Abstract
Background:
The aim of our analysis is to further characterise the prognostic relevance of early tumour shrinkage (TS) during VEGF-targeted therapy in mRCC, in order to explore whether this could define a group of patients with long-term survivorship.
Methods:
A hundred patients were stratified into five subgroups according to their change of tumour size with first treatment evaluation: −100% to −60% −59% to −30% and −29% to 0% TS or gain of tumour size from 1% to 19% and ⩾20% or occurrence of new lesions (i.e., progressive disease).
Results:
The median PFS and OS were 10.4 months and 28.2 months, respectively. The median OS stratified according to the subgroups as described above was 77.4, 33.5, 26.9, 30.0 and 14.3 months, respectively. Multivariate analysis revealed early TS as a prognostic marker (P=0.021; HR 1.624).
Conclusion:
The extent of TS defines a small proportion of patients with an excellent prognosis. Larger studies are warranted to define the relationship of long-term survivorship and extent of TS with targeted therapies.
Keywords: VEGF-targeted therapy, tumour shrinkage, first treatment evaluation, metastatic renal cell carcinoma (mRCC), deep remission
The implementation of targeted therapies inhibiting the vascular endothelial growth factor (VEGF) or the mammalian target of rapamycin (mTOR) pathway established new standards for the treatment of metastatic renal cell carcinoma (mRCC). To date, the tyrosine kinase inhibitors (TKIs) sunitinib and pazopanib as well as the monoclonal antibody bevacizumab (in combination with interferon) are considered as the standard of care for first-line treatment in patients with good and intermediate prognosis according to the MSKCC risk stratification. These agents are perceived of having similar efficacy, measured by the median progression-free survival (PFS) of sunitinib, pazopanib or bevacizumab with 11, 9.2 and 10.2 months and an objective response rate (ORR) of 31, 30 and 31%, respectively (Escudier et al, 2007; Motzer et al, 2007; Sternberg et al, 2010). Recently, treatment results of a new generation of TKIs were introduced. The use of tivozanib was associated with a median PFS of 12.9 months compared with 9.1 months (P=0.037) with sorafenib in treatment-naive patients (Motzer et al, 2012). Axitinib reached a median PFS of 10.1 months compared with 6.5 months with sorafenib (P=0.038), but the stratified hazard ratio came in at 0.72 failing its primary statistical endpoint (Hutson et al, 2013). ORR was 33% and 32% for tivozanib and axitinib in these studies, respectively. Even with these selective VEGF-receptor inhibitors, a major advancement in PFS and ORR may not be reached in mRCC. It remains unclear which measures would really advance the field of mRCC, but long-term survivorship is generally conceived as a proper outcome.
The relevance of objective tumour remission in mRCC was characterised by an analysis presenting the superior outcome for patients achieving an objective tumour remission with sunitinib treatment (Molina et al, 2012). At the same time, our group presented a tumour shrinkage (TS) of ⩾10% within the first 12 weeks of treatment as prognostic parameter for a longer PFS and OS (Grünwald et al, 2012). Another analysis presented data on initial tumour size reduction of primary tumours as independent predictor for OS in renal cancer, underscoring the relevance of early treatment evaluation (Abel et al, 2011). Even in Phase I studies, the change of tumour size according to Response Evaluation Criteria In Solid Tumours (RECIST) proved to correlate with prognosis, but boundaries for efficacy assessment have been questioned (Jain et al, 2012).
The aim of our analysis is to investigate if there is a prognostic relevance for early TS under VEGF-targeted therapy in mRCC, in order to explore whether this approach could define a group of patients with long-term survivorship.
Patients and methods
Clinical data of 100 mRCC patients treated with VEGF-targeted therapies between 2005 and 2013 were retrieved. No restrictions were made concerning the histological subtype, the performance status (ECOG) or the risk stratification scores according to the MSKCC and Heng Score (Oken et al, 1982; Motzer et al, 2002; Heng et al, 2009). Patient characteristics are shown in detail in Table 1. Medical records were analysed retrospectively in accordance with the declaration of Helsinki. Patients received computed tomography (CT) of the thorax and abdomen with contrast medium after two treatment cycles as standard procedure. In case of an impaired kidney function, a magnetic resonance imaging of the abdomen and native chest CT scans of the thorax were performed. The median time of follow-up (from the day of first treatment evaluation) was 22 months.
Table 1. Patient characteristics.
| N | |
|---|---|
| No. of patients |
100 |
|
Gender | |
| Female | 33 |
| Male |
67 |
| Median age beginning TKI treatment, years |
62 |
| Nephrectomy before systemic treatment |
95 |
|
Histology | |
| Clear cell | 90 |
| Non-clear cell histology |
10 |
|
Metastatic organs | |
| Bone | 24 |
| Liver | 27 |
| Lung | 71 |
| Lymph node |
54 |
|
Number of metastatic organ sites | |
| <3 | 50 |
| ⩾3 |
50 |
|
Best response | |
| CR | 6 |
| PR | 25 |
| SD | 48 |
| PD |
21 |
|
Tumour remission with first treatment evaluation | |
| 100–60% | 7 |
| 59–30% | 20 |
| 29–0% | 39 |
| Gain of 1–19% | 15 |
| ⩾20% and new tumour lesions |
19 |
|
ECOG | |
| 0 | 79 |
| 1 |
18 |
|
MSKCC | |
| Low | 22 |
| Intermediate | 54 |
| Poor | 5 |
| Missing |
19 |
|
Heng Score | |
| Low | 11 |
| Intermediate | 42 |
| Poor | 7 |
| Missing |
40 |
| Second-line treatment |
77 |
| mTOR |
45 |
| TKI |
32 |
| Previous immunotherapy | 41 |
Abbreviations: CR=complete response; ECOG=Eastern cooperative oncology group; mTOR=mammalian target of rapamycin; MSKCC=Memorial Sloan-Kettering Cancer Center; PD=progressive disease; PR=partial response; SD=stable disease; TKI=tyrosine kinase inhibitor.
After radiological appraisal, imaging of first treatment evaluation compared with baseline was re-evaluated by our study group. The response to systemic therapy was then evaluated according to the RECIST version 1.1, and the change of tumour size was defined by the fraction of decrease or increase of the sum of the largest diameter (SLD) of target lesions (Eisenhauer et al, 2009). For this purpose, 236 target lesions were defined and their response to treatment was analysed.
The early change of tumour size was defined as treatment response detected with first evaluation performed after two treatment cycles. After imaging was evaluated, patients were stratified into five subgroups according to our stratification strategy with TS from −100% to −60% −59% to −30% −29% to 0% or a gain of SLD from 1% to 19% and ⩾20% or the occurrence of new lesions (i.e., progressive disease).
The aim of evaluating early treatment response was to investigate the role of the change of tumour size as a prognostic marker. Our patient stratification strategy was performed to arrange our patients into multiple groups to analyse treatment responders without TS and treatment responders with TS and to introduce also a subgroup with excellent TS to further specify initial treatment responders.
The outcome of patients with objective tumour remission compared with patients achieving stable disease as best response was also compared to further discuss the role of tumour remission throughout the course of first-line treatment. Patients best response results throughout first-line VEGF-targeted therapy was already available with our data bank and was not re-evaluated by our study group.
Treatment regimens
First-line VEGF-targeted therapies consisted of sunitinib in 73 patients, sorafenib in 12 patients, axitinib in 8 patients, bevacizumab/interferon alpha in 3 and pazopanib in 4 patients. Before VEGF-targeted therapy, 40 patients received interferon alpha (IFN). Sunitinib was administered as 50 mg once daily on 28 consecutive days of a given 6-weeks cycle, and sorafenib was administered continuously 400 mg BID. Bevacizumab (Bev) was infused at 10 mg/kg intravenously every 2 weeks, with IFN (Bev/IFN) applied subcutaneously thrice a week with up to 9 Mio units. Pazopanib 800 mg was applied continuously once daily. Every patient received axitinib within the ‘Axitinib (AG-013736) With Or Without Dose Titration (Increase) In Patients With Kidney Cancer' (NCT00835978) clinical trial. Axitinib was administered according to the protocol, consisting of 5–10 mg twice daily (BID).
In case of significant toxicity, dose reductions were performed to avoid early treatment abort and for quality-of-life improvement. Sunitinib doses were reduced from 50 mg to 37.5 mg, and further to 25 mg OD if necessary. Sorafenib dose reductions consisted of a decrease to 200 mg BID. Pazopanib doses were reduced from 800 mg to 600 mg and further to 400 mg OD. Bev/IFN dose reduction consisted of IFN dosage decrease from 9 to 6 Mio or 3 Mio units. Dose reductions of axitinib were according to the protocol.
Of the 85 patients who already progressed under first-line therapy at the time of analysis, 73 received further treatment lines with an mTORi (n=44) or another TKI (n=29). Patients received 1–6 different treatment lines (median 3). Three patients died during first-line therapy, others deceased shortly after tumour progression. Best supportive care, that is, use of analgesics, antiemetic therapy and psychological assistance was provided to every patient ambulatory or by inpatient treatment.
Statistics
Statistical analyses were performed to detect prognostic factors that are significantly associated with the OS. Survival curves were calculated using the Kaplan–Meier method. Patients were censored at the date of last follow-up. OS was investigated from the day of initiation of first-line VEGF-targeted therapy to the time of death or censored at the date of last follow-up. Kaplan–Meier curves comparing the OS between patient characteristics were constructed and log-rank testing was used to compare these censored outcomes. The following patient characteristics were tested: change of tumour size with first treatment evaluation, MSKCC risk score before first-line TKI treatment, the Heng Score before first-line TKI treatment, ECOG performance status, histology, previous immunotherapy, number of metastatic organ sites before TKI therapy (<3 vs ⩾3 organ systems), and location of metastatic organ sites, for example, the lung, liver, bone and lymph nodes. Variables were found to be significant if a two-sided P-value was <0.05 on univariate testing. We also used the Cox proportional hazards model for multivariable analysis. The variables that reached statistical significance in this model were then deemed to be independent predictors for treatment outcome concerning the OS. Kaplan–Meier analysis was performed with the landmark method (Anderson et al, 1983). All statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS) 19 (Chicago, IL, USA).
Landmark analyses
The landmark analysis was performed for statistical analyses. The intention of the landmark method is to estimate in an unbiased way the time-to-event probabilities in each group conditional on the group membership of patients at a specific time point, the landmark time. Therefore patients who died before 3 and 6 months after TKI initiation were excluded from further statistical analyses. The 6-months landmark strategy was chosen by our study group, because 6 months were already explored to be a valid prognostic parameter for patients' outcome in previous studies (Heng et al, 2011; Seidel et al, 2012), while 3 months roughly implies the time period from treatment initiation to first treatment evaluation (median: 2.75 months in our analyses).
Results
Outcome and response of first-line VEGF-targeted therapy
The median OS from the start of first-line VEGF-targeted therapy was 28.2 months (95% CI 21.8–34.6). First-line VEGF-targeted therapy was associated with a median PFS of 10.4 months (95% CI 8.2–12.6).
Landmark analyses
A 3- and 6-months landmark strategy was performed for this analysis. Four patients were excluded from analysis for the 6-months landmark due to death before 6 months after treatment initiation. One patient died after 1.3 months and was therefore excluded for the 3-months landmark strategy. Also, 3 of the 4 patients had progressive disease with first treatment evaluation while 1 patient received a tumour remission of −3%.
Treatment outcome best response
Best response was SD in 48 patients, PR in 25 patients and CR in 6 patients. Twenty-one patients had PD at first tumour evaluation.
Change of tumour size with first treatment evaluation
The patients were stratified according to the initial change of tumour size. Seven patients reached a TS from −100% to −60%, 20 patients from −59% to −30% 39 patients reached from −29% to 0% and 15 patients gained tumour size from 1 to 19%, while 19 patients initially had a gain of tumour size of ⩾20% or occurrence of new lesions. Also, 8 patients had progressive disease due to new lesions, while 11 patients had gain of tumour size only. There was no difference concerning the OS between both the groups (P=0.36).
Toxicity analyses
Dose reductions due to adverse events were required in 30 patients. Systemic treatment with first-line VEGF-targeted therapies had to be permanently interrupted due to toxicity in 12 patients. Treatment cessation was caused by acute renal failure, mucositis, proteinuria, cardiac insufficiency, thrombopenia, alveolitis, angina pectoris, diarrhoea and hand–foot syndrome.
Association between response and OS
Univariate analyses
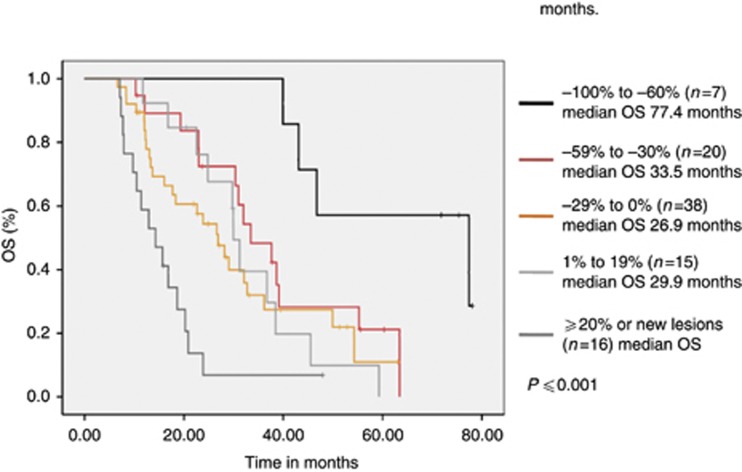
The Kaplan–Meier plots showed a statistical significant correlation between the change of tumour size with first treatment evaluation and OS (Figure 1). For the 6-months landmark, the median OS was 77.4 months (95% CI 30.6–124.2) in patients with a TS from −100% to −60%, 33.5 months (95% CI 24.8–42.2) for TS from −59% to −30%, 26.9 months (95% CI 20–33.7) for TS from −29% to 0% 30 months (95% CI 27.9–32.1) in patients with an increasing tumour size from 1% to 19%, and 14.3 months (95% CI 8.6–20.2) for patients with an increase of ⩾20% or new lesions (i.e., PD). As already depicted by the separation of the Kaplan–Meier curves, the log-rank test supported this observation by reaching significance (P⩽0.001). Further patient statistical investigations revealed the following patient characteristics to correlate with the OS: Patients with bone metastases (P=0.013) and a non-clear cell histology (clear cell differentiation vs others) (P⩽0.001) had an impaired prognosis (Table 2).
Figure 1.
Survival distribution of the different patient cohorts with TS from −100% to −60% −59% to −30% −29% to 0% and gain of tumour size from 1% to 19% and ⩾20% or occurrence of new lesions. Kaplan–Meier analysis with a landmark set to 6 months is shown. Also, n=1 and n=3 patients with a tumour remission from 0 to 29% and with progressive disease, respectively, were excluded from analyses due to death before 6 months. Results demonstrate that patients with major tumour remission (−100% to −60%) have a significantly prolonged OS. Others had a more homogenous course of the disease, while treatment non-responders exert the worst prognosis.
Table 2. Results of univariate and multivariate analysis for overall survival.
|
Results of univariate analyses for all prespecified prognostic factors with 6-months landmark | ||||
|---|---|---|---|---|
| Characteristic | Value | P value of univariate analyses | Median OS in months | 95% confidence interval |
| Initial tumour remission |
100–60% 59–30% 29–0% 0–20% gain; >20% gain |
<0.001 |
77.4 vs 33.5 vs 26.9 vs 29.9 vs14.3 |
30.60–124.18 and 24.78–42.16 and 20.0–33.71 and 27.88–32.06 and 8.56–20.04 |
| Histology |
Non-clear cell vs clear cell histologies |
<0.001 |
13 vs 30.3 |
9.86–16.10 and 26.70–34.04 |
| Heng Score |
Good vs intermediate vs poor |
<0.001 |
29.1 vs 26.9 vs 10.5 |
21.10–37.02 and 14.53–39.19 and 7.50–13.49 |
| Bone metastases |
Pts. with vs without bone metastases |
0.013 |
22.9 vs 31.9 |
14.36–31.54 and 23.84–40.14 |
| MSKCC score |
Good vs intermediate vs poor |
0.065 |
38.7 vs 23 vs 30 |
30.10–47.27 and 14.11–31.79 and 0 to 61.41 |
| ECOG performance status |
ECOG 0 vs ECOG 1 |
0.117 |
30.4 vs 23.9 |
25.24–35.46 and 17.60–30.15 |
| Previous cytokine therapy |
Pts. with vs without previous cytokine therapy |
0.219 |
31 vs 23.9 |
28.16–33.84 and 14.20–33.54 |
| Numer of metastatic organ sites |
<3 vs ⩾3 metastatic organ sites |
0.342 |
30.4 vs 28.2 |
21.18–39.52 and 21.30–35.10 |
| Lung metastases |
Pts. with vs without lung metastases |
0.497 |
24.8 vs 33.5 |
17.96–31.68 and 24.75–39.23 |
| Lymphnode metastases |
Pts. with vs without lymph node metastases |
0.991 |
24.8 vs 31.9 |
17.96–31.68 and 24.75–39.23 |
| Liver metastases | Pts. with vs without liver metastases | 0.923 | 36.7 vs 28.2 | 11.35–62.09 and 21.48–34.88 |
Abbreviations: ECOG=Eastern cooperative oncology group; MSKCC=Memorial Sloan-Kettering Cancer Center; OS=overall survival.
Univariate analysis revealed initial tumour shrinkage (P⩽0.001), histology (clear cell differentiation vs non-clear cell) (P⩽0.001), the Heng Score (P⩽0.001) and bone metastases (P=0.013) as prognostic variables.
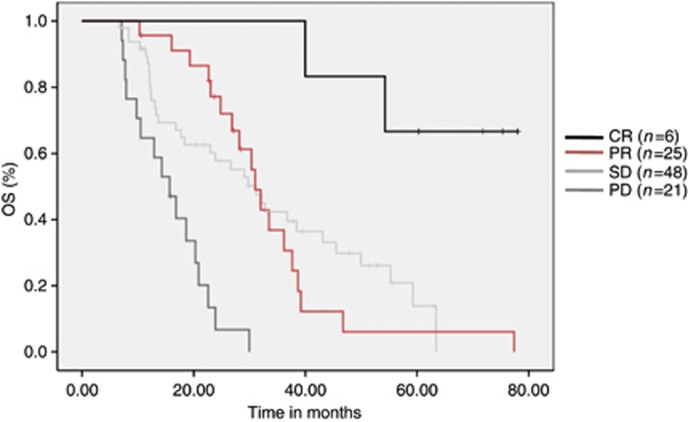
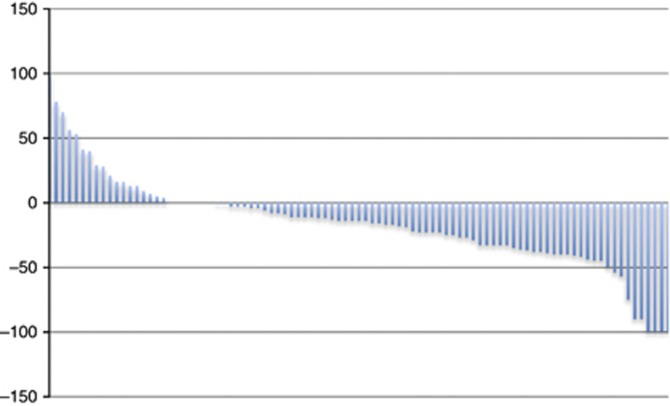
Patients achieving objective tumour remission (CR and PR) under first-line VEGF-targeted therapy (n=29) had a median OS of 36 months compared with 31 months in patients with stable disease (P=0.217; 95% CI 26.1–38.1; Figure 2). The TS of target lesions according to RECIST 1.1 is demonstrated by a waterfall plot for individual patients in Figure 3.
Figure 2.
Survival distribution according to best response during first-line VEGF-targeted therapy. Patients achieving objective tumour remission (CR and PR) under first-line VEGF-targeted therapy had a median OS of 36 months compared with 31 months in patients with stable disease (P=0.217; 95% CI 26.1–38.1). Altogether, patients with a PR, SD and PD had a median OS of 31, 31.2 and 15.7 months, respectively.
Figure 3.
Waterfall plot—Change of baseline in percentage with first treatment evaluation. Changes in tumour burden were quantifiable by RECIST for 92 patients included in this study. Of the 19 patients having PD, 8 progressed with new lesions at first restaging, unquantifiable by SLD. Patients with measurable changes had a range of tumour changes from complete disappearance to a 100% increase in SLD.
Multivariate analyses
Cox proportional hazard models at a landmark time of 6 months were used to assess the effect of prognostic factors on OS (Table 3). Multivariate analyses was performed comparing the following factors: change of tumour size with first treatment evaluation, stratified according to TS from −100% to −60% −59% to −30% −29% to 0% or a gain of SLD from 0% to 20% and ⩾20% or occurrence of new lesions, were compared with the MSKCC risk score, ECOG performance status, histology, previous immunotherapy, number of metastatic organ sites, location of metastatic organ sites, for example, the lung, bone and lymph nodes. Multivariate analyses confirmed the relevance of early TS as prognostic factor for OS (P=0.021; HR 1.624). Osseous lesions also proved to be a valid prognostic factor (P=0.042; HR 2.046). A less conservative approach applied the 3-months landmark strategy for multivariate analysis. Early TS and osseous lesions were confirmed to be prognostic factors with P=0.01; HR 1.58 and P=0.024; HR 2.26, respectively. In addition, the MSKCC score also proved to be a significant prognostic factor (P=0.04; HR 1.96).
Table 3. Multivariate analysis for the 6-months strategy confirmed the relevance of initial tumour shrinkage and osseous lesions as a prognostic factor for OS with P=0.021; HR 1.62 and P=0.042; HR 2.05, respectively.
|
Final multivariate cox proportional hazards models OS with 6-months landmark | ||||
|---|---|---|---|---|
| Characteristic | Value | P value of multivariate analyses | HR | 95% confidence interval |
| Initial tumour remission |
−100% to −60% −59% to −30% −29% to 0% 0 to 19% gain; ⩾20% gain |
0.021 |
1.624 |
1.06–2.09 |
| Bone metastases |
Pts. with vs without bone metastases |
0.042 |
2.046 |
1.03–4.08 |
| MSKCC score |
Good vs intermediate vs poor |
0.079 |
1.719 |
0.94–3.15 |
| Histology |
Non-clear cell vs clear cell histologies |
0.141 |
0.422 |
0.13–1.33 |
| Lymphnode metastases |
Pts. with vs without lymph node metastases |
0.217 |
0.695 |
0.34–1.28 |
| ECOG performance status |
Eastern cooperative oncology group (ECOG) ECOG 0 vs ECOG 1 |
0.371 |
0.672 |
0.28–1.61 |
| Previous cytokine therapy |
Pts. with vs without previous cytokine therapy |
0.641 |
0.859 |
0.46–1.62 |
| Number of metastatic organ sites |
<3 vs ⩾3 metastatic organ sites |
0.823 |
0.932 |
0.50–1.73 |
| Lung metastases |
Pts. with vs without lung metastases |
0.892 |
1.047 |
0.54–2.04 |
| Liver metastases | Pts. with vs without liver metastases | 0.978 | 1.010 | 0.49–2.10 |
Abbreviations: HR=hazards ratio; MSKCC=Memorial Sloan-Kettering Cancer Center; OS=overall survival.
A second strategy of multivariate analysis tested the three variables: change of tumour size with first treatment evaluation, the MSKCC risk score and osseous lesions with a 6-months landmark. Multivariate analyses also proved osseous lesion and early TS as valid prognostic factors with P⩽0.001; HR 1.640 and P=0.043; HR 1.884, respectively (Table 4).
Table 4. A second strategy tested the most frequent prognostic parameters for metastatic renal cancer, testing initial TS with osseous lesions and the MSKCC score.
|
Final multivariate cox proportional hazards models OS with 6-months landmark | ||||
|---|---|---|---|---|
| Characteristic | Value | P value of multivariate analyses | HR | 95% confidence interval |
| Initial tumour remission |
100–60% 59–30% 29–0% 0–20% gain; >20% gain |
<0.001 |
1.640 |
1.26–2.12 |
| Bone metastases |
Pts. with vs without bone metastases |
0.043 |
1.884 |
0.89–2.41 |
| MSKCC score | Good vs intermediate vs poor | 0.138 | 1.462 | 1.02–3.47 |
Abbreviations: HR=hazards ratio; MSKCC=Memorial Sloan-Kettering Cancer Center; OS=overall survival; TS=tumour shrinkage.
Discussion
With the introduction of targeted therapies, tumour assessment using RECIST criteria has been challenged by many. Choi et al (2007) established the decrease of density and a lower threshold of TS as a more reliable system to assess tumour response in GIST. The controversy opened a discussion on the topic of TS and whether the 30% cutoff for objective response applies to targeted therapies. A more recent study supports the relevance of objective remission on the clinical outcome, which correlated with OS and PFS in mRCC, while a 10% tumour remission cutoff has been shown to achieve a prognostic value (Abel et al, 2011; Grünwald et al, 2012). Patients with disease stabilisation, however, are believed to attain benefit to a similar extent. Therefore within this study, we assessed the fraction of TS at first assessment on overall survival, incorporating landmark analyses.
Our results demonstrate that patients with a TS of at least 60% benefit most and achieve prolonged OS in comparison with others. Patients with intrinsic resistance attain the worst outcome, as already described previously (Busch et al, 2011). As demonstrated in Figure 1, patients with other degrees of TS achieve similar benefit from systemic treatment, underscoring the clinical relevance of disease control independently from remission. Certainly, the study size limits the analytical threshold for detection of differences within this group of patients. Our results are supported by other reports on prolonged treatment duration in patients with CR as best response to targeted therapies (Johannsen et al 2011; Albiges et al 2012). Both reports describe a favourable clinical outcome and have not reached median overall survival, yet.
When RECIST criteria were implied, there was no difference in patients achieving an objective tumour remission compared with patients with stable disease as best response as demonstrated in Figure 2. Therefore our data support the notion that the depth of remission is associated with a superior overall survival. However, the cohort of patients with such an immense benefit remains small and applies to only 7 % in our study population. Certainly, the study size limits our conclusions, but it also provides a rational that long-term remissions are achievable in mRCC with VEGFR-targeted therapies, associated with an improved overall survival.
The bulk part (74%) of our study cohort achieved tumour size changes between shrinkage by 59% to the gain of size up to 19%. To further refine the clinical outcome of these patients, novel measures of tumour assessment may be necessary. The Choi response criteria include changes in tumour attenuation on CT, which reflect tumour density. In 2010, van der Veldt et al (2010) analysed the Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cancer treated with sunitinib. Hence, investigators were able to describe that in patients with PR the Choi criteria had a significantly better predictive value for PFS and OS than RECIST criteria. On this basis, density measurements may add additional predictive value, especially for patients without significant TS.
It is debatable whether superior TS represents a favourable treatment effect or merely tumour biology. It certainly questions how we perceive advancement in the field of mRCC. The era of targeted therapies has brought a new level of efficacy to the field of mRCC. The occurrence of ORR is not required to achieve clinical benefit from targeted agents. But further clinical advancement may not apply to the whole group of patients, as seen with contemporary clinical trials. It becomes obvious that additional increments in treatment efficacy or prognosis will apply to subgroups only, which has initiated a discussion about end points and selection criteria for clinical trials. The one size fits-all approach of recent years has abandoned the goal to achieve durable remissions, which have been a prerequisite for long-term survivorship in the era of immunotherapies. As indicated in our study, it may be worthwhile to aim for maximal TS to achieve a prognostic impact instead of providing moderate efficacy to the whole group of patients. Especially with the novel targets currently being explored in clinical trials, this aim may become more apparent in the near future.
Our study explores early TS as a prognostic tool in mRCC and shows that the depth of remission may define a subset of patients with superb response to VEGF-targeted therapies, at least in mRCC. The limitation of our analysis is the retrospective design and the limited number of patients. Additional studies will have to validate our findings. It will be important for future clinical development how we define our end points in clinical trials in order to achieve impact on the clinical outcome in mRCC.
Acknowledgments
We thank Dr Hendrik Eggers for providing data.
Jonas Busch is an advisor to Pfizer, Mologen and Bayer and has given lectures for Pfizer. Steffen Weikert is an advisor to Bayer, Pfizer, Novartis, Mologen and Genentech and has given lectures for Pfizer, Novartis, Astellas and GSK. Viktor Grünwald is a consultant and receives honoraria from GSK, Pfizer, Roche, Novartis and Bayer. The other authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Abel EJ, Culp SH, Tannir NM, Tamboli P, Matin SF, Wood CG. Early primary tumor size reduction is an independent predictor of improved overall survival in metastatic renal cell carcinoma patients treated with sunitinib. Eur Urol. 2011;60:1273–1279. doi: 10.1016/j.eururo.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiges L, Oudard S, Negrier S, Caty A, Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, Laguerre B, Legouffe E, Kohser F, Dietrich PY, Theodore CA, Escudier B. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30:482–487. doi: 10.1200/JCO.2011.37.2516. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- Busch J, Seidel C, Weikert S, Wolff I, Kempkensteffen C, Weinkauf L, Hinz S, Magheli A, Miller K, Grünwald V. Intrinsic resistance to tyrosine kinase inhibitors is associated with poor clinical outcome in metastatic renal cell carcinoma. BMC Cancer. 2011;11:295. doi: 10.1186/1471-2407-11-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, AVOREN Trial investigators Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- Grünwald V, Seidel C, Fenner M, Woike M, Kalanovic D.2012Use of earyl tumor shrinkage as a response to VEGF inhibitors as a predictor of progression-free survival (PFS) and overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol 30(15(Suppl; abstr 4631). [Google Scholar]
- Heng DY, Xie W, Bjarnason GA, Vaishampayan U, Tan MH, Knox J, Donskov F, Wood L, Kollmannsberger C, Rini BI, Choueiri TK. Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer. 2011;117:2637–2642. doi: 10.1002/cncr.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- Hutson TE, Gallardo J, Lesovoy V, Al-Shukri S, Stus V, Bair AH, Rosbrook B, Bycott PW, Tarazi JC, Kim S, Vogelzang NJ, Sammons BC.2013Axitinib versus sorafenib as first-line therapy in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol 31(6(Suppl; abstr LBA348). [Google Scholar]
- Jain RK, Lee JJ, Ng C, Hong D, Gong J, Naing A, Wheler J, Kurzrock R. Change in tumor size by RECIST correlates linearly with overall survival in phase I oncology studies. J Clin Oncol. 2012;30:2684–2690. doi: 10.1200/JCO.2011.36.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen M, Staehler M, Ohlmann CH, Flörcken A, Schmittel A, Otto T, Bex A, Hein P, Miller K, Weikert S, Grünwald V. Outcome of treatment discontinuation in patients with metastatic renal cell carcinoma and no evidence of disease following targeted therapy with or without metastasectomy. Ann Oncol. 2011;22:657–663. doi: 10.1093/annonc/mdq437. [DOI] [PubMed] [Google Scholar]
- Molina MM, Zhang J, Lin X, Niculescu L, Korytowsky B, Matczak E, Wiltshire R, Motzer RJ.2012Sunitinib objective response (OR) in metastatic renal cell carcinoma (mRCC): analysis of 1,059 patients treated on clinical trials J Clin Oncol 30(15(Suppl; abstr 4542). [Google Scholar]
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Nosov D, Eisen T, Bondarenko IN, Lesovoy V, Lipatov ON, Tomczak P, Lyulko AA, Alyasova A, Harza M, Kogan M, Alexeev BY, Sternberg CN, Szczylik C, Zhang J, Strahs LA, Esteves B, Slichenmyer WJ, Berkenblit A, Hutson TE.2012Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial J Clin Oncol 30(15(Suppl; abstr 4501). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarbá JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- Seidel C, Busch J, Weikert S, Steffens S, Fenner M, Ganser A, Grünwald V. Progression free survival of first line vascular endothelial growth factor-targeted therapy is an important prognostic parameter in patients with metastatic renal cell carcinoma. Eur J Cancer. 2012;48:1023–1030. doi: 10.1016/j.ejca.2012.02.048. [DOI] [PubMed] [Google Scholar]
- van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer. 2010;102:803–809. doi: 10.1038/sj.bjc.6605567. [DOI] [PMC free article] [PubMed] [Google Scholar]