Abstract
Background
ADHD is a common and highly heritable neurodevelopmental disorder with a complex aetiology. The identification of candidate intermediate phenotypes that are both heritable and genetically linked to ADHD may facilitate the detection of susceptibility genes and elucidate aetiological pathways. Very low-frequency (VLF; <0.5Hz) electroencephalographic (EEG) activity represents a promising indicator of risk for ADHD, but it is currently unclear whether it is heritable or genetically linked to the disorder.
Methods
Direct-current (DC)-EEG was recorded during a cognitive activation condition in 30 monozygotic and dizygotic adolescent twin pairs concordant or discordant for high ADHD symptom scores, and 37 monozygotic and dizygotic matched-control twin pairs with low ADHD symptom scores. Structural equation modelling was used to quantify the genetic and environmental contributions to the phenotypic covariance between ADHD and VLF activity.
Results
ADHD was significantly associated with reduced VLF power during cognitive activation, which suggests reduced synchronisation of widespread neuronal activity. VLF power demonstrated modest heritability (0.31) and the genetic correlation (−0.80) indicated a substantial degree of overlap in genetic influences on ADHD and VLF activity.
Conclusions
Altered VLF activity is a potential candidate intermediate phenotype of ADHD, which warrants further investigation of underlying neurobiological and genetic mechanisms.
Keywords: ADHD, EEG, very low-frequency activity, endophenotype, genetics, heritability
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent neurodevelopmental disorders and is characterised by impairing levels of inattentive, impulsive and hyperactive symptoms. ADHD persists beyond childhood in around 65% of cases and is associated with high levels of clinical, psychosocial and economic burden (Faraone, Biederman, & Mick, 2006; Kendall, Taylor, Perez, & Taylor, 2008). Family and twin studies suggest ADHD is under substantial genetic influence, with an average heritability estimate of 0.76 (Faraone, et al., 2005). Candidate gene association studies support the role of genetic factors; however these studies have shown inconsistency and non-replication of findings, which suggests a complex genetic inheritance with a small risk conferred by individual genetic variants (Faraone, et al., 2005).
One strategy to facilitate the detection of susceptibility genes and elucidate aetiological pathways is the identification of neurobiological processes that underlie the disorder and potentially mediate between genes and behaviour (Gottesman & Gould, 2003). In order to be useful for genetic research, these intermediate phenotypes, or endophenotypes, must be associated with the disorder, be heritable and share genetic effects with the disorder (Gottesman & Gould, 2003). Several candidate endophenotypes have been reported for ADHD (Kuntsi, McLoughlin, & Asherson, 2006; McLoughlin, Kuntsi, Brandeis, & Banaschewski, 2005). Electrophysiological abnormalities as measured by electroencephalography (EEG), in particular, are among the most promising indicators of increased genetic risk for ADHD with consistent associations and moderate to high heritability (McLoughlin, et al., 2005; Tye, McLoughlin, Kuntsi, & Asherson, 2011).
Investigations of brain function in ADHD have recently extended to include very low-frequency activity (VLF; <.05 Hz) that can be measured using direct-current (DC)-coupled EEG recordings. Spontaneous VLF fluctuations synchronise activity across functionally specific but widespread distributed neural networks, demonstrating a high degree of coherence within these circuits (Balduzzi, Riedner, & Tononi, 2008; Buszaki & Draguhn, 2004; Fransson, 2005; Vanhatalo, et al., 2004). It has been suggested that VLF activity represents a reflection of the brain’s default-mode network (DMN) that is typically characterised by a low-frequency BOLD signal (Fox, et al., 2005; Sonuga-Barke & Castellanos, 2007). Specifically, based on findings from fMRI studies, VLF fluctuations are posited to reflect toggling between two anti-correlated brain networks: a task-negative network that is active during wakeful resting states and characterised by these slow oscillations, and the task-positive network that activates during goal-oriented activity (Fox, et al., 2005; Sonuga-Barke & Castellanos, 2007). In addition, EEG studies have reported that VLF activity modulates the activity of higher frequency bands, suggesting regulation of gross cortical excitability (Monto, Palva, Voipio, & Palva, 2008; Vanhatalo, et al., 2004). During an active task condition, slow cortical activity can also be generated in response to stimuli, and as such reflect event-related rather than spontaneous activity. These time-locked slow cortical potentials have been posited as an index of conscious perception (He & Raichle, 2009).
Abnormalities in VLF activity are associated with several neuropathological disorders (Broyd, et al., 2009). Adults with attentional problems demonstrated reduced spontaneous VLF power and reduced rest-to-task VLF attenuation (Helps, Broyd, James, Karl, & Sonuga-Barke, 2009; Helps, James, Debener, Karl, & Sonuga-Barke, 2008), which was replicated in a sample of adolescents with ADHD (Helps, et al., 2010). In addition, reduced rest-to-task VLF attenuation has been associated with poor task performance (Helps, et al., 2010) and similar slow fluctuations in task performance (Helps, et al., 2009; Monto, et al., 2008), which may underlie the deficits in task performance exhibited in ADHD (Castellanos, et al., 2005). This is supportive of the default-mode interference (DMI) hypothesis, which proposes that VLF activity usually exhibited at rest persists during cognitive activation in ADHD producing periodic lapses of attention (Fox, et al., 2005; Sonuga-Barke & Castellanos, 2007). Measurement of VLF activity during a cognitive task allows investigation of this brain-behaviour association and its persistence during activation states that are altered in ADHD (Sergeant, 2000).
Twin research conducted on frequency bands above 1Hz suggests that EEG is highly heritable (Smit, Posthuma, Boomsma, & Geus, 2005). A preliminary study of affected sibling pairs with ADHD indicated high sibling correlations of 0.53 to 0.76 during a cognitive activation condition (Loo & Smalley, 2008) though smaller estimates (0.22 to 0.61) have been reported in a larger study of multiplex families with ADHD (Loo, et al., 2010). These studies have used family designs that are unable to discriminate between genetic and environmental influences. The twin design however allows separation of these effects by utilising the different levels of genetic relatedness between monozygotic (MZ; 100%) and dizygotic (DZ; 50%) twin pairs (Neale & Cardon, 1992).
No twin study on VLF activity or its genetic overlap with ADHD has been conducted to date. This study aims to evaluate VLF activity as a potential intermediate phenotype of ADHD during a key developmental period by 1) estimating the heritability of VLF activity 2) quantifying the strength of the phenotypic relationship of VLF activity with ADHD symptoms and 3) examining the genetic and environmental overlap with ADHD symptoms. We measured VLF activity during a cognition activation condition (cued continuous performance test (CPT-OX)); (Doehnert, Brandeis, Straub, Steinhausen, & Drechsler, 2008; McLoughlin, et al., 2010; Valko, et al., 2010) in adolescent MZ and DZ twin pairs (12-15 years old) concordant and discordant for high and low ADHD symptom scores. Structural equation modelling was applied enabling separation of the phenotypic covariance between these two parameters (i.e. ADHD and VLF activity) into genetic and environmental components (Toulopoulou, et al., 2007). Significant heritability and genetic overlap between ADHD and VLF activity would support this measure as a candidate endophenotype for the disorder, reflecting mediating processes on ADHD (Gottesman & Gould, 2003) or pleiotropic effects of genes (Kovas & Plomin, 2006).
METHODS
Sample
The sample was selected from the Twins’ Early Development Study (TEDS), a birth cohort study of all twins born in England and Wales between 1994 and 1996 (Trouton, Spinath, & Plomin, 2002). Zygosity was determined using a zygosity questionnaire that has been shown to have 95% accuracy (Price, et al., 2000); For cases where zygosity was unclear from this questionnaire, DNA testing was conducted. The TEDS sample is representative of the general population in terms of parental education, ethnicity and employment status (Kovas, Haworth, Dale, & Plomin, 2007).
The Neurophysiological Study of Activity and Attention in Twins (NEAAT) subset used in this study consisted of 67 male twin pairs in groups of 22 pairs concordant for high levels of ADHD symptoms (MZ: 11; DZ: 11), 8 pairs discordant for ADHD symptoms (MZ: 2; DZ: 6) and 37 control pairs concordant for low levels of ADHD symptoms (MZ: 21; DZ: 16). Twin pairs were selected based on an analysis of symptom development over time using the program MPLUS (Supplementary Material S1). This identified sub-groups of individuals who have been stably high or stably low at ages 8, 12 and 14, using the 18 DSM-IV ADHD items from the Long Version of the Conners’ Parent Rating Scale (Conners, Sitarenios, Parker, & Epstein, 1998a). This ensured that the selected twin pairs were consistently concordant or discordant for high levels of ADHD symptoms (corresponding to a clinical diagnosis) or unaffected controls who had consistently low ADHD symptoms. Demographic characteristics are given in Table 1. Participating families gave their written informed consent and the study was approved by King’s College London Psychiatry, Nursing and Midwifery Reseach Ethics Sub-Committee (PNM/08/09-89).
Table 1. Raw scores and mean comparisons of demographic characteristics adjusted for genetic relatedness.
|
All ADHD versus all
controls |
|||||
|---|---|---|---|---|---|
| Mean | SD | t | p | ||
| Age (years) | MZ Control (n=44) | 14.40 | 1.05 | −2.72 | <.001 |
| DZ Control (n=38) | 14.62 | 0.70 | |||
| MZ ADHD (n=24) | 14.04 | 0.63 | |||
| DZ ADHD (n=28) | 14.03 | 0.75 | |||
| IQ (n= Raven’s matrices, | MZ Control (n=25,26) | 103.28 | 13.82 | −2.29 | .04 |
| WISC-III-PI multiple choice | DZ Control (n=23,25) | 103.51 | 12.93 | ||
| vocabulary subtests)a | MZ ADHD (n=6,6) | 91.88 | 16.15 | ||
| DZ ADHD (n=7,8) | 96.80 | 15.13 | |||
| Parent Conners (T-score) b | |||||
| DSM-IV symptom subscale: | MZ Control (n=44) | 43.03 | 3.45 | 12.60 | <.001 |
| Inattention | DZ Control (n=38) | 42.18 | 2.49 | ||
| MZ ADHD (n=24) | 57.13 | 8.41 | |||
| DZ ADHD (n=28) | 54.18 | 9.10 | |||
| DSM-IV symptom subscale: | MZ Control (n=44) | 44.73 | 2.72 | 10.74 | <.001 |
| Hyperactivity/Impulsivity | DZ Control (n=38) | 43.86 | 1.44 | ||
| MZ ADHD (n=24) | 62.21 | 11.61 | |||
| DZ ADHD (n=28) | 59.50 | 12.84 | |||
| Teacher Conners (T-score) c | |||||
| DSM-IV symptom subscale: | MZ Control (n=25) | 45.92 | 6.76 | 5.57 | <.001 |
| Inattention | DZ Control (n=23) | 46.61 | 6.61 | ||
| MZ ADHD (n=11) | 64.55 | 10.97 | |||
| DZ ADHD (n=15) | 51.87 | 11.35 | |||
| DSM-IV symptom subscale: | MZ Control (n=25) | 48.00 | 8.62 | 1.86 | .07 |
| Hyperactivity/Impulsivity | DZ Control (n=23) | 46.48 | 5.08 | ||
| MZ ADHD (n=11) | 57.36 | 9.11 | |||
| DZ ADHD (n=15) | 49.27 | 7.67 | |||
General cognitive ability was assessed at age 14 as part of ongoing TEDS web-based data collection. The twins were tested on the WISC-III-PI vocabulary multiple choice subtests (Wechsler, 1992) and Raven’s standard and advanced progressive matrices (Raven, et al., 1996). Missing scores were imputed from multiple IQ subtest scores across ages 7, 12 and 14 using the ICE command in Stata for each outcome variable. A g score was created with equal weights for the two tests by summing their standardized scores within the NEAAT sample. Further information about g as measured in TEDS can be found elsewhere (e.g.(Haworth, et al., 2010). Measures of g and IQ correlate highly and both provide an index of general intelligence (Jensen, 1998) and as such IQ can be calculated from standardised g using the formula IQ=(g*15+100).
Long Version of the Parent Conners’ Rating Scale T scores (Conners, et al., 1998a) collected on the day of testing.
Long Version of the Teacher Conners’ Rating Scale T scores (Conners, Sitarenios, Parker, & Epstein, 1998b). Teachers were contacted following completion of the testing session.
Task and stimuli
The cued-CPT (flanker version; (Doehnert, et al., 2008; McLoughlin, et al., 2010; McLoughlin, et al., 2011; Valko, et al., 2010) consists of a black letter array formed of a centre letter flanked on each side by distractor letters, presented in four identical blocks of 100 letter arrays each. Subjects were instructed to ignore the distractor letters and attend only to the centre letter. There were 11 different centre letters (O, X, H, B, C, D, E, F, G, J and L) subtending approximately 0.5 degrees. Centre letters ‘X’ and ‘O’ were flanked by the incompatible letter ‘O’ or ‘X’ and distractor letters were flanked by either ‘X’ or ‘O’. The letter arrays were presented briefly (150ms) every 1.65s in a pseudo-random sequence at the centre of a computer monitor, at the viewing distance of 120cm. Duration of the task was 11 minutes. Subjects were seated on a height-adjustable chair in a video-monitored testing cubicle. They were instructed to respond only to cue-target sequences (i.e. XOX-OXO) by pressing a button as quickly as possible with the index finger of their preferred hand. The task was practised and comprehension ascertained prior to task performance. If necessary, participants were told to minimise eye movements or blinks. Participants completed 6 minutes of resting EEG prior to completing the cued CPT-OX. The task was followed by other tasks not reported here.
Scoring overt performance
Performance measures in the cued CPT task included target reaction time (MRT, mean latency of responding in ms after target onset), within-subject variability in reaction times (SD-RT), and the coefficient of reaction time variability (CV, SD-RT/MRT). MRT and SD-RT were calculated across correctly answered target trials. Hits were characterized by target-OXOs that were detected between 200 and 1500 ms after stimulus onset. False alarms were responses to letters other than target-OXO. Errors were broken down into subcategories (omission errors, total commission errors, and O-not-X commission errors).
Other measures
General cognitive ability was assessed at age 14 as part of ongoing TEDS web-based data collection. The twins were tested on the WISC-III-PI vocabulary multiple choice subtests (Wechsler, 1992) and Raven’s standard and advanced progressive matrices (Raven, Court, & Raven, 1996). Missing scores (Table 1) were imputed from multiple IQ subtest scores across ages 7, 12 and 14 using the ICE command in Stata version 10 statistical software (Stata Corp, College Station, Texas) for each outcome variable. A g score was created with equal weights for the two tests by summing their standardized scores within the NEAAT sample. Further information about g as measured in TEDS can be found elsewhere (e.g.(Haworth, et al., 2010). Measures of g and IQ correlate highly and both provide an index of general intelligence (Jensen, 1998) and as such IQ can be calculated from standardised g using the formula IQ=(g*15+100).
EEG recording and analysis
EEG was recorded using 62 channel DC-coupled recording system (extended 10-20 montage). Electrode impedance was kept below 5KOhm. The reference electrode was positioned at FCz. Vertical and horizontal electrooculograms (EOGs) were simultaneously recorded from electrodes above and below the left eye and at the outer canthi. The signal was digitized at 500Hz sampling rate, stored and analyzed offline.
Data were analysed in Brain Vision Analyzer (2.0). The signal was re-referenced offline to the average reference and downsampled to 256Hz. We applied 0.02-4Hz (12dB/Oct) Butterworth filters. Ocular artifacts were removed from the data using biased infomax Independent Component Analysis (ICA (Jung, et al., 2000) The extracted independent components were manually inspected and ocular artefacts were removed by back-projection of all but those components. Continuous EEG was segmented into 50 second epochs. Segments with artifacts exceeding 120μV peak-to-peak in any channel were rejected. A DC-Detrend command was executed to remove linear drifts from the data. Fast-Fourier Transform analysis was performed on the data from each of the electrodes for each participant. 50-second Hanning windows were used and power was calculated. The current study focused on the frequency band 0.02-0.2Hz that has been implicated previously in ADHD (Helps, et al., 2010). In order to allow comparison with other studies investigating familial effects on higher frequency bands in ADHD (Loo, et al., 2010), VLF power was compared across frontal (Fz, F3, F4), central (Cz, C3, C4), parietal (Pz, P3, P4) and, in addition, occipital (Oz, O1, O2) scalp electrode locations.
Statistical analysis
Two participants were excluded from analysis due to excessive artifact (DZ control) and extreme commission errors (n=37) indicative of insufficient task engagement (MZ ADHD).
For the analysis of performance data, the effects of age and general intelligence were regressed out of the data using Stata. All measures had pronounced heterogeneity of variance and skewed distributions and were log-transformed (optimised minimal skew through the lnskew0 command in Stata).
Preparation of EEG Data Prior to Model Fitting
The effects of age and general intelligence were regressed out of the data using Stata due to significant associations with both ADHD (Table 1) and VLF activity (age r= −0.12 to −0.19; g r= −0.26 to −0.33). Although general cognitive ability is confounded by the presence of psychiatric disorder, given that age and IQ are highly predictive of cognitive performance, regressing out these effects before twin modelling ensures straightforward interpretation; any reported associations between VLF activity and ADHD are independent of general cognitive ability and neurodevelopmental changes. Simultaneous analyses of dichotomous and continuous data could not be performed in the Mx program so both ADHD, which was scored as a dichotomous attribute, and the VLF measure, which was scored as a continuous variable, were modelled as threshold traits. Accordingly, each VLF measure was ordinalised into 5 equal classes in terms of proportions, which should capture most of the information in the continuous data. In order to provide a normal distribution for a more successful ordinal cut and to fulfil twin model assumptions, outliers were removed separately for each VLF parameter (±3 standard deviations from the mean) and log-transformed (optimised minimal skew through the lnskew0 command in Stata). The Mx software for structural equation modelling (Neale, Boker, Xie, & Maes, 1999)) was then used to estimate polychoric correlations and genetic model parameters using maximum likelihood statistics, while correcting for the selected nature of the sample.
Comparison of means
The comparisons of mean values were analyzed by means of a regression command in Stata that allows for non-independent observations (e.g. twin pairs) by using a robust cluster command to estimate standard errors. The association between the VLF activity and ADHD symptom scores, and VLF activity and performance measures, was investigated using Pearson’s product moment correlation coefficient on transformed residuals. Performance measures for correlation analysis were selected on the basis of trends toward case-control differences.
Twin correlations
Twin correlations between VLF activity and ADHD were estimated by fitting a constrained correlational model to the observed MZ and DZ data to produce (i) one overall within-twin across-trait correlation regardless of zygosity, i.e. between ADHD and VLF, (ii) the MZ and (iii) the DZ cross-twin within-trait correlation for VLF, and (iv) the MZ and (v) DZ cross-twin cross-trait correlation, by comparing one twin’s ADHD score and the co-twin’s VLF score. The MZ and DZ cross-twin correlations were fixed according to the point estimates for ADHD derived from the heritability estimate of a meta-analysis (rMZ= 0.76, rDZ= 0.38; (Faraone, et al., 2005), due to the uncertain ascertainment process for twins concordant and discordant for ADHD symptoms (see below Accounting for Selection; (Toulopoulou, et al., 2007)).
Genetic model fitting
A more sophisticated approach to the analysis of twin data is using structural equation models that model correlations between variables within individuals and across twins using relationships between observed and latent variables (Rijsdijk & Sham 2002). Liability-threshold models were used for both ADHD and VLF variables (Falconer & Mackay, 1996). The liability-threshold models for the dichotomized ADHD phenotype (affected versus non-affected) assume that risk is normally distributed on a continuum and that the disorder occurs only when a certain threshold is exceeded (Neale & Kendler, 1995). Both affected and unaffected individuals were assumed to be part of the same distribution of liability to the disorder, with each individual being either below or above the threshold.
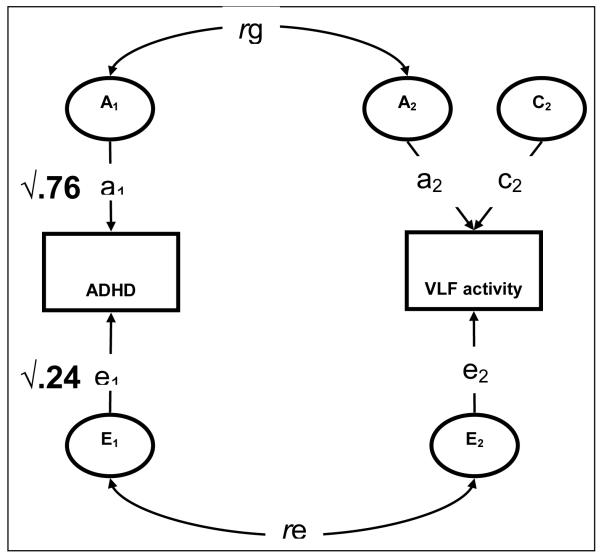
Through examination of the differences in correlations between MZ and DZ twin pairs, the variance of VLF activity can be decomposed into additive genetic (A), shared environmental (C) and unique environmental (E) components, in order to estimate (1) the heritability of VLF activity and (2) genetic and environmental correlations of ADHD with VLF activity. The applied ACE bivariate correlated factors liability-threshold model is illustrated in Figure 1. The parameter estimates from the model can be used to estimate the correlation between the genetic factors for ADHD and VLF activity (rg), which is an index of shared genetic effects between these parameters, and similarly for correlations of unique environmental factors (re). As the rg and re correlations do not take into account the heritability of either trait, it is possible for a large genetic correlation to actually explain a very small portion of the observed covariation between these two traits. Combining the information from the rg and re with the heritabilities of each trait, we can establish the genetic (rph-a) and unique environmental (rph-e) contributions to the total phenotypic correlation (rph) between ADHD and VLF activity (Toulopoulou, et al., 2007).
Figure 1. Constrained correlated factors bivariate model for ADHD and VLF activity1.
1 Circles represent latent additive genetic (A), shared environmental (C) and non-shared environmental (E) factors. a1, a2, e1, e2, c2 represent genetic and environmental path estimates. rg and re represent genetic and environmental correlations between ADHD and VLF activity, Parameters for ADHD (heritability and unique environmental estimates) are fixed values according to meta-analysis (Faraone, et al., 2005).
Accounting for Selection
Because the data are from twins selected for high or low ADHD symptoms scores, rather than a random sample, the heritability of ADHD cannot be estimated. Selected samples are more efficient and can be more powerful when studying low prevalence disorders (Neale, Eaves, & Kendler, 1994), but model fitting analyses will usually require an ascertainment correction. Since selection is based on ADHD and blind to VLF values, the required ascertainment correction will depend only on the model for ADHD and therefore the model parameters for ADHD were fixed to constant values supported by a meta-analysis of 20 studies of ADHD (model 1: h2=0.76, c2=0, e2=0.24; (Faraone, et al., 2005); univariate twin modelling of DSM-IV based ADHD scores on the Conners’ scores at age 8 in the TEDS sample (model 2: h2=0.89, c2=0, e2=0.11 (Ronald, Simonoff, Kuntsi, Asherson, & Plomin, 2008); and at age 12 in the TEDS sample (model 3: h2=0.73, c2=0.13, e2=0.14; C. Greven, unpublished data). In these models, the variance components for VLF activity, as well as their relationship with ADHD, are free parameters to be estimated from the data (Figure 1). The different parameter estimates for ADHD from the different models had no effect on the variance components for VLF activity and thus we only report those of the first model. In addition, we fixed the ADHD prevalence rate to a lifetime risk of 5% (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007).
RESULTS
Results from the regression and correlation analyses
Regression analyses indicated no significant differences between ADHD and control twins for cognitive performance measures (Table 2). VLF power was highest in central regions and was significantly reduced for central and parietal locations in the ADHD group, with the strongest association with central locations (Table 3). Therefore, to reduce the total number of variables and subsequent multiple testing bias VLF power at central regions was used in correlations between symptom scores and performance measures, and in twin modelling analyses in order to minimise heteroscedascity. Significant associations between VLF power and symptom scores were found in the control sample only (Table 4), suggesting increased symptoms of inattention and hyperactivity/impulsivity are associated with increased VLF power in typically developing adolescents. Reduced VLF activity was significantly associated with increased response variability in the ADHD group only (Table 5). All other associations were non-significant.
Table 2. Summary statistics of raw scores and mean comparisons adjusted for genetic relatedness for task performance measures based on age- and IQ-regressed scoresa.
|
All ADHD versus all
controls |
|||||
|---|---|---|---|---|---|
| Mean | SD | t | p | ||
| MRT (msec) | MZ Control (n=44) | 411.36 | 60.04 | 1.27 | .21 |
| DZ Control (n=38) | 379.18 | 47.00 | |||
| MZ ADHD (n=23) | 441.30 | 75.18 | |||
| DZ ADHD (n=28) | 407.93 | 56.01 | |||
| SD-RT (msec) | MZ Control (n=44) | 95.27 | 42.46 | 1.88 | .06 |
| DZ Control (n=38) | 77.08 | 30.13 | |||
| MZ ADHD (n=23) | 128.09 | 64.38 | |||
| DZ ADHD (n=28) | 102.00 | 38.91 | |||
| CV (SD-RT/MRT) | MZ Control (n=44) | 0.23 | .08 | 1.95 | .06 |
| DZ Control (n=38) | 0.20 | .07 | |||
| MZ ADHD (n=23) | 0.28 | 0.12 | |||
| DZ ADHD (n=28) | 0.25 | 0.09 | |||
| Commission errors (n) | MZ Control (n=44) | 2.64 | 2.64 | 1.21 | .22 |
| DZ Control (n=38) | 3.79 | 3.44 | |||
| MZ ADHD (n=23) | 4.78 | 4.35 | |||
| DZ ADHD (n=28) | 3.79 | 4.15 | |||
| O-not-X commission errors (n) | MZ Control (n=44) | 1.91 | 2.63 | −0.69 | .49 |
| DZ Control (n=38) | 2.32 | 2.19 | |||
| MZ ADHD (n=24) | 2.74 | 3.40 | |||
| DZ ADHD (n=28) | 2.39 | 3.02 | |||
| Omission errors (n) | MZ Control (n=44) | 0.68 | 0.86 | 1.43 | .16 |
| DZ Control (n=38) | 0.92 | 1.32 | |||
| MZ ADHD (n=22) | 1.87 | 1.98 | |||
| DZ ADHD (n=28) | 1.82 | 2.58 | |||
Abbreviations: MRT: mean reaction time in milliseconds; SD-RT: within-subject variability in RTs in milliseconds; CV: coefficient of variation (SD-RT/MRT); MZ: monozygotic; DZ: dizygotic
Table 3. Summary statistics and mean comparisons adjusted for genetic relatedness for VLF power based on transformed age- and IQ-regressed scores with extreme outliers removeda.
|
All ADHD versus all
controls |
|||||
|---|---|---|---|---|---|
| Mean | SD | t | p | ||
| VLF frontal | MZ Control (n=43) | 4.36 | .36 | −1.88 | .07 |
| DZ Control (n=37) | 4.36 | .40 | |||
| MZ ADHD (n=21) | 4.10 | .45 | |||
| DZ ADHD (n=27) | 4.28 | .48 | |||
| VLF central | MZ Control (n=44) | 5.29 | .18 | −3.03 | .003 |
| DZ Control (n=37) | 5.28 | .19 | |||
| MZ ADHD (n=20) | 5.17 | .14 | |||
| DZ ADHD (n=26) | 5.18 | .17 | |||
| VLF parietal | MZ Control (n=44) | 4.92 | .27 | −2.25 | .03 |
| DZ Control (n=37) | 4.87 | .23 | |||
| MZ ADHD (n=21) | 4.79 | .33 | |||
| DZ ADHD (n=27) | 4.74 | .27 | |||
| VLF occipital | MZ Control (n=44) | 4.34 | .46 | 1.99 | .05 |
| DZ Control (n=38) | 4.28 | .37 | |||
| MZ ADHD (n=21) | 4.20 | .51 | |||
| DZ ADHD (n=26) | 4.10 | .38 | |||
Abbreviations: VLF: very low frequency power; MZ: monozygotic; DZ: dizygotic
Table 4. Correlations between VLF power at central scalp locations and symptoms of ADHD (Pearson’s product moment correlation on transformed age and IQ-regressed scores)a.
| Control | ADHD | |||||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |||
| Inattention b | 0.41* | 0.19 | 0.70* | −0.01 | 0.04 | −0.05 |
| Hyperactivity/Impulsivity b | 0.44* | 0.34* | 0.71* | −0.09 | −0.22 | 0.02 |
p<.01,
p<.1
Abbreviations: MZ: monozygotic; DZ: dizygotic
ADHD symptom scores based on the Long Version of the Parent Conners’ Rating Scale (Conners, et al., 1998a) collected on the day of testing.
Table 5. Correlations between VLF power at central locations and performance measures (Pearson’s product moment correlation on transformed age and IQ-regressed scores)a.
| Control | ADHD | |||||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |||
| SD-RT | 0.07 | 0.02 | 0.11 | −0.21+ | −0.46* | 0.20 |
| CV | 0.08 | 0.06 | 0.09 | −0.28* | −0.49* | 0.04 |
p<.05,
p<.1
Abbreviations: MRT: mean reaction time in milliseconds; SD-RT: within-subject variability in RTs in milliseconds; CV: coefficient of variation (SD-RT/MRT); MZ: monozygotic; DZ: dizygotic
Results from the twin modelling analyses
The MZ cross-twin within-trait correlation for VLF power (r=0.37; 95%CI, 0.002 to 0.64) was greater than the DZ cross-twin within trait correlation for VLF power (r=0.21; 95%CI, −0.21 to 0.56) which suggests that genetic effects contribute to VLF power. As the MZ correlation for VLF power deviated from 1, this suggests that unique environmental influences contribute substantially to VLF power. Further, the DZ cross-twin within-trait correlation for VLF power was slightly more than half the MZ correlations, suggesting the presence of shared environmental effects. The MZ cross-twin cross-trait correlation between ADHD and VLF power (r= −0.42; 95%CI, −0.65 to −0.09) was greater than the DZ cross-twin cross-trait correlation (r=−0.14; 95%CI, −0.39 to 0.13), suggesting genetic effects contribute to the association between reduced VLF power and ADHD. The DZ cross-trait cross-twin correlation is less than half the MZ correlation, suggesting that non-additive genetic dominance effects may contribute to this overlap.
Structural equation modelling indicated that genetic factors accounted modestly for the total variation in VLF power (h2 =0.31; 95%CI, 0.01 to 0.64). Shared environment did not significantly explain individual differences in VLF power (c2 =0.06; 95%CI, 0 to 0.44), whereas unique environmental effects (incorporating also measurement error) accounted for a moderate part of the variance in VLF power (e2 =0.63; 95%CI, 0.38 to 0.92).
The extent to which ADHD and VLF activity share the same genetic and unique environmental effects is given by the correlations rg and re, respectively. Significant genetic correlations (rg=−0.80; 95%CI, −1.00 to −0.15) indicated substantial genetic overlap between ADHD and reduced VLF power. Unique environmental effects did not have a significant overlap with ADHD (re=0.41; 95%CI, −0.61 to 0.96). The negative phenotypic correlation (rph) suggested that increased liability to ADHD was associated with reduced VLF power (−0.23; 95%CI, −0.44 to −0.01). Due to the moderate to high heritabilities and moderate genetic correlation, the phenotypic correlation between ADHD and VLF power appears to be largely attributable to genetics (rph-a= −0.39; 95%CI, −0.60 to −0.08). Unique environmental contributions to phenotypic variance were non-significant (rph-e= 0.16; 95%CI, −0.24 to 0.42).
DISCUSSION
This study aimed to evaluate very low-frequency (VLF) neuronal activity as an intermediate phenotype of ADHD in a sample of adolescent monozygotic and dizgotic twin pairs concordant and discordant for ADHD symptoms. Genetic analyses showed that VLF activity demonstrates modest heritability, with no evidence of significant shared environmental effects. Structural equation modelling revealed a significant phenotypic association between high ADHD symptom scores during adolescence and reduced VLF activity during cognitive activation. Genetic factors were the main source of this association and unique environmental factors were not significant. This is the first study to support VLF activity as an electrophysiological marker of genetic risk in ADHD.
Heritability of VLF activity during a cognitive activation condition is consistent with reports of high heritability (Smit, et al., 2005) and high sibling similarity in an ADHD sample (Loo, et al., 2010) in higher frequency bands. This suggests that VLF activity has a genetic basis combined with a moderate contribution from unique environmental effects. The substantial genetic correlation between ADHD and VLF activity indicates that they are substantially influenced by the same genes, supporting VLF activity as a putative intermediate phenotype of the disorder. This finding is an important step in understanding the neurobiological pathways involved in the disorder and potentially to facilitate in the detection of susceptibility genes. For example, findings suggest a catecholaminergic deficiency underlies abnormal VLF oscillations, which is also widely reported in ADHD (Castellanos, et al., 2005). Indeed dopamine reuptake inhibitors (e.g. methylphenidate), which are used as treatment for ADHD, have been found to modulate slow oscillations in subcortical structures (Ruskin, et al., 2001). Causal tests of mediation are necessary to identify if these electrophysiological markers mediate aetiological effects on ADHD, rather than pleiotropic (or epiphenomenal) processes (Walters & Owen, 2008).
The phenotypic association between ADHD and VLF activity is consistent with previous studies reporting reduced VLF power at rest in ADHD (Helps, et al., 2010; Helps, et al., 2008). In addition, previous studies report an association between increased rest-to-task VLF attenuation and a higher number of inattention symptoms in typical adults (Helps, et al., 2009) and a clinical ADHD group (Helps, et al., 2010). In the present study, higher VLF activity was associated with increased levels of inattention and hyperactivity/impulsivity in the control group. Such discrepancies between control and ADHD participants may reflect neuropathological differences between groups, and also the robustness of the longitudinal method employed for group selection, compared to investigating symptom scores at the single time-point of data collection.
Overall, the findings for both groups support spontaneous VLF activity, typically associated with resting periods as exhibited by slow fluctuations of the BOLD signal, as present during cognitive activation, suggesting it represents a continuous process that is present during both resting and goal-oriented periods (Fransson, 2006). VLF activity are proposed to be an index of information integration or functional connectivity through synchronisation over widely distributed neuronal networks (Biswal, Yetkin, Haughton, & Hyde, 2005; He & Raichle, 2009). The association between ADHD and reduced VLF power in this study may therefore reflect reduced synchronisation of these widespread circuits. As VLF activity is proposed to relate to the DMN, one of several widely distributed networks as defined by fMRI (Sonuga-Barke & Castellanos, 2007), these findings suggest that individuals with ADHD demonstrate reduced DMN synchronisation during cognitive activation, or abnormal toggling between the anti-correlated introspective task-negative and extroceptive task-positive networks producing impairment to the functions they each serve (Fox, et al., 2005; Fransson, 2005; Sonuga-Barke & Castellanos, 2007).
The measurement of VLF activity during this cognitive task may include both spontaneous VLF activity and event-related VLF slow cortical potentials time-locked to the cue stimulus (the contingent negative variation). Although in this particular study there is limited evidence for a relationship between spontaneous VLF activity and event-related VLF activity (see Supplementary Material S2), it is still important that future work considers this relationship, particularly as without correction for multiple testing there is a small yet significant correlation between these measures. Along with the significant correlation between VLF activity and delta activity, such phenotypic overlap may suggest that the genetic contributions reported may be shared across frequency bands. In accordance, VLF phase has been shown to correlate with the magnitude of higher frequency bands suggesting a modulation of gross cortical excitability (Monto, et al., 2008; Vanhatalo, et al., 2004). Further work investigating phase locking with higher frequency bands as a measure of synchronisation will increase our understanding of the mechanisms underlying this overlap, particularly when used in genetically sensitive designs.
Advances in EEG source localisation techniques are likely to provide further insight into the relationship between the BOLD signal and EEG; recent work using sLORETA identified differential localisation of rest-task VLF attenuation dependent on ADHD status, in the absence of case-control differences in rest-task attenuation itself (Broyd, Helps, & Sonuga-Barke, 2011). This highlights potential topographical and source differences between control and clinical populations. In accordance with this, in the current study we identified group differences in VLF activity at central and parietal scalp locations only, and genetic effects emerged primarily for central scalp locations. Localisation differences may reflect the measure of VLF activity used (i.e. a comparison of VLF activity between rest and task, as opposed to VLF activity during the task itself) and task-specific demands that particular brain processes/regions subserve. Examination of changes over time and space in VLF activity in well-validated and robust EEG/ERP paradigms is required.
For measures of task performance, phenotypic associations with ADHD were non-significant. This is contrary to findings reporting slower and more variable reaction-times in ADHD (Klein, Wendling, Huettner, Ruder, & Peper, 2006; Kuntsi, et al., 2010) and increased omission errors (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). The lack of a group difference in the performance data is likely to be due to the use of the CPT-OX with flankers which was not specifically designed to optimally measure cognitive performance. Another possible reason for our lack of cognitive performance findings is our use of a population sample, rather than a clinical sample, albeit one selected for low and high ADHD symptom scores. One study reported increased RT variability in a population sample using ADHD symptom scores (similar to the study design here), but the data was collected at a younger age, in a larger sample and using task conditions that maximise RT variability, such as a slow event rate and lack of rewards (Kuntsi, Wood, Van der Meere, & Asherson, 2009). The greater association between ADHD and neurophysiological markers compared to task performance suggests EEG is a more sensitive index of genetic loading for ADHD than the cognitive performance measures, at least as derived from the task used in this study.
According to the default-mode interference hypothesis, a failure to fully attenuate VLF activity in cognitive activation conditions contributes to attentional lapses associated with ADHD (Sonuga-Barke & Castellanos, 2007). VLF activity was not associated with task performance measures in this study across the whole sample, but when collapsing the sample by ADHD status and zygosity, significant associations were found between reduced VLF power and increased response variability in the ADHD group only. This suggests reduced synchronisation during a task is associated with poor performance in ADHD, and is in accordance with previous studies that report associations between rest-task attenuation and increased errors and variability (Helps, et al., 2010). Further work incorporating analysis of trial-to-trial changes in the synchronicity between task performance and VLF activity is required (Castellanos, et al., 2005; Helps, et al., 2009) and linking this to optimal measures of RT variability will help to clarify this association.
Certain limitations must be taken into consideration. Firstly the sample size is relatively small for a twin study, such that although the estimates are modest to large, the confidence intervals around these estimates are also large introducing an overlap in the confidence intervals for MZ and DZ twins. This might suggest that the contribution from genetics is not significant; nevertheless combining the significant MZ correlations with the significant mean correlation differences suggests a familial nature that is likely to be of a genetic basis based on the fixed parameters used. This limited statistical power also restricted the estimation of shared environmental (as suggested by cross-twin within-trait correlations for VLF power) and non-additive genetic influences (as suggested by cross-twin cross-trait correlations between ADHD and VLF power). We acknowledge such influences may have contributed to the estimates of heritability and genetic overlap between ADHD and VLF activity in our study, although the genetic contribution presented is not overestimated. The substantial contribution of unique environmental influences on VLF activity may reflect error or instability in the measure. Reliability sets an upper limit on the estimates of heritability; any deviations from perfect reliability will increase measurement error and therefore unique environmental influences (Kuntsi, Rogers, et al., 2006). Studies of test-retest reliability are required to investigate the stability of both phenotypic associations and genetic influences on VLF activity (de Geus, 2002). In addition, the imputation of a relatively substantial proportion of general cognitive ability as a potential confounding influence may introduce further error arising from predicted scores, although the use of a robust multiple-imputation method is likely to have reduced this error variance. As we do not know whether the underlying causes of altered VLF activity are shared or disorder-specific, particularly as abnormalities are reported in several other neuropathological disorders such as autism and schizophrenia (Broyd, et al., 2009), it is important that future work directly compares VLF activity profiles across disorders.
In conclusion, applying genetic model fitting for the first time to VLF neuronal activity in ADHD, we have demonstrated modest heritability of VLF activity and substantial genetic overlap between ADHD and VLF activity. This supports the relevance of this novel measure of physiological arousal to our understanding of ADHD and indicates that lower VLF activity during cognitive activation is a potential intermediate phenotype for ADHD. This provides a basis for identification of specific genes that influence both ADHD and VLF activity, and warrants the investigation of the underlying mechanisms of VLF activity, in order to provide insight into the pathophysiological underpinnings of ADHD.
Supplementary Material
Growth Mixture Modelling on Longitudinal TEDS ADHD data
Associations between VLF power and higher frequency bands
Key points.
The identification of neurobiological processes that mediate between genes and behaviour may elucidate the aetiological pathways underlying complex psychiatric disorders such as ADHD.
The current study investigated genetic effects on very low-frequency (VLF) neuronal activity in adolescent twin pairs concordant or discordant for high or low ADHD symptom scores.
Subjects with high ADHD symptom scores demonstrated reduced VLF power that was associated with poor performance.
VLF activity demonstrated moderate heritability and shared genetic influences with ADHD symptoms, suggestive of an ideal intermediate phenotype of the disorder.
Investigating these processes in this key developmental period elucidates the pathophysiological underpinnings of ADHD, and may help in understanding their impact on social and academic functioning.
Acknowledgements
The authors would like to gratefully acknowledge the participating families and all staff involved in this study, in particular Chloe Booth, Sarah Lewis, Stuart Newman, the TEDS research team and the Director of TEDS, Robert Plomin. The PI (GM) was supported by a fellowship from the National Institute for Health Research.
References
- Balduzzi D, Riedner BA, Tononi G. A BOLD window into brain waves. Proceedings of the National Academy of Sciences. 2008;105(41):15641–15642. doi: 10.1073/pnas.0808310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 2005;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Helps SK, Sonuga-Barke EJS. Attention-Induced Deactivations in Very Low Frequency EEG Oscillations: Differential Localisation According to ADHD Symptom Status. PLoS ONE. 2011;6(3):e17325. doi: 10.1371/journal.pone.0017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszaki G, Draguhn A. Neuronal Oscillations in Cortical Networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of Attention-Deficit/Hyperactivity Disorder-Related Intra-Individual Variability. Biological Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. The Revised Conners’ Parent Rating Scale (CPRS-R): Factor Structure, Reliability, and Criterion Validity. Journal of Abnormal Child Psychology. 1998a;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners C, Sitarenios G, Parker J, Epstein J. Revision and restandardization of the Conners’ Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998b;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- de Geus EJC. Introducing genetic psychophysiology. Biological Psychology. 2002;61(1-2):1–10. doi: 10.1016/s0301-0511(02)00049-2. [DOI] [PubMed] [Google Scholar]
- Doehnert M, Brandeis D, Straub M, Steinhausen H-C, Drechsler R. Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: is there neurophysiological evidence for specific effects? Journal of Neural Transmission. 2008;115(10):1445–1456. doi: 10.1007/s00702-008-0104-x. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Addison Wesley Longman Ltd; Harlow: 1996. [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36(02):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function?: Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Haworth CMA, Wright MJ, Luciano M, Martin NG, De Geus E, Van Beijsterveldt C, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry. 2010;15(11):1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends in Cognitive Sciences. 2009;13(7):302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJS. Altered spontaneous low frequency brain activity in Attention Deficit/Hyperactivity Disorder. Brain Research. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, James CJ, Karl A, Sonuga-Barke EJS. The Attenuation of Very Low Frequency Brain Oscillations in Transitions from a Rest State to Active Attention. Journal of Psychophysiology. 2009;23(4):191–198. [Google Scholar]
- Helps SK, James CJ, Debener S, Karl A, Sonuga-Barke EJS. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. Journal of Neural Transmission. 2008;115(2):279–285. doi: 10.1007/s00702-007-0825-2. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: the science of mental ability. Praeger; Wesport: 1998. [Google Scholar]
- Jung T-P, Makeig S, Humphries C, Lee T-W, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. [PubMed] [Google Scholar]
- Kendall T, Taylor E, Perez A, Taylor C, Guideline Development Group Diagnosis and management of attention-deficit/hyperactivity disorder in children, young people, and adults: summary of NICE guidance. British Medical Journal. 2008;337:a1239. doi: 10.1136/bmj.a1239. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-Subject Variability in Attention-Deficit Hyperactivity Disorder. Biological Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kovas Y, Haworth CMA, Dale PS, Plomin R. The genetic and environmental origins of learning abilities and disabilities in the early school years. Monographs of the Society for Research in Child Development. 2007;72(3):1–144. doi: 10.1111/j.1540-5834.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends Cogn Sci. 2006;10(5):198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, McLoughlin G, Asherson P. Attention deficit hyperactivity disorder. NeuroMolecular Medicine. 2006;8(4):461–484. doi: 10.1385/NMM:8:4:461. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Börger NA, Meere J. v. d., Rijsdijk FV, et al. Reaction time, inhibition, working memory and performance: genetic influences and their interpretation. Psychological Medicine. 2006;36(11):1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood A, Rijsdijk F, Johnson K, Andreou P, Albrecht B, et al. Separation of cognitive impairments in attention deficit hyperactivity disorder into two familial factors. Archives of General Psychiatry. 2010;67:1159–1166. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Van der Meere J, Asherson P. Why cognitive performance in ADHD may not reveal true potential: Findings from a large population-based sample. Journal of the International Neuropsychological Society. 2009;15(04):570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Hale T, Hanada G, Macion J, Shrestha A, McGough JJ, et al. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with attention deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(4):368–377. [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Smalley SL. Preliminary report of familial clustering of EEG measures in ADHD. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(1):107–109. doi: 10.1002/ajmg.b.30575. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, et al. Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behavioral and Brain Functions. 2010;6(1):66. doi: 10.1186/1744-9081-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G, Asherson P, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, et al. Cognitive-electrophysiological indices of attentional and inhibitory processing in adults with ADHD: familial effects. Behavioral and Brain Functions. 2011;7:26. doi: 10.1186/1744-9081-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G, Kuntsi J, Brandeis D, Banaschewski T. Electrophysiological parameters in psychiatric research: ADHD. Psychiatry. 2005;4(12):14–18. [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. Very Slow EEG Fluctuations Predict the Dynamics of Stimulus Detection and Oscillation Amplitudes in Humans. Journal of Neuroscience. 2008;28(33):8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical Modeling. 5th ed Department of Psychiatry, Box 126 MCV; Richmond, VA 23298: 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer; Dordrecht: 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. American Journal of Human Genetics. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research and Human Genetics. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary scales. Oxford University Press; Oxford: 1996. [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Shenker A, Freeman LE, Baek D, Walters JR. Drugs used in the treatment of attention-deficit/hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine autoreceptor action. Biological Psychiatry. 2001;49(4):340–350. doi: 10.1016/s0006-3223(00)00987-2. [DOI] [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: an empirical approach to Attention-Deficit Hyperactivity Disorder. Neuroscience & Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Smit D, Posthuma D, Boomsma DI, Geus EJC. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42(6):691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, et al. Substantial Genetic Overlap Between Neurocognition and Schizophrenia: Genetic Modeling in Twin Samples. Archives of General Psychiatry. 2007;64(12):1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Research and Human Genetics. 2002;5:444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- Tye C, McLoughlin G, Kuntsi J, Asherson P. Electrophysiological markers of genetic risk for attention deficit hyperactivity disorder. Expert Reviews in Molecular Medicine. 2011;13 doi: 10.1017/S1462399411001797. null-null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko L, Doehnert M, Müller UC, Schneider G, Albrecht B, Drechsler R, et al. Differences in Neurophysiological Markers of Inhibitory and Temporal Processing Deficits in Children and Adults with ADHD. Journal of Psychophysiology. 2010;23(4):235–246. [Google Scholar]
- Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Molecular Psychiatry. 2008;12(10):886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children - third edition UK (WISC-IIIUK) manual. The Psychological Corporation; London: 1992. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth Mixture Modelling on Longitudinal TEDS ADHD data
Associations between VLF power and higher frequency bands