol doses of 0.0, 1.25, 2.5 or 3.5 g/kg were administered intragastrically (i.g.), as described in Pautassi et al., (2008). The KOR agonist spiradoline mesylate (U62,066E; Sigma-Aldrich, St. Louis, MO) was administered intraperitoneally (i.p.) at doses of 0.0, 1.0 or 5.0 mg/kg/10ml. These doses were chosen based on a previous study (Pautassi et al., 2012). Tap water and saline were used as vehicle.
Stress Exposure
On PD28 or PD70, animals from the acute restraint stress group were removed from their home cage and confined for 90 minutes in restraint tubes made of clear acrylic (Braintree Scientific, Braintree, MA) (Experiment 1), as described in Varlinskaya and Spear (2012). Animals in the social deprivation group were removed from their homecage and individually placed in a new cage (standard maternity cage with fresh bedding) for 90 (Experiments 1a and 2) or 180 (Experiment 2) minutes. Adolescent and adults assigned to the non-manipulated condition remained in their holding cages with a littermate until the ethanol challenge. These moderate stressors were chosen based on the notion that validity of stress-related research can be enhanced through animal models that rely on ethologically relevant situations (Miczek et al., 2008). Social stress should be particularly relevant in adolescent rats because they are particularly sensitive to social interactions at that age (e.g., Douglas et al, 2004). It has been suggested that moderate stressors induce behavioral activation, and facilitate drug intake; whereas more intense stress results in behavioral suppression and less engagement in drug taking (Miczek et al., 2008).
Assessment of ethanol-induced locomotor activity (LMA) in adolescent and adult rats
Immediately after stress exposure all animals were given ethanol (1.25, 2.5 or 3.5 g/kg; i.g.) or vehicle (0.0 g/kg) and returned to their holding cages until the onset of testing 5 minutes later. For testing, each animal was individually placed in the center of a 40 X 40 X 30 cm open field and its activity recorded over 15 minutes (post-administration time of 5–20 min). Total distance travelled (cm) was measured in a minute-by-minute basis using a VersaMax Animal Activity Monitoring System (Accuscan Instruments, Columbus, OH), as described in Pautassi et al., 2012.
Determination of Blood and Brain Ethanol Concentrations and Corticosterone and Progesterone Levels (Experiment 1b and 3)
In Experiment 1b, adolescent and adult rats were decapitated at 7.5 min after ig. administration of 3.5 g/kg ethanol, trunk blood samples collected using a plastic heparinized tube and brains quickly removed. Blood and brain samples were frozen and stored at −80 °C until BECs and BrECs were determined as previously described (Silveri and Spear 2000) via headspace gas chromatography (HP 5890 series II Gas Chromatograph, Wilmington, DE). In Experiment 3, trunk blood samples (200µl) were collected for analysis of BECs immediately after the assessment of ethanol-induced LMA. Samples were centrifuged for 20 min at 3000 rpm and then analyzed via an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) as described in Pautassi et al. (2008). BECs and BrECs scores were expressed as mg/dl (mg%). All animals were given high-dose ethanol prior to hormonal measurements, thus an effect of ethanol on stress could not be assessed. For hormone assays samples were centrifuged at 2°C for 20 min at 3000 rpm and plasma aliquots collected and stored at −80°C. Plasma CORT levels were determined by radioimmunoassay using RIA kits (ICN Biomedicals, Inc.; Orangeburg, NY).
Experimental designs
Experiment 1a assessed the effects of stress on sensitivity to ethanol-induced LMA. A 2 (age: adolescents or adults) × 3 (stress exposure: non-manipulated control, 90 minutes of acute restraint stress or social deprivation stress) × 4 (ethanol dose: 0.0, 1.25, 2.5 or 3.5 g/kg) factorial design was used, with 12 animals in each group.
Experiment 1b measured BECs and BrECs, CORT and PROG levels in groups (n = 7–8) of adolescents and adults exposed to 90 min of social deprivation and in a group of non-manipulated adolescents. All animals had been given 3.5 g/kg ethanol 7.5 min before trunk blood collection. Experiment 2 assessed the effects of varying the length of social deprivation stress on LMA in adolescent and adult male rats treated with the higher dose of ethanol. A 2 (age: adolescents or adults) × 3 (stress exposure: 0 [non-manipulated control], 90 or 180 minutes of social deprivation before test) × 2 (ethanol dose: 0.0 or 3.5 g/kg) factorial design was used, with 12 and 11 animals in each adolescent and adult group, respectively.
Experiments 1 and 2 indicated that adolescents were relatively insensitive to social deprivation and restraint stress. Experiment 3 explored reactivity to pharmacological stress at adolescence. Specifically, Experiment 3 assessed the effects of U62,066E, a potent kappa opioid receptor agonist, on subsequent ethanol-induced LMA in adolescent rats. A 3 (U62,066E dose: 0.0, 1.0 or 5.0 mg/kg) × 2 (ethanol dose: 0.0 or 2.5 g/kg) factorial was used, with 9–10 animals in each group. Ethanol-treated animals were assessed for BECs.
Data analysis
Preliminary ANOVAs indicated significant age-related differences in basal and ethanol-induced motor activity. Specifically, a four-way mixed ANOVA conducted on locomotor activity scores from Experiment 1a (Age × Ethanol dose × Stress exposure × Time at test) revealed a significant main effect of Age (F1, 264 = 11.09, p < 0.001), a significant Age × Ethanol dose interaction (F3, 264 = 17.40, p < 0.001), and a significant Age × Ethanol dose × Time at test interaction (F42, 3696 = 3.48, p < 0.001). Posthocs indicated that adults exhibited significantly greater overall locomotion scores than adolescents. Moreover, post-hocs indicated that adolescent but not adult rats exhibited ethanol-induced motor activation when compared with their vehicle-treated counterparts, a phenomenon that was significantly more pronounced during the first 5-min of testing. Age-related differences in ethanol-induced LMA at this time were further analyzed through z-scores, with separate transformations conducted at each age using the appropriate 0.0 g/kg group as the reference group. An Age and Ethanol dose factorial ANOVA of these standardized values likewise revealed a significant main effect of Age and a significant Age × Ethanol dose interaction, F1, 210 = 81.16, p < 0.001, F2, 210 = 4.56, p < 0.05, with adolescents again significantly more sensitive to ethanol-induced locomotion than adults, age differences that increased with dose. Mean (± SEM) standardized activity scores in adolescents and adults were as follows: 1.83±0.28, 2.76±0.32, 3.03±0.73 and 0.04±0.20, 0.05±0.27,−1.10±0.26; for animals given 1.25, 2.5 and 3.5 g/kg ethanol, respectively. Based on these baseline differences and using the strategy followed by Varlinskaya and Spear (2012), subsequent analyses were separately conducted at each age.
Ethanol-induced locomotor activity (distance travelled in a minute-by-minute basis) was analyzed at each age by separate three-way mixed-factor ANOVAs. In Experiment 1a and 2, the between group factors were Stress exposure (non-manipulated, 90 min acute restraint stress or 90 min social deprivation) and Ethanol dose (0.0, 1.25, 2.5 or 3.5 g/kg in Experiment 1; 0.0 or 3.5 g/kg in Experiment 2). In Experiment 3 U62,066E (0.0, 1.0 or 5.0 mg/kg) and Ethanol dose (0.0 or 2.5 g/kg) were used as between factors. Time at test (min 5 to 19 post-administration) was the within-group factor.
A one-way ANOVA was conducted to analyze BECs, BrECs, CORT and PROG levels in Experiment 1b. The three groups compared were adolescents and adults exposed to 90 min of social deprivation and a group of control, non-manipulated adolescents. Blood ethanol concentrations in Experiment 3 were analyzed through a one-way ANOVA (grouping factor: U62,066E dose).
The loci of significant main effects or significant interactions were analyzed through Tukey’s post hoc tests or planned comparisons. Alpha level was p < .05 in all experiments.
Results
Effects of ethanol and stress on locomotor activity (LMA) in adolescent and adult rats (Experiment 1a)
Adolescents
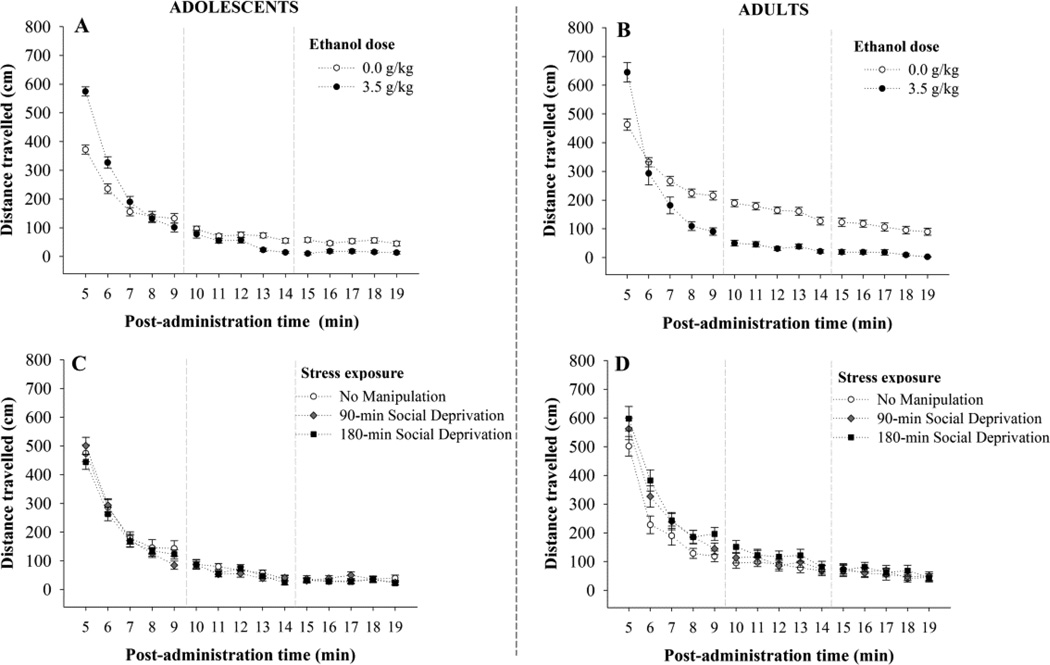
As shown in Figure 1, panel A, adolescents exhibited dose-response, ethanol-induced LMA. These activating effects of ethanol were more pronounced during the initial testing minutes. Stress exposure did not significantly affect spontaneous or ethanol-induced locomotion. The statistical analysis yielded significant main effects of Ethanol dose and Time at test (F3, 132 = 3.19 and F14, 1848 = 451.72; respectively, ps < .01) and a significant interaction between these factors (F42, 1848 = 9.11, p < .01). Planned comparisons indicated that animals given 3.5 or 2.5 g/kg exhibited significantly higher LMA scores than vehicle-treated controls during the first five testing minutes (i.e. post-injection minutes 5–9), whereas those given 1.25 g/kg ethanol exhibited significantly greater activity than controls during minutes 5 to 7. Animals treated with the highest ethanol dose showed significantly greater LMA than those given 1.25 g/kg during minutes 5 and 6 post-administration and significantly greater LMA than animals given 2.5 g/kg during minute 5. There were no significant main effects or significant interactions involving Stress. Locomotion scores in adolescents as a function of Stress conditions are depicted in Figure 1, panel C.
Figure 1.
Effects of Ethanol (0.0, 1.25, 2.5 or 3.5 g/kg; i.g.) and Stress on locomotor activity (LMA) in adolescent and adult rats exposed to 90 min of restraint-induced or social deprivation-induced stress, or no stress. The figure depicts distance travelled as a function of Ethanol dose (panels A and B) and Stress exposure (panels C and D) in adolescent (panels A and C) and adult (panels B and D) rats from minutes 5 to 19 post-administration. Each of the 24 groups was composed by 12 subjects. Vertical bars indicate the standard error of the means (SEM).
Adults
The effects of ethanol on locomotion were markedly different in adults than those observed in adolescents (see Figure 1, panel B). Animals given 2.5 g/kg ethanol exhibited a transient increase in locomotion, which was followed by a long-lasting motor depressing effect at this and the 3.5 g/kg dose. The ANOVA yielded significant main effects of Ethanol dose, Stress exposure and Time at test (F3, 132 = 23.67, F2, 132 = 6.99, F14, 1848 = 377.01; respectively, ps < .01). Ethanol dose × Time at test, and Stress exposure × Time at test interactions (F42, 1848 = 6.59, F42, 1848 = 3.98, respectively, p’s < .01) also achieved significance. Planned comparisons indicated that animals given 2.5 g/kg ethanol exhibited greater locomotion than control subjects, but only during the first testing minute. This mild activating effect of ethanol rapidly turned to motor depression. Planned comparisons indicated that adults given 3.5 g/kg ethanol exhibited significantly less motor activity than vehicle-treated subjects from post-administration minute 6 until termination of testing, with those given 2.5 g/kg ethanol having significantly lower scores than control counterparts during post-administration time 8–14 minutes. The 1.25 g/kg ethanol dose exerted neither activating nor depressing effects.
Further analysis of the Stress × Time at test interaction through planned comparisons revealed that adult rats exposed to social, but not restraint, stress exhibited overall heightened locomotion than control subjects from minutes 5 to 10 postadministration. This effect of Social stress, depicted in panel D of Fig. 1, did not differ for rats given ethanol or vehicle.
Blood and Brain Ethanol Concentration, and Corticosterone and Progesterone levels (Experiment 1b)
Blood ethanol concentrations (ethanol dose: 3.5 g/kg) were slightly, yet significantly higher, in non-stressed adolescents than in adults exposed to 90 min of social deprivation, F2, 19 = 3.73, p < .05. Posthoc tests indicated that non-stressed adolescents and those given 90 min of social deprivation exhibited similar blood ethanol levels, and that non-stressed adolescents had higher BECs than adults exposed to 90 min of social deprivation. On the other hand, the ANOVA for brain ethanol concentrations revealed no differences across groups. CORT and PROG levels were significantly higher in adolescents whether or not they were exposed to social deprivation than in adults given 90 min of social deprivation (F2, 19 = 6.45, p < .01, F2, 19 = 9.06, p < .01; respectively). Means and SEM for BrECs, BECs, CORT and PROG levels can be found in Table 1.
Table 1.
Blood and Brain Ethanol Concentrations, and Corticosterone and Progesterone Levels in socially-deprived adolescent and adult rats, and in non-manipulated adolescents (Experiment 1b)
| Blood ethanol concentrations (mg/dl) |
Brain ethanol concentrations (mg/dl) |
Corticosterone levels (ng/mL) |
Progesterone levels (ng/mL) |
|
|---|---|---|---|---|
| Non-manipulated adolescents | 160.94±17.68 | 138.89±18.50 | 309.71±48.07 | 20.07±2.94 |
| Adolescents given 90 min of social deprivation | 147.67±16.81 | 138.17±16.57 | 320.62±16.18 | 22.50±2.07 |
| Adults given 90 min of social deprivation | 104.21±8.09 | 129.74±14.95 | 186.28±12.32 | 9.14±1.98 |
Blood and Brain Ethanol Concentrations (mg/dl), and Corticosterone and Progesterone Levels (ng/ml), in adolescent and adult rats exposed to 90 min of social deprivation and in a group of non-manipulated adolescents. All animals had been given 3.5 g/kg ethanol (i.g.) at termination of stress exposure. Trunk blood was collected at 7.5 min post-administration time. Values are expressed as mean ± SEM.
Ethanol-induced LMA after social deprivation in adolescent and adult male rats (Experiment 2)
Experiment 2 further explored the consequences of social stress in adolescents and adults and potential interactions between ethanol’s motor effects and stress. The focus of the Experiment was on social deprivation (90 or 180 min pre-testing), the stressor that in Experiment 1a altered overall motor activity in adults, but not in adolescents. The ethanol dose employed was the one that induced, among those tested on Experiment 1a, maximal activating and sedative effects in adolescent and adult rats, respectively (i.e., 3.5 g/kg). The hypothesis was that the greater length of social deprivation (180 min, instead of the 90 min employed in Exp. 1a) would reveal stress effects or stress-induced potentiation of ethanol-induced LMA in adolescents.
Adolescents
The ANOVA revealed a significant main effect of time at Test and significant interaction between Ethanol dose and Time at test (F14, 924 = 312.05; F14, 924 = 23.55, both p < 0.01). Planned comparisons indicated that ethanol-induced motor stimulating effects during the initial minutes of testing. Ethanol-treated adolescents also exhibited, when compared to vehicle-treated subjects, greater reduction in motor activity from post-administration minute 13 through termination of assessment (Figure 2, panel A). Stress did not exert a significant main effect nor was it involved in significant interactions (Figure 2, panel C).
Figure 2.
Effects of Ethanol (0.0 or 3.5 g/kg) and social deprivation Stress on locomotor activity (LMA) in adolescent and adult rats. The figure depicts distance travelled as a function of Ethanol dose (panels A and B) and Stress exposure (panels C and D) in adolescent (panels A and C) and adult (panels B and D) rats from minutes 5 to 19 post-administration. Each adolescent and adult group was composed by 12 and 11 subjects, respectively. Vertical bars indicate the standard error of the means (SEM).
Adults
The ANOVA yielded significant main effects of Ethanol dose, Stress exposure and Time at test (F1,60 = 54.27, F2,60 = 4.56, F14,840 = 189.73; respectively, ps < .01) and significant Ethanol dose × Time at test, and Stress exposure × Time at test interactions (F14,840 = 16.34, F28,840 = 1.86, respectively, p < .01). Planned comparisons indicated ethanol-induced motor activation during the initial testing minute. This stimulating effect was short-lived. Ethanol-treated subjects exhibited significantly less motor activity than controls from post-administration minute 7 until termination of the assessment (see Figure 2, panel B). In Figure 2, panel D, effects of Social stress on overall locomotion scores are depicted. Adults exposed to 180 min of social deprivation exhibited significantly higher overall LMA scores than non-manipulated control subjects at post-administration minutes 5, 6, 8, 9 and 10.
Ethanol-induced LMA in adolescent rats after pharmacological stress (Experiment 3)
Experiment 3 assessed the effects of an alternative source of stress on spontaneous and ethanol-induced LMA on adolescents. Pharmacological stress was induced by administering the kappa agonist U62,066E, 15 min before 2.5 g/kg ethanol. LMA was assessed 5–19 min after the ethanol intubation and blood samples were taken at termination of the test for determination of BECs.
The ANOVA revealed significant main effects of U62,066E dose, Ethanol dose and Time at test (F2,53 = 30.08, p < .001, F1,53 = 11.27, p < .005, F14,742 = 217.51, p < .001; respectively). The U62,066E dose × Time at test interaction (F28,742 = 7.60, p < .001 ) and the Ethanol dose × Time interaction (F14,742 = 9.35, p < .001) achieved significance; as did the three-way interaction among U62,066E, Ethanol dose and Time at test (F28,742 = 1.56, p < .05). As observed in Fig. 3 and confirmed by planned comparisons, ethanol-treated subjects exhibited greater motor activation than animals given only vehicle (0.0 g/kg); and U62,066E injection decreased overall locomotion scores whether accompanied by ethanol or not.
Figure 3.
Effects of Ethanol (0.0 or 2.5 g/kg) and U62,066E (0.0, 1.0 or 5.0 mg/kg, i.p.; panels A, B and C, respectively) administration on locomotor activity (LMA) in adolescent rats. Distance travelled (cm) was assessed from minutes 5 to 19 post-administration of ethanol. Each group was composed by 10 animals, except the ethanol 0.0 g/kg / U62,066E 0.0 mg/kg, which had 9 animals. Vertical bars indicate the standard error of the means (SEM).
To understand the significant three-way interaction, repeated measures ANOVAs (between-group factors: Ethanol dose) were conducted for each dose of U62,066E and for ethanol- and saline treated subjects (between-group factors: U62 dose). In the absence of U62,066E (see figure 3, panel A), ethanol induced a significant motor activating effect during post-administration minutes 5 to 9 (F14,238 = 8.34, p < .001). This activating effect of ethanol was significantly inhibited in animals given 1.0 or 5.0 mg/kg of U62,066E (F14,252 = 1.27, p > .20, F14,252 =3.34, p < .01; respectively). Animals given ethanol and the highest dose of U62,066E exhibited a transient increase in motor activity during the initial testing minute, when compared to counterparts given 0.0 g/kg ethanol after 5.0 mg/kg of U62,066E. The ANOVAs for ethanol (F28,378 = 4.46, p < .001) and vehicle-treated subjects (F28,364 = 4.93, p < .001) and subsequent planned comparisons indicated that the motor suppressive effect of U62,066E on spontaneous or ethanol-induced motor activity was significantly greater in animals given 5.0 mg/kg than in those given 1.0 mg/kg U62,066E.
A one-way ANOVA indicated that U62,066E injected 15 min before ethanol did not significantly alter BECs. Means and SEM for BECs in animals that received 0.0, 1.0 and 5.0 mg/kg U62,066E were 169.99±32.10, 124.5±40.21 and 150.05±36.67 mg%, respectively.
Discussion
The expectations were that adolescents would show greater sensitivity to ethanol-induced LMA and stress than adults; and that stress would exacerbate the motor stimulant effect of ethanol. These hypotheses were only partially corroborated. Ethanol did induce motor activation in adolescents but it induced motor depression in adults. The sedative effect observed in adults was long-lasting and clearest with relatively high ethanol doses. Adult, but not adolescent, rats were affected by 90 or 180 min of social deprivation stress. Acute restraint stress was surprisingly ineffective in altering spontaneous or ethanol-induced locomotion at either age.
The most striking age-related difference was that adolescents were uniquely sensitive to the motor stimulating effects of ethanol and much more resistant than adults to the sedative effect of ethanol. Ethanol-induced sedation in adults occurred shortly after ethanol administration and was much more pronounced and long lasting than that found in adolescents. These results parallel work by Varlinskaya et al. (2010), in which adolescents were more sensitive than adults to social facilitation induced by low-dose ethanol but more resistant to social-suppressing effects of higher ethanol doses. A synergistic relationship could be postulated, in which greater sensitivity to motor activating effects of ethanol facilitates investigation of social counterparts. Facilitation of social interaction by ethanol (Monahan and Lannutti 2000), in turn, could further increase arousal, motor activation and likelihood of drinking alcohol. Evidence supporting these relationships can be drawn from the dependence of both phenomena on the integrity of the endogenous opioid system (Pautassi et al. 2012; Varlinskaya and Spear 2009).
Significant age-related differences in overall locomotion were found, with adults exhibiting significantly greater distance travelled than adolescents. This raises the possibility of adults not exhibiting stimulant effects of ethanol because of a potential ceiling effect. Similarly, perhaps the lower levels of overall locomotion protected adolescent from exhibiting pronounced ethanol-induced sedation.
Another source of stress (Sperling et al. 2010) applied to adolescents in this study was the activation of the endogenous kappa opioid receptor (KOR) system via the kappa agonist U62,066E (Experiment 3). Previous studies have revealed that KOR agonism increased locomotion in infants (Duke et al. 1997; Pautassi et al. 2012) but decreased locomotion in adults (Ukai and Kameyama 1985). This may be related to the functional switch in the motivational consequences of the kappa opioid receptor (from appetitive to aversive) during development (Petrov et al. 2006; Nizhnikov et al., 2012). New information derived from the present study is that, unlike preweanlings, adolescents exhibited an adult-like pattern of responding to KOR activation: administration of U62,066E induced a drastic, dose-response decrease in spontaneous and ethanol-induced LMA.
It could be argued that the significance of the stimulation of LMA obtained in the present study is questionable because the effect is short-lived and restricted to the first minutes of testing. Previous studies in adolescent and adult Wistar rats and adult Swiss mice (Acevedo et al. 2010, 2012; Faria et al. 2008; Quoilin et al. 2010, 2012) concur, however, that the expression of acute motor stimulation and motor sensitization after repeated ethanol treatment is regularly found during the initial testing minutes.
It has been suggested that adolescents are more sensitive to stressful situations than adults. Early work (Stone and Quartermain 1997) found greater anxiogenic response in a plus maze, in 29-day than in in 56-day old mice subjected to social stress. More recently, Doremus-Fitzwater et al. (2009) observed that adolescents were more sensitive than adults to several physiological consequences of restraint stress, including body weight alterations and stress-induced elevations in corticosterone levels. Intriguingly, in the present study adult, but not adolescent, rats were sensitive to relatively brief (90 or 180 min) and acute social deprivation. The direction of the stress effect was in agreement with previous literature indicating that acute and chronic stress exert differential effects (see Katz et al., 1981).
Overall, stress did not exert a significant effect on ethanol-induced LMA. This finding is in contrast to previous studies indicating that adolescents are more sensitive to ethanol/stress interactions than adults. Procedural differences may account for these seemingly disparate data. Studies that found greater sensitivity to ethanol following stress in adolescents typically measured social behavior, for instance showing that ethanol reduced the anxiety induced by acute stress in in adolescent but not adult rats, thus suggesting an interaction between stress and the anxiolytic effect of ethanol (Varlinskaya & Spear, 2012). Studies assessing stress and age effects on conventional tests of ethanol’s motivational consequences have yielded more inconsistent results.Anderson et al. (2010) found reduced ethanol-induced conditioned taste aversion in adolescent than in adults, yet similar to the present work stress did not exert a significant effect on conditioned aversion by ethanol. It is possible that adolescents may be more sensitive to ethanol-stress interactions in situations featuring a strong social component. This should not be a surprise, since adolescents place greater incentive value on social stimuli than adults (Willey and Spear, 2012).
One caveat of this study is that the testing environment was novel. Unfamiliarity may have resulted in additional stress which, in turn, could interact with previous exposure to social and restraint stress. The lack of response of adolescents to restraint or social stress (Exp. 1a and 2) could be related to differential reactivity to the additional stress of the arena. Yet, prior work has shown that pre-test habituation of animals to the testing environment would likely preclude ethanol-induced motor activation in this species (Arias et al., 2009). Moreover, use of habituation in the present study may have had different effects in adolescents and adults, given evidence that cognitive processing of contextual stimuli changes through ontogeny. When a conditional stimulus (CS) and a context are simultaneously conditioned, the CS strongly potentiates the context conditioning in preweanlings, induces overshadowing in adults, while producing an intermediate effect in adolescents (Brasser & Spear, 2004).
Motor stimulation by ethanol has been considered an analogue of ethanol-induced reinforcement (Quoilin et al. 2012). Human subjects exhibiting positive family history of alcoholism exhibit heightened sensitivity to the stimulant effects of ethanol. Substantial overlap between neurobiological underpinnings of ethanol-induced locomotion and reinforcement has been also claimed (Robinson and Berridge 2008). In infant and adolescent rats the time courses of these phenomena are coincident (Arias et al. 2008; Molina et al. 2007; Nizhnikov et al. 2009); and adolescent female rats that were more sensitive to ethanol's locomotor-activating effects showed greater ethanol self-administration than counterparts less sensitive to these effects of ethanol (Acevedo et al., 2010). The present study indicates differences in ethanol-induced motor stimulation and sedation between adolescents and adults.
Acknowledgments
Acknowledgments, Funding sources and Relationship with the Organizations that sponsored the research: This work was a collaborative project between the Research Foundation of SUNY Binghamton and Instituto Ferreyra and was supported by National Institute on Alcohol Abuse and Alcoholism grants AA011960, AA013098, AA015992, and AA017823 to NES, R01 AA018026 and P50 AA017823 to Linda P. Spear and grants PIP CONICET (Argentina) 2010–2012, SECYT-UNC 2012 and Fundación Florencio Fiorini 2012 to RMP.
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. 2010;52:424–440. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo MB, Nizhnikov ME, Spear NE, Molina JC, Pautassi RM. Ethanol-induced locomotor activity in adolescent rats and the relationship with ethanol-induced conditioned place preference and conditioned taste aversion. Dev Psychobiol. 2012 May 16; doi: 10.1002/dev.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. 2012 Dec 28; doi: 10.1016/j.neuroscience.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Solari AC, Mlewski EC, Miller S, Haymal B, Spear NE, Molina JC. Social isolation and stress related hormones modulate the stimulating effect of ethanol in preweanling rats. Behav Brain Res. 2010;29:64–70. doi: 10.1016/j.bbr.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiol Learn Mem. 2004;81:46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Giuliano RJ, Wicha NY, Graham RJ. Acute stress and event-related potential correlates of attention to alcohol images in social drinkers. Stud Alcohol Drugs. 2010;73:761–771. doi: 10.15288/jsad.2012.73.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;22:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social vs. solate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Duke MA, Meier TL, Bolanos CA, Crawford CA, McDougall SA. Paradoxical effects of kappa-opioid stimulation on the locomotor activity and Fos immunoreactivity of the preweanling rat: role of dopamine receptors. Behav Neurosci. 1997;111:1114–1122. doi: 10.1037//0735-7044.111.5.1114. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology. 1995;117:216–224. doi: 10.1007/BF02245190. [DOI] [PubMed] [Google Scholar]
- Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, Giorgetti Britto LR, Camarini R. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;6:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Re. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Masur J, Oliveira de Souza ML, Zwicker AP. The excitatory effect of ethanol: absence in rats, no tolerance and increased sensitivity in mice. Pharmacol Biochem Behav. 1986;24:1225–1228. doi: 10.1016/0091-3057(86)90175-9. [DOI] [PubMed] [Google Scholar]
- McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol Behav. 2010;9:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan JL, Lannutti PJ. Alcohol as social lubricant alcohol myopia theory, social self-esteem and social interaction. Hum Commun Res. 2000;26:175–202. [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear NE. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–45. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Varlinskaya EI, Rahmani P, Spear NE. Ontogenetic differences in ethanol's motivational properties during infancy. Alcohol. 2012;46:225–234. doi: 10.1016/j.alcohol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Early role of the κ opioid receptor in ethanol-induced reinforcement. Physiol Behav. 2012;105:1231–1241. doi: 10.1016/j.physbeh.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev. 2009;33:953–974. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Nizhnikov ME, Varlinskaya EI, Spear NE. Dynorphin A(1–13) and responsiveness of the newborn rat to a surrogate nipple: Immediate behavioral consequences and reinforcing effects. Behavioural Brain Research. 2006;180:1–14. doi: 10.1016/j.bbr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav. 1997;57:487–493. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deminière JM, Kharoubi M, Moal ML, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology (Berl) 2010;212:501–512. doi: 10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Developmental differences in ethanol-induced sensitization using postweanling, adolescent, and adult Swiss mice. Psychopharmacology (Berl) 2012;219:1165–1177. doi: 10.1007/s00213-011-2453-7. [DOI] [PubMed] [Google Scholar]
- Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, Rice KC, Riley AL. Genetic and early environmental contributions to alcohol’s aversive and physiological effects. Pharmacol Biochem Behav. 2008;91:134–139. doi: 10.1016/j.pbb.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities and intake: Setting the stage for alcohol use disorder? Child Development Perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl) 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiology & Behavior. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Ukai M, Kameyama T. Multi-dimensional analyses of behavior in mice treated with U50,488H,a purported kappa (non-mu) opioid agonist. Brain Res. 1985;337:352–356. doi: 10.1016/0006-8993(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012;227:224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2012;226:613–618. doi: 10.1016/j.bbr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]