Abstract
Angiogenesis is a feature of numerous pathologies including cancer and inflammatory conditions and as such is key therapeutic target for the treatment of disorders where excessive or insufficient formation of new blood vessels occurs. The study of angiogenesis in vivo provides many challenges, however the growth of new blood vessels in vitro from aortic explants has provided a highly useful model for the study of this process. In this manuscript we examine the critical factors which can affect this assay and demonstrate that aortas from both female rats and mice exhibit a reduced angiogenic response to males. These findings have implications not only for the experimental design of angiogenesis experiments but also in the use of therapies targeting angiogenesis in the treatment of pathologies, such as cancer.
Keywords: Angiogenesis, Tumour angiogenesis, Aortic ring, Gender, Endothelial cell
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing ones, occurs primarily during embryonic development and growth in order to remodel the primary network of vascular endothelial cells formed during vasculogenesis into a vast network of arteries, veins and capillaries that will form the blood circulatory system of the adult. Angiogenesis occurs during wound healing where angiogenic capillary sprouts invade the fibrin/fibronectin-rich wound clot and within a few days organize into a microvascular network throughout the granulation tissue. Additionally, in the female, angiogenesis happens during the reproductive cycle and pregnancy. In healthy unchallenged individuals angiogenesis is controlled by a fine balance of pro- and anti- angiogenic modulators. In particular conditions, such as tissue injury, the balance is tipped in favour of pro-angiogenic factors such as VEGF, FGF and angiopoietins. The quiescent vessels sense and respond to these molecules initiating the angiogenic process. A sequence of coordinated events culminate in the formation of a new capillary network: first, a so-called tip cell migrates from the vessel into the ECM guided by growth factor gradients; following the tip cell, stalk cells proliferate and eventually form the vessel lumen; mural cells such as pericytes and/or smooth muscle cells are recruited around the newly formed vessel and are necessary for its stabilisation. Angiogenesis involves the remodelling of the extracellular space through the action of proteases secreted by ECs and mural cells and is characterized by many interactions between adhesion receptors on the EC surface and ECM molecules [1-3].
Abnormal angiogenesis (either excessive or insufficient) is a common feature of many diseases such as cancer, cardiovascular and skin disorders, ischemia, retinopathies and arthritis and the importance of angiogenesis in these pathologies has led to considerable efforts to understand the mechanisms associated with new blood vessel formation in the hope that novel therapeutic targets for treatment can be established [1,4]. So far angiogenic and anti-angiogenic therapies have been developed but their efficiencies have rarely met expectations; a deeper understanding of the mechanisms underlying the angiogenic process is therefore crucial for designing better therapeutic strategies.
The aortic rings model of angiogenesis has been used to study new blood vessel formation [5-7]. Thoracic aortas from either rats or mice are dissected, sectioned and embedded in either a Collagen I or Matrigel matrix and angiogenesis is stimulated by the addition of pro-angiogenic factors such as VEGF or FGF. Angiogenic sprouts (new vessels) are visualised after 4-8 days in culture. In this study we describe the key factors that can affect this assay and show that the angiogenic response is different between males and females. This data has implications not only for experimental design of angiogenesis studies but also on the potential dosing of therapeutic interventions between male and female patients.
Materials and Methods
Aortic ring assay
Thoracic aortas dissected from cervically dislocated 180-200 g Wistar rats (Harlan laboratories) or C57BL/6 mice (Charles River) were sliced into 0.5 mm sections and incubated overnight in serum free OptiMEM (Invitrogen) at 37°C, 10% CO2. Male and female rats and mice were aged matched unless stated otherwise and animals were fed a standard chow diet and housed under a 12hour light and dark cycle. Aortic rings were embedded in type I collagen (1mg/ml) in E4 media (Invitrogen). Wells were supplemented with OptiMEM with FBS (PAA) VEGF (R and D systems) at the concentrations indicated and incubated at 37°C, 10% CO2. Emergent angiogenic sprouts from rat and mouse aortas were counted after 4 days and 8 days in culture respectively. All animal experiments were conducted in accordance with the British Home Office regulations (Scientific Procedures) Act 1986, United Kingdom.
Imaging
Images were captured using an Olympus IX81 inverted microscope with a Hamamatsu Orca-ER digital camera. Image acquisition was performed using the CellM software (Olympus) and micrographs were prepared for publication using Adobe Photoshop.
Statistical analysis
Statistical analysis was performed using the Graphpad Prism software Version 6.0.
Results
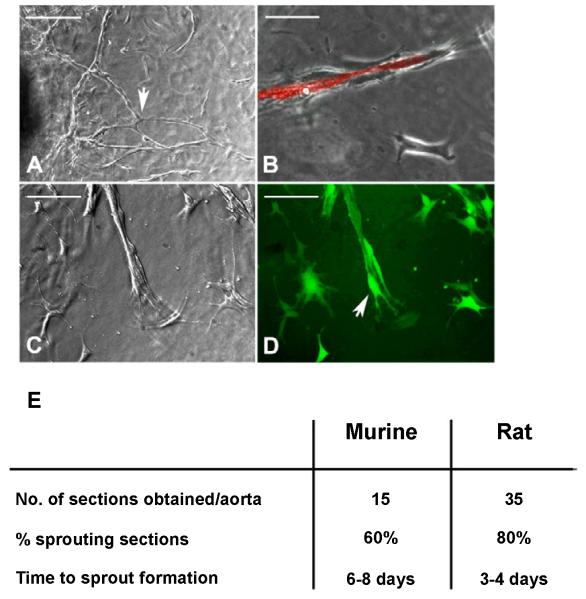
The aortic ring model of angiogenesis is an ex vivo model and has been used extensively to study factors which affect angiogenesis particularly with regard to tumour angiogenesis [5-7]. The model entails growing blood vessels from sections of aorta. The resultant structures form branching tube like structures composed of both endothelial cells and pericytes (Figures 1A-1D). Rat aortas (Figure 1A) produce longer more branched sprouts than those obtained from murine aortas (Figures 1B-1D). Staining using a fluorescently labelled endothelial cell specific lectin from the bacteria Bandeiraea simplicifolia reveals, that the sprouts produced consist of vessel-like structures composed of endothelial cells surrounded by perivascular cells. These perivascular cells express the pericyte marker α-smooth muscle actin (αSMA) and envelop the newly formed vessel, as shown in Figure 1C and 1D in which sprouts have been induced from aortas from transgenic mice in which eGFP is under the control of the αSMA promoter.
Figure 1. Angiogenic sprouts from both rat and mouse aortas from microvessels consisting of endothelial cells surrounded by pericytes.
(A) Vessel out growth from a rat aortic ring seeded in Collagen I, in the presence of 1% FBS and 10ng/ml VEGF (Scale bar 200μ). Sprouts from rat aortic rings are longer in length and tend to be more branched (arrow).
(B) Murine angiogenic sprout stained with Bandeiraea simplicifolia (BS) lectin (red) showing endothelial cells comprising the microvessel surrounded by pericytes (Scale bar 100μ).
(C and D) Angiogenic sprouts from aortas from transgenic mice expressing eGFP under the control of the αSMA promoter. GFP expressing pericytes (green) are observed surrounding ECs (D).
(E) Table comparing the use of mouse and rat aortas for the study of angiogenesis.
Aortas from both rats and mice are used in these studies; rat aortas are longer and wider, enabling the collection of a greater number of rings and are more angiogenic in that they produce more sprouts and the chances of a given aortic section producing angiogenic sprouts is far higher than that observed in mice. Mice aortas are smaller, more susceptible to damage during dissection, particularly fat removal and there is a far higher chance of a given section failing to produce an angiogenic response (Figure 1E). The use of murine tissues is of course favourable owing to the number of genetically modified mice that are now available. Many factors can affect the aortic rings assay, and these can have major implications for the interpretation of the experimental data. The source of critical reagents can have an effect on the magnitude of the angiogenic response in these assays and it is inevitable that there will be differences between laboratories. We tried to standardize an aortic ring assay by examining the effect of VEGF, FBS and gender on the angiogenic response in tissues from both rats and mice (Figure 2).
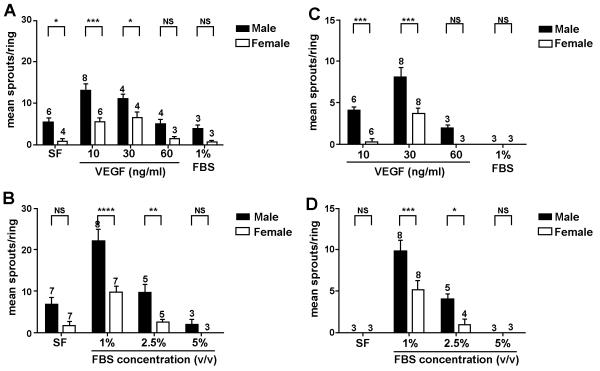
Figure 2. In all conditions tested aortas from females gave a reduced angiogenic response compared to males.
(A) VEGF promotes angiogenesis in rat aortas embedded in collagen I in serum free conditions. Age matched Male and female rat aortic rings were seeded as described and supplemented with the concentrations of VEGF indicated.
(B) High level of FBS can be inhibitory to the angiogenic response from both male and female rat aortas. Rings were embedded in collagen I and supplemented with 10ng/ml VEGF and the concentrations (v/v) of FBS indicated.
(C) The formation of angiogenic sprouts from murine aortas requires VEGF.
(D) High levels of serum also inhibit angiogenic sprout formation from murine vessels.
Murine aortic explants were supplemented with 30ng/ml VEGF and the concentrations of FBS indicated. In (A-D) angiogenic sprouts were counted 4 days (rat) and 8 days (mouse) post embedding. At least 10 rings/animal, were counted and the mean calculated, data presented for each condition represents the mean of mean sprout formation obtained from at least 3 different animals (n-numbers for each condition are shown above data bars). Error bars represent the standard error of the mean and significance between male and female samples at each condition was calculated using a 2-way ANNOVA comparison.
Optimization of the rat aortic ring assay
As mentioned above rat aortas generally give a stronger angiogenic response than mice, indeed angiogenic sprouting can be observed in rings from male animals seeded in collagen only in the absence of FBS or VEGF. This response can be increased in the presence of 10ng/ml of VEGF. A similarly high response is observed in the presence of 30 ng/ml of VEGF although there is a reduction in angiogenic sprouts when 60ng/ml of VEGF is added. Interestingly in all conditions the angiogenic response from aortic rings from female rats is greatly reduced. There is still a response to increased concentrations of VEGF but this is significantly reduced when compared to samples from male rats.
In a second set of experiments we tested the effect of foetal bovine serum on sprout formation from rat aortic rings. The combination of 1% serum and 10 ng/ml VEGF elicited the highest angiogenic response in both male and female aortic rings, although as previously the response was significantly lower in females versus males. Increased serum concentration (2.5% and 5% v/v) resulted in an inhibition of this response. One of the issues with the aortic rings assay is that in addition to angiogenic sprouts emerging from the ring section there is also a significant amount of fibroblasts and smooth muscle cells. Higher serum concentrations tend to favour the emergence of fibroblasts and when this is excessive it appears to be inhibitory to sprout formation.
Optimization of the mouse aortic ring assay
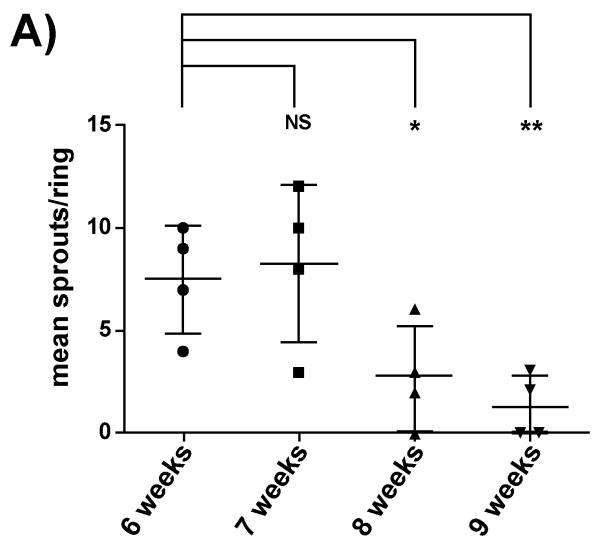
The use of mouse aortas for the study of angiogenesis is more technically challenging. Unlike rat aortic rings murine sections do not sprout in serum free conditions and require both serum and VEGF to elicit a response. We have found that the best response is obtained from rings treated with 1% serum and 30ng/ml of VEGF. Higher concentrations of VEGF (>30 ng/ml) and serum (>1%v/v) are largely inhibitory to the response. As in the rat system higher serum levels favour the emergence of fibroblasts and smooth muscle cells which is not conducive to new blood vessel formation. As is also the case aortas from female mice give a significantly lower angiogenic response than males, regardless of the treatment. The use of murine aortic ring is also dependant on the age of the mice used (Figure 3). Aortas from younger mice give a more robust angiogenic response than that observed from older mice (Older than 8 weeks).
Figure 3. In the murine model of angiogenesis aortas from older mice show a reduced angiogenic response.
(A) Male mice (aged as indicated) were embedded in Collagen I and supplemented with 1% FBS and 30 ng/ml VEGF. Mean sprout formation was calculated from at least 10 rings each point represents the mean value from one animal. Error bars represent the standard error of the mean and significance was calculated using individual student T tests compared to the 6 week value.
Discussion
In the work presented here we have examined in detail the factors which can affect a rodent model of angiogenesis. Although the effects of serum and VEGF are well established in this model, we also highlight the fact that the angiogenic response in this assay is greatly different between males and female donors. Results clearly showed a decreased angiogenic response in both female mice and rats when compared to their male counterparts. This difference was persistent throughout all the experiments regardless of the different conditions (VEGF or serum concentration).
Gender differences in cellular responses particularly inflammation have been identified [8]; however, differences in angiogenesis are less well characterised, particularly in an ex vivo model such as the aortic ring model where the tissues are isolated from the normal hormonal factors present in vivo. The reason why female samples are less angiogenic is not clear; however, it is intriguing to speculate that angiogenesis, like other physiological processes or disorders might have an epigenetic component [9-11]. Sexual dimorphism in angiogenic response could be caused by a different hormone-induced DNA methylation or histone modification in male and female resulting in different gene regulation. Microarray-based epigenetic profiles might shed some light on this hypothesis. Angiogenesis has been shown to be affected by gene dosage affects and it may be that sex linked genes contribute to this effect. This observation is highly relevant to gender specific pathologies such as testicular and ovarian cancer. Moreover, these findings are of considerable importance both in terms of experimental design relating to the study of angiogenesis but also for the development of angiogenic and anti-angiogenic therapies and represent the start point for further investigation.
Acknowledgements
The bulk of this work was supported by a grant from the William Harvey Research Foundation. JRW is supported by Arthritis Research UK grant No. 19702 and GDR by Barts and the London School of Medicine and Dentistry.
Abbreviations
- FGF
Fibroblast Growth Factor
- VEGF
Vascular Endothelial Growth Factor
- ECM
Extracellular Matrix
- EC
Endothelial Cell
- FBS
Foetal Bovine Serum
References
- 1.Ramjaun AR, Hodivala-Dilke K. The role of cell adhesion pathways in angiogenesis. Int J Biochem Cell Biol. 2009;41:521–530. doi: 10.1016/j.biocel.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 4.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 5.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, et al. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 6.Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Methods Enzymol. 2008;443:119–136. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172–183. doi: 10.1016/j.mvr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero-Bosagna C, Savenkova M, Haque MM, Nilsson E, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLoS One. 2013;8:e59922. doi: 10.1371/journal.pone.0059922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takasugi M, Hayakawa K, Arai D, Shiota K. Age- and sex-dependent DNA hypomethylation controlled by growth hormone in mouse liver. Mech Ageing Dev. 2013 doi: 10.1016/j.mad.2013.05.003. [DOI] [PubMed] [Google Scholar]