Sir,
We read with interest the article by Loupakis et al (2013) entitled ‘Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab' published in the June 2013 issue of the British Journal of Cancer. This paper clearly underlines the positive impact of FOLFOXIRI plus bevacizumab, on the extent of both tumour regression and necrosis, in resected liver metastases from colorectal cancer (CRC). The authors conclude that the addition of bevacizumab leads to a high ‘histopathologic activity' as compared to FOLFOXIRI or XELOXIRI alone. These data are important as pathologic response is considered as a new outcome end point by some authors, representing a prognostic parameter and a marker of sensitivity to preoperative treatments (Rubbia-Brandt et al, 2007; Blazer et al, 2008). Indeed, the higher the histopathologic response, the longer the survival (Rubbia-Brandt et al, 2007; Blazer et al, 2008). In this setting, we would like to mention several points that may be clinically relevant. First of all, pathologic complete response (pCR) was defined, in the study by Loupakis et al (2013), as the absence of tumour cells replaced by fibrosis and/or necrosis. This pCR definition corresponds to the grade 0 of the classification proposed by Blazer et al (2008), which is based exclusively on the percentage of residual tumour cells whatever the type of regression. However, in the Tumour Regression Grade (TRG) classification as proposed by Rubbia-Brandt et al (2007), fibrosis, but not necrosis, is considered as a characteristic feature of cellular response. According to these authors, the necrosis seen in CRC liver metastases is linked to spontaneous evolution of the tumour, involving insufficient vascular supply, and not to the treatment itself, thus excluding this characteristic from the TRG. In contrast, Li Chang et al (2012) recently showed a particular type of necrosis, so-called ‘infarct-like necrosis' (ILN), characterised by large confluent areas of eosinophilic cytoplasmic remnants, located centrally within a lesion and surrounded by a rim of fibrosis with foamy macrophages (Li Chang et al, 2012). This necrosis is morphologically different from the so-called ‘dirty necrosis', usually seen in CRC, containing nuclear debris in a patchy distribution. In this study, ILN was only seen in preoperatively treated CRC liver metastases and never observed in untreated patients who underwent primary resection of CRC liver metastases. In addition, Li Chang et al (2012) also noticed that ILN was significantly associated with chemotherapy plus bevacizumab treatment, although this feature was not specific and was also encountered with chemotherapy alone. Moreover, progression-free survival and overall survival were longer in patients with CRC whose liver metastases showed ILN as compared with CRC patients whose metastases lacked this feature. Besides the well-designed work by Loupakis et al (2013), several studies concerning preoperative treatment of liver metastases have already reported a higher percentage of necrotic areas in tumours treated with bevacizumab (Klinger et al, 2010; Wicherts et al, 2011). However, the precise type of necrosis involved in tumour response was not reported.
Our team recently confirmed the previous findings of Li Chang et al (2012), but on a larger population of bevacizumab-treated patients and in the setting of first-line metastatic treatment. We retrospectively reviewed archival liver CRC metastases from 91 patients who underwent secondary resection after preoperative treatment. On the basis of tumour availability, three group of patients with liver metastases were identified: a control group of chemonaive metastases (n=29), a group with metastases treated with chemotherapy (CT) alone (n=31) and a group with metastases treated with CT and bevacizumab (n=31). The frequency of ILN was statistically different among the three groups (P<0.001, Table 1). Infarct-like necrosis was observed in 53% of liver metastases of treated patients (CT only and CT+bevacizumab), but never in those of untreated patients (P<0.001). Moreover, the rate of ILN lesions was much higher in the CT plus bevacizumab group than in the CT group (68% vs 39%, P=0.02). The histologic features observed in our series of ILN cases were similar to those described in the study by Li Chang et al (2012) (Figure 1). Taken together, these data indicate that ILN is a particular feature of tumour response induced by the preoperative treatment. Therefore, it would be interesting, in the study by Loupakis et al (2013), to determine which type of necrosis is present in the liver metastases of the control arm (i.e., without any preoperative treatment) and to confirm that ILN might be a ‘bevacizumab-related effect'.
Table 1. Frequency of ILN in liver metastases from colorectal cancer according to treatment.
| No treatment, N=29 | CT only, N=31 | CT+Beva, N=31 | P-valuea | |
|---|---|---|---|---|
| Presence of ILN |
0 (0%) |
12 (39%) |
21 (68%) |
P<0.001 |
| Lack of ILN | 29 (100%) | 19 (61%) | 10 (32%) |
Abbreviations: Beva=bevacizumab; CT=chemotherapy; ILN=infract-like necrosis.
χ2-test.
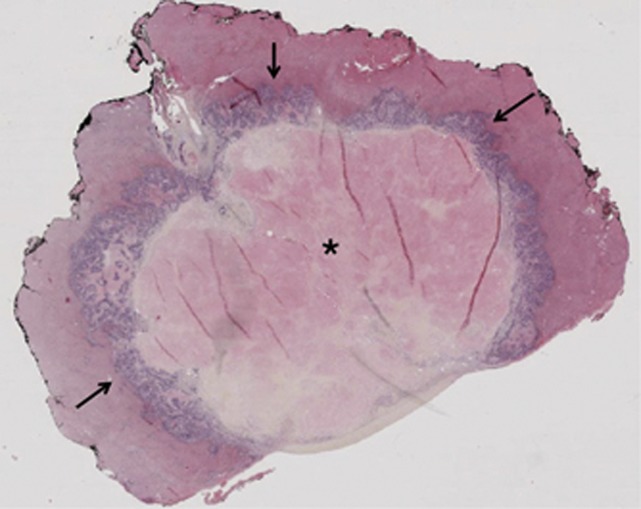
Figure 1.
Subcapsular liver metastasis resected after preoperative treatment combining chemotherapy and bevacizumab. Typical well-limited lesion with central infarct-like necrosis (*), surrounded by fibrosis and residual tumour (arrows).
Finally, we would like to point out the correlation established between the histologic features and the radiologic patterns of CRC liver metastases after preoperative treatment, and skilfully discussed by Loupakis et al (2013). Indeed, size criteria do not seem to be accurate enough to properly evaluate the radiologic impact of treatment combining chemotherapy and bevacizumab, and morphologic characteristics are thought to be more useful parameters (Chun et al, 2009). In this setting, Maru et al (2010) suggested measuring the tumour thickness at the tumour–normal liver interface as a novel pathologic indicator of chemotherapy response in liver CRC metastases. They found that tumour thickness correlated better with radiologic response, according to morphologic characteristics than by RECIST criteria. Tumour thickness also correlated with pathologic response assessed according to the Blazer classification. In addition, greater thickness predicted shorter recurrence-free survival and this parameter remained statistically significant in multivariate analysis. Interestingly, tumour thickness was significantly smaller in patients treated with bevacizumab than in patients who were not.
In conclusion, this promising work by Loupakis et al (2013) highlights the impact of intensive preoperative treatments on the pathologic response of CRC liver metastases. Moreover, it equally strengthens the need for more precise and rational classification of this response, especially with anti-angiogenic therapies. Indeed, identifying ILN may represent an important contribution from pathologists when assessing chemotherapy and targeted treatment response in both clinical and research settings.
Acknowledgments
We would like to acknowledge the editorial assistance of Nikki Sabourin and Julie Courraud.
The authors declare no conflict of interest.
References
- Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H, Charnsangavej C, Loyer EM. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B, Gruenberger T. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059–2065. doi: 10.1245/s10434-010-0972-9. [DOI] [PubMed] [Google Scholar]
- Li Chang HH, Leeper R, Chan G, Quan D, Driman DK. Infarct-like necrosis: a distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am J Surg Pathol. 2012;36:570–576. doi: 10.1097/PAS.0b013e31824057e7. [DOI] [PubMed] [Google Scholar]
- Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, Cremolini C, Salvatore L, Morvillo M, Antoniotti C, Marmorino F, Masi G, Falcone A. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer. 2013;108:2549–2556. doi: 10.1038/bjc.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru D, Kopetz S, Boonsirikamchai P, Agarwal A, Chun YS, Wang H, Abdalla EK, Kaur H, Charnsangavej C, Vauthey JN, Loyer EM. Tumour thickness at the tumour-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B. Importance of histological tumour response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- Wicherts DA, de Haas RJ, Sebagh M, Saenz Corrales E, Gorden DL, Levi F, Paule B, Azoulay D, Castaing D, Adam R. Impact of bevacizumab on functional recovery and histology of the liver after resection of colorectal metastases. Br J Surg. 2011;98:399–407. doi: 10.1002/bjs.7368. [DOI] [PubMed] [Google Scholar]