Abstract
Background:
The objective of this study is the effectiveness of multidisciplinary rehabilitation on treatment-related adverse effects after completed radiotherapy in patients with prostate cancer (PCa).
Methods:
In a single-centre oncology unit in Odense, Denmark, 161 PCa patients treated with radiotherapy and androgen deprivation therapy were randomly assigned to either a programme of two nursing counselling sessions and two instructive sessions with a physical therapist (n=79) or to usual care (n=82). Primary outcome was Expanded Prostate Cancer Index Composite (EPIC-26) urinary irritative sum-score.
Before radiotherapy, pre-intervention 4 weeks after radiotherapy, and after a 20-week intervention, measurements included self-reported disease-specific quality of life (QoL; EPIC-26, including urinary, bowel, sexual, and hormonal symptoms), general QoL (Short-form-12, SF-12), pelvic floor muscle strength (Modified Oxford Scale), and pelvic floor electromyography. Intension-to-treat analyses were made with adjusted linear regression.
Results:
The intervention improved, as compared with controls, urinary irritative sum-score 5.8 point (Cohen's d=0.40; P=0.011), urinary sum-score (d=0.34; P=0.023), hormonal sum-score (d=0.19; P=0.018), and the SF-12 Physical Component Summary, d=0.35; P=0.002. Patients with more severe impairment gained most. Pelvic floor muscle strength measured by electromyography declined in both groups, P=0.0001.
Conclusion:
Multidisciplinary rehabilitation in irradiated PCa patients improved urinary and hormonal symptoms, and SF-12 physical QoL.
Keywords: radiotherapy, physical therapy, pelvic floor, nursing, quality of life, urinary irritative symptoms
Prostate cancer (PCa) is the most frequent male malignancy in the Western world (Ferlay et al, 2010). The development of treatment with radiotherapy combined with androgen deprivation therapy (ADT) has, in locally advanced or high-risk PCa, increased the 10-year survival rates from ∼60% to >70% (Widmark et al, 2009). Following these improvements in survival, a growing interest has emerged in evaluating the impact of the overall treatment on quality of life (QoL) (Miller et al, 2005; Sanda et al, 2008), and clinical attention has been directed towards how the adverse effects of the treatment may be counteracted (Johansen, 2007; Armes et al, 2009; World Health Organization, 2012). Adverse effects are categorised into acute disorders occurring within 6 months of radiotherapy or late complications after 6 months or more (Grise and Thurman, 2001).
In particular, urinary irritative problems causing frequency, nocturia, urgency or urge incontinence are of major concern for these patients (Michaelson et al, 2008; Budaus et al, 2012). The incidence of acute urinary tract symptoms after intensity-modulated radiotherapy is estimated to occur in one or even two out of every four patients (Budaus et al, 2012). Furthermore, the risk of late urinary adverse effects is increasing in patients with acute disorders. In a follow-up study from the United States of America with 1571 patients who experienced acute urinary symptoms during treatment, the risk of having grade 2 (CTC 3.0) late adverse effects after 10 years was found to be significantly increased from 12 to 35% (Zelefsky et al, 2008).
In randomised trials, home-based training of pelvic floor exercises has been confirmed as an effective non-invasive treatment of post-prostatectomy incontinence, showing significantly decreased duration and degree of incontinence (Van Kampen et al, 2000; Filocamo et al, 2005). However, this concept has not been investigated in a randomised designed study following treatment of PCa with radiotherapy and ADT (Cockle-Hearne and Faithfull, 2010), and especially not with urinary irritative problems as the primary end point. Here, we present data from the first RCT to investigate a multidisciplinary rehabilitation programme comparing usual care with psychosocial support from nurses and counselling in pelvic floor exercises primarily to reduce urinary irritative problems and secondarily to increase overall QoL.
Materials and Methods
Setting and participation
The study called RePCa was approved by the local Scientific Research Ethics Committee (File no. S-20090142), the Danish National Data Protection Agency (File no. 2012-41-1175), and registered by ClinicalTrials.gov (Study number, NCT01272648). All participants provided written informed consent.
Design
The design was organised as a two-armed randomised, controlled trial recruiting from 226 patients referred to curative radiotherapy from 1 February 2010 to 31 January 2012 at Odense University Hospital, Denmark. A total of 209 patients fulfilled the inclusion criteria and were eligible for participation as shown in the CONSORT diagram (Boutron et al, 2008), Figure 1. The patients were informed about the RePCa study from a project nurse at the first meeting at the Department of Oncology.
Figure 1.
CONSORT-Flow chart. .
Inclusion criteria: men ⩾18 years old with biopsy-documented adenocarcinoma of the prostate.
Exclusion criteria: former prostatectomy, not able to speak Danish, or included in other protocols.
Information about Gleason score, TNM-staging, prostate-specific antigen values, and comorbidity was obtained from the medical records, and patients were registered in risk groups as described by D'amico et al (1998) with regard to determining the treatment plan.
Intensity-modulated radiotherapy used a prescription dose of 78 Gy in 39 fractions given in five fractions per week. Inter-fractional prostate displacement was corrected by daily IGRT using implanted gold markers. Target volume was the prostate gland including the proximal 2 cm of the seminal vesicles in high-risk patients and adding a 7-mm margin. Three patients in each randomisation group received pelvic radiotherapy due to metastatic lymph nodes. Androgen deprivation therapy was started 3 months before radiotherapy; in T3 patients, ADT is given for up to 3 years.
Patients were randomly assigned to the intervention group or usual care (control group) in a ratio of 1 : 1 after the completion of radiotherapy. The randomisations were externally handled by the Department of Clinical Research at Odense University Hospital, Denmark, and the allocation sequence was concealed from the research team.
Intervention
The intervention was based on the following definition: ‘Rehabilitation is a focused and temporary process of cooperation between the patient, relatives, and the professionals. The purpose for the patient is to achieve an independent and meaningful life, even if he has, or is at risk of a significant decline in his physical, mental, or social functions. Rehabilitation is based on the patient's whole life situation and consists of a coordinated, interconnected, and knowledge-based effort' (Rehabiliteringsforum, 2004).
The intervention took place in an outpatient setting at the Department of Oncology and the Department of Rehabilitation. At 4 weeks post radiation, pre-intervention data were obtained from all patients in the study and the intervention was initiated.
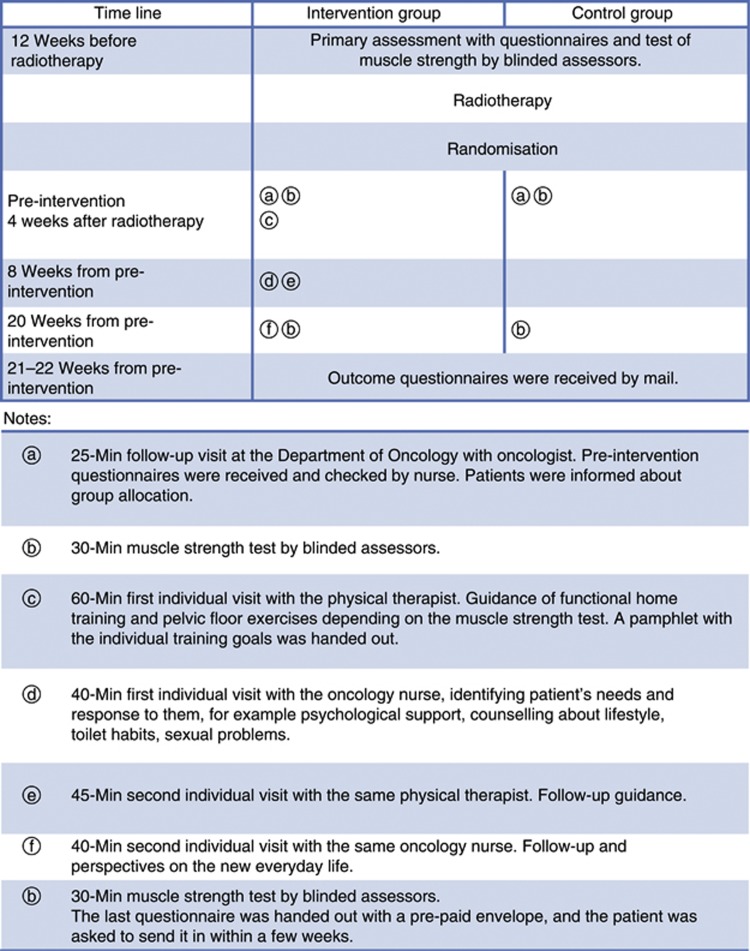
The control group received usual care during follow-up. The usual care consisted of one physician visit 4 weeks after radiotherapy. No systematic education for the control group was provided during the trajectory. In addition to usual care, the patients in the intervention group were instructed in an individually suited multidisciplinary programme during two nursing counselling sessions and during two additional sessions of counselling by physical therapists aiming the exact need of each individual patient; Figure 2. The patient was recommended to bring his spouse along for all counselling and instructions in order to increase understanding of and compliance with the exercises suggested.
Figure 2.
Graphical depiction of the intervention in a randomised rehabilitation study with 161 participants with PCa, Odense, Denmark. .
The above-mentioned intervention was provided by dedicated staff members at each site. The seven project oncology nurses engaged in the intervention activities were specially trained and qualified radiation therapists, and the two physical therapists had more than 10 years of clinical experience in the instruction and training of men in order to address incontinence, including pelvic floor training. This group of nine staff members were all enrolled in a 6-day course with seven 45 min lectures per day containing the topics PCa and treatment, the male perspective, incontinence and the pelvic floor, sexuality, depression and fear of recurrence, social support, and finally the method of motivational interviewing (Miller and Rollnick, 2002) that was used as a communicative platform. To ensure consistency in the intervention, the staff had 12 supportive, 60-min reinforcing sessions every second month provided by a motivational interview trainer. Communication between the multidisciplinary staff members was provided during structured documentation in the patients files.
Nursing counselling
The nursing counselling sought to provide psychological support and enable identification of problems regarding the disease experienced by the patient and his spouse, Figure 2. In accordance with the framework for nursing (Benner and Wrubel, 1989), the nurses initiated the dialogue based on the patients needs. With this approach and secondarily with the professional knowledge of possible themes important for PCa patients written in a guideline, the nurses identified information needs about adverse effects, established an individual rehabilitation plan based on the patients' personal goals, and, if needed, provided advice on lifestyle changes concerning smoking, alcohol, general fitness, diet, weight control, and further suggested solutions to other problems, for example, toilet habits, sexuality, and psychological problems. The project oncology nurses had the authority to refer patients in the intervention group to medical specialists, public/community rehabilitation centres, sexologists, and social workers.
Physical therapy counselling
The physical therapy identified the individual patient's need for increased pelvic floor muscle function and general physical activity level; Figure 2. Symptoms related to pelvic floor muscle function were explored, for example, urination control, flatulence, and defecation. If necessary, the patient was guided by biofeedback, a visual signal presenting the pelvic floor strength to the patient (Dorey, 2006).
A written pamphlet was created specifically for the purpose of this study and explained how PCa treatment affects physical and general health. The self-training home programme consisted of pelvic floor muscle exercises integrated in daily activities, for example, during driving the car, walking, or working in the garden. The exercises for the major muscle groups included muscle endurance and strength and balance exercises, for example, three sessions of 10–12 repetitions for each muscle group. General physical activities were recommended to inactive patients for at least 30 min per day. The agreement about the self-training programme was noted in the pamphlet. The second session was used as a follow-up on the individual goals of each patient.
Primary and secondary outcome measures
Study outcomes were preliminary assessed before radiotherapy, as pre-intervention measurements at 4 weeks post radiation and after the 20 weeks of intervention; Figure 2. The 20 weeks of intervention were used to allow for muscle training and a change from ‘being a patient' to ‘being a cancer survivor'.
The primary outcome was defined as the urinary irritative sum-score based on the Expanded Prostate Cancer Index Composite (EPIC-26). The irritative sum-score was derived from four items regarding pain, bleeding, weak stream, or frequent urination. Secondary outcomes included QoL arising from the Medical Outcome Study Short-form-12 (SF-12), urinary incontinence, bowel, sexual and hormonal sum-scores as measured by the EPIC-26, and assessment of the pelvic floor by clinical examination and electromyography.
EPIC-26
The disease-specific validated EPIC-26 consists of domains concerning urinary symptoms, bowel symptoms, sexual function, and symptoms related to ADT. The internal consistency and test-retest reliability for EPIC-26 (Cronbach's alpha>0.70 and r>0.69) for all domains support its validity. For each domain, a sum-score is constructed. In addition, two urinary scales that distinguish irritative/obstructive symptoms and incontinence were obtained. For the primary outcome, the urinary irritative sum-score, Cronbach's alpha was 0.74 and r >0.80 (Szymanski et al, 2010). Expanded Prostate Cancer Index Composite items are answered on a 5-point Likert scale from no problems to severe problems. Scores are transformed linearly to a scale of 0–100, with higher scores indicating better QoL (Wei et al, 2000; Szymanski et al, 2010). A low inter-scale correlation observed between SF-12 and EPIC domains supports the concurrent use of EPIC with SF-12 (Wei et al, 2000).
SF-12
The generic extensively validated QoL questionnaire SF-12 (Ware et al, 1996) includes eight concepts: physical functioning, role limitations due to physical health problems, body pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The reliability of SF-12 was tested in two data sets and showed a test-retest summary measure of 0.89 in the United States of America and 0.86 in the United Kingdom. Results are expressed in two meta-scores: the Physical Component Summary and the Mental Component Summary. These meta-scores are standardised to the population normative values of the United States of America, with a mean score of 50 and a s.d. of 10. Higher scores represent better health (Ware et al, 1996).
Pelvic floor assessment
A standardised protocol ensuring a correct and reproducible technique was used for all tests. This protocol was developed after pilot tests of seven patients, including subject lateral positioning, exact wording of instructions, and avoidance of muscles other than the pelvic floor. Pilot test data were not included in this study.
In all patients, a correct pelvic floor muscle contraction was confirmed on digital rectal evaluation by the assessor before making the test. The instruction used for each contraction was ‘squeeze and lift' the pelvic floor. Muscle strength was measured by the ability to contract. Digital evaluation was done according to the modified 6-point Oxford Scale (Dorey, 2007). Surface anal electromyography (EMG) was performed with NeuroTrac MyoPlus (Verity Medical Ltd., Hampshire, England) with an Anuform analprobe (Patterson Medical UK Ltd, Nottinghamshire, UK). The three assessors of the pelvic floor function were blinded and independent of the research team. The patients were told not to give the assessors information about group assignments.
All data entry was done by the first author. A quality control was made with the procedure as recommended by King and Lashley: the first 10 questionnaires in the data set were controlled, and then every 10 questionnaire until errors occurred. Further, each questionnaire was controlled until 10 were without errors (King and Lashley, 2000).
Sample size calculation and statistical analyses
The power calculation was based on a two-sided t-test on difference between programs in the mean reduction in the EPIC-26 urinary irritative sum-score (Michigan, 2012) corresponding to a Cohens d of 0.5 (mean change divided by s.d.). We applied a significance level of 5% and power of 80%. The sample size of 160 patients was found by taking into account a maximal drop-out rate of 20%.
Socio-demographic and clinical characteristics were described using means for continuous variables and frequencies for categorical variables. Differences regarding disease-specific and general QoL (continuous) between intervention and control groups were tested with multiple linear regression models adjusted for pre-intervention scores. Differences in the strength of pelvic floor muscles were tested with the Wilcoxon rank-sum test. Inter-rater reliability between assessors was tested in a random example of 15 records with Kappa statistics. Groups were analysed with intention-to-treat according to the allocated group.
Correlations between variables were tested with linear regression models. We checked the model assumptions, that is, linearity, normality, homogeneity, and serial correlation of residuals, by relevant scatter plots followed by visual inspection.
As only one primary end point was selected and secondary end points have to be tested in future studies, adjustments for multiple comparisons were not made. P-values <0.05 were considered statistically significant and were reported two-sided.
In some of the returned questionnaires, data were missing. According to the methods described for EPIC urinary irritative sum-score and incontinence sum-score and SF-12, all questions should be answered to be analysed. Questionnaires with insufficient data were therefore removed from the analysis. The EPIC domains: urinary, bowel, sexual, and hormonal sum-scores allow one missing answer (Ware et al, 1996; Wei et al, 2000). Statistics were calculated with STATA 11 (StataCorp LP, College Station, TX, USA).
Results
A number of 226 patients were screened for eligibility, but 48 patients refused to participate because of several reasons; Figure 1. Groups were balanced at pre-intervention, and we had some information of the patients who refused; Table 1. Patient flow before and after randomisation of 161 patients is shown in Figure 1, leaving 153 patients (95%) for the analysis. The attrition rate was 5% as four patients dropped out, one died, and three were lost to follow-up. A total of 71 out of 79 patients in the intervention group (90%) completed the entire intervention programme that included two nursing counselling and two physical therapy counselling sessions, but data from all patients were analysed according to the allocated group. Data (150 out of 153; 98%) regarding the primary end point urinary irritative symptoms were completed.
Table 1. Socio-demographic and biological pre-intervention characteristics of 161 participants and 48 refusers with primary prostate cancer included in a randomised controlled trial after radiotherapy and androgen deprivation therapy, 2010–2012 Odense, Denmark.
| Intervention group (n=79) | Control group (n=82) | Refusers (n=48) | |
|---|---|---|---|
|
Socio demographic characteristics | |||
| Age at first radiotherapy date (years), mean (s.d.) | 68.2 (4.8) | 69.0 (5.2) | 68.7 (6.4) |
| Weight (kilo) reported at pre-intervention mean (s.d.) | 84.7 (12.7) | 86.3 (14.8) | 85.9 (15.6) |
| Unknown | 1 | 3 | 7 |
| Body mass index (BMI) kg m−2 mean (s.d.) | 27.1 (3.6) | 27.4 (4.1) | 27.2 (4.6) |
| Unknown |
1 |
3 |
7 |
|
Social status reported at pre-intervention | |||
| Living alone | 10 (13%) | 12 (15%) | 8 (19%) |
| Living with a spouse | 68 (87%) | 67 (85%) | 33 (81%) |
| Unknown |
1 |
3 |
7 |
|
Education (years) | |||
| 9–10 | 31 (40%) | 36 (46%) | 21 (52%) |
| 12–13 | 24 (31%) | 23 (29%) | 12 (30%) |
| 13–16 | 18 (23%) | 18 (23%) | 6 (15%) |
| 18–20 | 5 (6%) | 2 (2%) | 1 (3%) |
| Unknown |
1 |
3 |
8 |
|
Smoking status at pre-intervention | |||
| Never smoker | 33 (42%) | 32 (41%) | 12 (29%) |
| Past smoker | 30 (39%) | 35 (44%) | 20 (49%) |
| Current smoker | 15 (19%) | 12 (15%) | 9 (22%) |
| Unknown |
1 |
3 |
7 |
|
Medical characteristics | |||
| PSA pre-treatment serum mean ng ml−1 (s.d.) |
21.5 (17.7) |
19.8 (16.8) |
21.6 (18.4) |
|
Gleason score | |||
| <7 | 10 (13%) | 17 (21%) | 10 (21%) |
| 7 | 47 (59%) | 44 (54%) | 25 (52%) |
| >7 |
22 (28%) |
21 (25%) |
13 (27%) |
|
Degree of malignancy | |||
| T1 | 8 (10%) | 9 (11%) | 6 (12%) |
| T2 | 34 (44%) | 35 (43%) | 23 (48%) |
| T3 | 36 (46%) | 38 (46%) | 19 (40%) |
| Unknown |
1 |
|
|
|
Risk group | |||
| Low | 1 (1%) | 3 (4%) | 6 (12%)a |
| Intermediate | 19 (24%) | 13 (16%) | 8 (17%) |
| High | 59 (75%) | 65 (80%) | 34 (71%) |
| Unknown |
|
1 |
|
|
Hormone therapy | |||
| No | 3 (4%) | 2 (3%) | 5 (11%)b |
| Androgen deprivation therapy (ADT) | 76 (96%) | 79 (97%) | 42 (89%) |
| Unknown |
|
1 |
1 |
|
Patient-reported comorbidity at pre-intervention | |||
| Charlson index score 1 | 20 (25%) | 19 (23%) | 13 (27%) |
| Score 2 | 4 (5%) | 5 (6%) | 1 (2%) |
| Score 3 | 0 | 0 | 0 |
| Score 6 | 0 | 0 | 0 |
Abbreviation: PSA=prostate-specific antigen values.
Fisher's exact test P=0.028
Fisher's exact test P=0.049
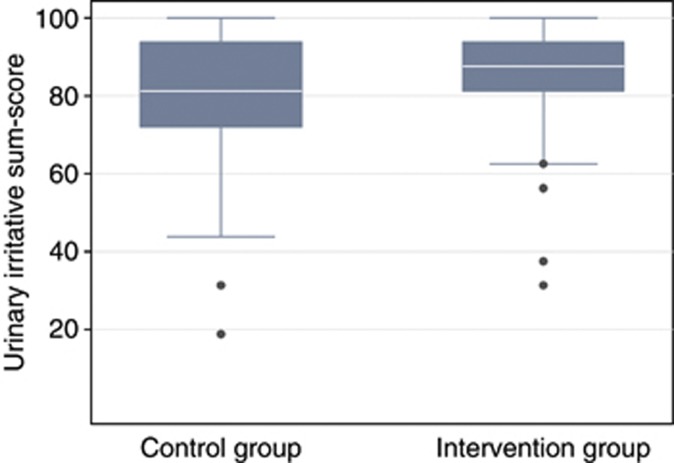
Multiple linear regression adjusted for pre-intervention scores showed that PCa patients in the intervention group benefitted significantly with regard to urinary irritative symptoms with 5.8 point, Cohen's d=0.40; P=0.011 (Tables 2 and 3 and Figure 3). No significant interactions between groups and pre-intervention scores were seen. Covariates (age, body mass index, risk group, prostate-specific antigen values, education, smoking, and marital status) showed no significant correlation with the urinary irritative sum-score.
Table 2. Mean changes in QoL scores among 161 Danish prostate cancer survivors included in a randomised rehabilitation study, Odense, Denmark.
| Effect (coefficient)a | CI (95%) | Pb | |
|---|---|---|---|
|
Intervention group vs control group | |||
|
SF-12 | |||
| Physical QoL (PCS) | 3.6 | 1.3; 5.8 | 0.002 |
| Mental QoL (MCS) |
0.7 |
−1.6; 3.0 |
0.549 |
|
EPIC domains | |||
| Urinary | 4.5 | 0.6; 8.4 | 0.023 |
| Incontinence | 2.6 | −1.8; 6.9 | 0.242 |
| Irritative | 5.8c | 1.4; 10.3 | 0.011 |
| Bowel | 3.0 | −1.9; 8.0 | 0.224 |
| Sexual | 3.6 | −0.9; 8.0 | 0.117 |
| Hormonal | 4.8 | 0.8; 8.8 | 0.018 |
Abbreviations: CI=95% confidence interval; EPIC=Expanded Prostate Cancer Index Composite; MCS=Mental Component Summary; PCS=Physical Component Summary; QoL=quality of life.
Multiple linear regression analysis adjusted for pre-intervention scores.
Reported P-values are two-sided and P<0.05 was considered statistically significant.
Example: if the patient is in the intervention group, his mean QoL score (0–100 scale) regarding urinary irritative sum-score increases with 5.8 point compared with the controls.
Table 3. Pre-intervention level and changes in disease-specific QoL scores (EPIC), and general QoL scores (SF-12) in intervention and control groups at 4 weeks (pre-intervention) and 24 weeks after radiotherapy (post intervention) among 161 survivors with primary prostate cancer included in (RePCa) a prospective randomised rehabilitation study, 2010–2012 Odense, Denmark.
|
EPIC and SF-12 mean QoL (score 0–100)a |
|||||
|---|---|---|---|---|---|
| Intervention group, n=79 | Control group, n=82 | ||||
|
QoL |
Pre-intervention |
Difference at 6 months |
Pre-intervention |
Difference at 6 months |
|
| Mean (s.d.) |
4 Weeks after radiotherapy |
Between follow-up and pre-intervention |
4 Weeks after radiotherapy |
Between follow-up and pre-intervention |
Cohen's db |
|
EPIC domain | |||||
| Urinary irritative | 67.7 (18.7) | 17.6 (18.1) | 68.1 (18.7) | 11.6 (16.5) | 0.40 |
| Urinary incontinence | 82.5 (17.5) | 7.3 (14.9) | 82.2 (21.7) | 4.9 (15.8) | |
| Urinary sum-score | 73.5 (14.5) | 13.3 (13.9) | 73.1 (17.4) | 9.0 (12.9) | 0.34 |
| Bowel sum-score | 77.6 (19.9) | 9.7 (20.3) | 77.0 (20.0) | 7.6 (16.4) | |
| Sexual sum-score | 15.0 (19.5) | −1.3 (13.7) | 14.0 (20.1) | −4,1 (16.4) | |
| Hormonal sum-score |
71.6 (18.8) |
2.3 (13.2) |
72.8 (18.9) |
−2.8 (12.8) |
0.19 |
|
SF-12 domain | |||||
| PCS | 47.4 (8.3) | 1.9 (6.7) | 47.5 (9.0) | −1.7 (7.9) | 0.35 |
| MCS | 53.0 (8.7) | 2.3 (7.1) | 51.7 (9.6) | 2.3 (8.9) | |
Abbreviations: EPIC=Expanded Prostate Cancer Index Composite; MCS=Mental Component Summary; PCS=Physical Component Summary; QoL=quality of life; SF-12=Short-form-12.
Higher scores indicating better QoL. Note: SF-12 is standardised to the population normative values of the United States of America , with a mean score of 50 and a s.d. of 10.
Cohen's d effect size=group mean differences at patients post intervention divided by mean s.d.
Figure 3.
Box-plot median urinary irritative sum-score post intervention in a randomised rehabilitation study with 161 participants with PCa, Odens, Denmark. The whiskers show the lower/upper adjacent value and the box shows 25th–75th percentile. The dots show outliers.
Physical Component Summary and Mental Component Summary correlated with improved urinary irritative sum-score, r=0.55; P=0.015 and r=0.58; P<0.001, respectively.
Subanalysis showed that the improvement of the urinary sum-score was most pronounced in patients living alone (12.0 point, d=0.83; P=0.021), that pre-intervention urinary bother (score >3) indicating moderate-to-severe problems gained (13.1 point, d=0.90; P=0.034), and a pre-intervention urinary irritative sum-score below the study mean value of 68 point predicted a higher intervention effect with (10.1 point, d=0.70; P=0.031). Urinary sum-score, hormonal sum-score, and physical QoL (Physical Component Summary) improved significantly in the intervention group compared with controls, whereas pelvic floor muscle strength measured by digital evaluation (Modified Oxford Scale 0–6) did not change significantly during the study period at post intervention in the intervention and the control groups, Table 4. Muscle strength measured by EMG declined concurrently (P=0.001), 31.3–24.7 μV in the intervention group and 31.6–23.3 μV in the control group, with no significant differences between the two groups. At post intervention in the overall population, a significant correlation was found between muscle strength measured by digital evaluation and EMG (ρ=0.5698, P<0.001).
Table 4. Pelvic floor muscle strength in prostate cancer patients treated with radiotherapy in RePCa: a randomised controlled rehabilitation study.
|
Pelvic floor muscle strength |
Intervention group,
n=79 |
Control group,
n=82 |
|
|||
|---|---|---|---|---|---|---|
| Mean (95%CI) | Study population pre-radiation | Pre-intervention | Post intervention | Pre-intervention | Post intervention | P-valuea |
| No. of participants |
n=156 |
n=73 |
n=68 |
n=75 |
n=71 |
|
| Digital evaluationb |
4.0 (3.8;4.1) |
3.8 (3.5;4.0) |
3.9 (3.6;4.1) |
3.8 (3.6;4.1) |
3.7 (3.5;4.0) |
NS |
| Static strength no. of seconds to hold one contraction |
34.4 (31.1;37.7) |
35.2 (30.2;40.2) |
36.1 (31.0;41.2) |
31.6 (26.6;36.6) |
32.9 (28.0;37.8) |
NS |
| Dynamic strength no. of contractions during 60 s |
22.2 (19.9;24.5) |
21.2 (17.7;24.7) |
22.7 (18.9;26.5) |
19.1 (15.9;22.3) |
19.2 (15.9;22.6) |
NS |
| No. of participants |
n=156 |
n=72 |
n=66 |
n=71 |
n=71 |
|
| EMG average activity (μV) |
38.2 (34.8;41.6) |
31.3 (27.9;34.8) |
24.7 (21.7;27.7) |
31.6 (27.3;35.9) |
23.3 (20.4;26.2) |
NS |
| EMG average rest (μV) | 6.8 (6.2;7.5) | 5.8 (5.0;6.6) | 4.8 (4.0;5.5) | 5.5 (4.9;6.1) | 4.9 (3.6;6.2) | NS |
Abbreviations: CI=class interval; EMG=electromyography.
Post-intervention differences between groups. Wilcoxon Sign-rank test. Reported P-values are two-sided and P<0.05 was considered statistically significant.
Measured by Modified Oxford Scale 0–6.
In the intervention group compared with the control group, the urinary irritative sum-score improved in patients with impaired <5 pelvic floor strength at pre-intervention Modified Oxford Scale, (7.4 point, d=0.51; P=0.012) as did the urinary irritative sum-score in patients with low EMG at pre-intervention,<21 μV (9.2 point, d=0.63; P=0.012).
In the digital evaluation, inter-rater reliability among the three assessors had Kappa values of 0.72, 0.83, and 0.84.
The time used for the intervention for each patient was estimated to 4 h including time for documentation.
Discussion
We found that the multidisciplinary rehabilitation had a significant effect compared with usual care on the primary outcome: urinary irritative symptoms, among a large sample of irradiated PCa patients. As secondary outcomes, we observed benefits of the intervention in improvement in overall urinary and hormonal symptoms, and physical QoL. Our intervention improved urinary irritative symptoms by > 5 points on a 0–100 scale compared with a control group, which is considered clinically significant (Osoba et al, 1998). The clinical meaningfulness was furthermore confirmed by the patients as they experienced the intervention useful in everyday life, as stated in focus groups (Dieperink et al, 2013).
Consistent with our finding, Faithfull et al (2011) in a small phase II trial including 22 irradiated patients observed significant improvements, with a median score change of 5, on lower urinary tract symptoms following an intervention consisting of a programme almost the same as used in this study (self-management programme comprising pelvic floor exercises, bladder training, patient education, and problem solving). However, Faithfull et al (2011) included only patients with moderate-to-severe symptoms, and their pelvic floor exercises were conducted as group sessions, whereas we included all patients with an individualised intervention aiming at the targeted and exact need of each individual patient. Our results are parallel to those from two randomised studies (N=102 and 300) among PCa patients treated with prostatectomy using a single component pelvic floor exercise programme to improve incontinence (Van Kampen et al, 2000; Filocamo et al, 2005). Ribeiro et al (2010) found in a controlled study in prostatectomy patients a positive effect of pelvic floor muscle training and biofeedback. They concluded that training improved not only recovery of urinary incontinence but also voiding symptoms and pelvic floor muscle strength. Ribeiro et al (2010) also included an extensive review and discussion of the available studies on prostatectomy patients. They described how a more precise comparison of the studies was difficult because of methodological differences among these. As the two treatments of PCa, prostatectomy, or radiotherapy have different adverse effects, a direct comparison with our data is even more difficult.
Pelvic floor function is closely related to bladder capacity or voiding dysfunction.
Previous research has examined pelvic floor muscle strength in men treated with prostatectomy, but there is no gold standard of measurement (Messelink et al, 2005). The pelvic floor strength measured by digital evaluation was stable during the period, but as measured by EMG, the pelvic floor strength declined significantly. Thus, EMG may be more sensitive in detecting changes in muscle strength, although the signals from the surface EMG must be made with caution owing to the risk of cross talk from other muscles (Herrington, 1996). Furthermore, studies show that ADT may cause a decline in large muscle groups (Williams et al, 2005; Alibhai et al, 2010), and perhaps this includes the muscles of the pelvic floor. Hence, the self-reported urinary improvements were in some contradiction to the clinical measurements of the pelvic floor, as the pelvic floor strength was stable as judged by repeated digital evaluation and declined as judged by EMG. Therefore, no clearcut causal relationship between the subjective dimensions of QoL and the objective measurements of the pelvic floor were seen. Pelvic floor muscle strength must be of some importance for the urinary irritative sum-score, as the intervention improved urinary irritative sum-score especially in patients with impaired muscle strength after radiotherapy. However, the exact relationship between the pelvic floor function and urinary irritative symptoms are still to be investigated. As this is the first study that investigates the pelvic floor in PCa patients treated with radiotherapy, it remains to be seen whether the finding can be reproduced in future studies.
The patients in the control group met the blinded assessors only when being tested. However, patients were able to obtain information from the internet or elsewhere, and this may, in theory, have watered down the intervention. Pelvic floor exercises are difficult to learn without instructions (Messelink et al, 2005), and consequently, we offered the intervention group meticulous instructions in order to give the best opportunity to counteract adverse effects, which may have been superior to information from other sources, for example, the internet.
Our recently published cross-sectional survey including 317 PCa patients (Dieperink et al, 2012) showed that risk factors (for example, smoking, severe obesity BMI >30, and the condition of living alone) were associated with increased risk of late adverse effects after radiotherapy. The present study showed that patients living alone had a better outcome after intervention. These results imply a need for integration of not only the adverse effects but also personal factors as lifestyle and life conditions during intervention.
Screening before intervention may identify patients with a potential for improvement and the motivation to make an effort. The present study showed that patients with pre-intervention objective (i.e., digital evaluation <5 or EMG<21) or subjective (i.e., EPIC irritative sum-score below 68 points) impairment improved the most. Therefore, screening with these measures may be considered in future rehabilitation intervention studies.
This study has a number of advantages. The accrual procedures made it possible to obtain information about the majority of the patients who denied participation, as 41 out of 48 filled out questionnaires before radiotherapy. This group of patients differed from patients included in the randomisation by having a statistically significant, but marginally lower D'Amico risk. We find it unlikely that it was related with not joining the study. In addition, this group included more patients living alone, and they had a lower but not significant level of education and higher incidence of smoking than did the randomised patients. Taken together, these factors should be taken into account if the intervention tested in this study is used as a standard of care (Holm et al, 2013), and health care professionals should make special efforts to include these groups in rehabilitation studies. Internal validity was maintained because of the randomisation and the homogeneity of the groups. The study provided good feasibility with a high inclusion rate and few drop-outs, although one important limitation was that we did not monitor the patients' compliance to the recommended self-training home exercises. In future studies, monitoring has to be considered at least with patient-reported outcomes, for example, exercise logbooks. Although we have the calculated time consumption used for the intervention, we did not make an exact cost-effectiveness analysis, but this could be recommended in future studies. Another limitation was that the design including psychosocial support and physical instructions do not allow blinding as a possibility. Instead, a trusting relation between patients and professionals is considered as an important motivation and may influence patient-reported outcome. Owing to concerns about the impact of radiotherapy on the pelvic floor muscles, the intervention started 1 month after radiotherapy, although we are aware of the recommendation that rehabilitation is started at diagnosis. Some of the patients complained of rectal tenderness when pelvic floor strength was tested, and this indicates that this was a correct decision. However, the intervention did not have any negative effects on the outcomes measured, and only few patients dropped out. The relative unrestricted inclusion and exclusion criteria, the uniform treatment protocol, and the fact that men who attended the study were living in cities as well as in rural areas in Denmark, allow generalisation of the results because the study sample was representative of a population of irradiated PCa patients. However, only one patient included in the study was not an ethnic Dane, and this limits the generalisation from the findings into a broader context. Furthermore, the design permits only causal conclusions about the combined programme of nursing and physical therapy counselling and not about the components separately.
Based on the results of this study, it can be recommended that patients treated with radiotherapy of the prostate may be offered a combined nurse–physiotherapist intervention programme, especially patients with impairments within urinary irritative function. Timing, duration, and more focus on the empowerment aspects of this intervention need further study.
Acknowledgments
We owe special thanks to the cancer patients who participated in the study for their valuable contributions. We are indebted to all colleagues who took part in the study. The study was supported by the Odense University Hospital (OUH) Research Foundation, the University of Southern Denmark, the Danish Cancer Society, CIRRO – the Lundbeck Foundation Center for Interventional Research in Radiation Oncology, the Department of Oncology, OUH, the Mette Hede Nielsen Foundation, the Danish Nurses Organization Research Foundation, and the Propa Vita Foundation.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Alibhai SM, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, Krahn M, Fleshner NE, Warde P, Canning SD, Klotz L, Naglie G. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, Palmer N, Ream E, Young A, Richardson A. Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol. 2009;27:6172–6179. doi: 10.1200/JCO.2009.22.5151. [DOI] [PubMed] [Google Scholar]
- Benner P, Wrubel J. The Primacy of Caring. Stress and Coping in Health and Illness. Addison-Wesley Publishing Company; 1989. [Google Scholar]
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- Budaus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, Wiegel T. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Cockle-Hearne J, Faithfull S. Self-management for men surviving prostate cancer: a review of behavioural and psychosocial interventions to understand what strategies can work, for whom and in what circumstances. Psychooncology. 2010;19:909–922. doi: 10.1002/pon.1657. [DOI] [PubMed] [Google Scholar]
- D'amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- Dieperink KB, Hansen S, Wagner L, Johansen C, Andersen KK, Hansen O. Living alone, obesity and smoking: important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncol. 2012;51:722–729. doi: 10.3109/0284186X.2012.682627. [DOI] [PubMed] [Google Scholar]
- Dieperink KB, Wagner L, Hansen S, Hansen O. Embracing life after prostate cancer. A male perspective on treatment and rehabilitation. Eur J Cancer Care. 2013;22:549–558. doi: 10.1111/ecc.12061. [DOI] [PubMed] [Google Scholar]
- Dorey G. Pelvic Dysfunction in Men. Diagnosis and Treatment of Male Incontinence and Erectile Dysfunction. John Wiley & Sons Ltd: United Kingdom; 2006. [Google Scholar]
- Dorey G. A clinical overview of the treatment of post-prostatectomy incontinence. Br J Nurs. 2007;16:1194–1199. doi: 10.12968/bjon.2007.16.19.27357. [DOI] [PubMed] [Google Scholar]
- Faithfull S, Cockle-Hearne J, Khoo V. Self-management after prostate cancer treatment: evaluating the feasibility of providing a cognitive and behavioural programme for lower urinary tract symptoms. BJU Int. 2011;107:783–790. doi: 10.1111/j.1464-410X.2010.09588.x. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Filocamo MT, Li Marzi V, Del Popolo G, Cecconi F, Marzocco M, Tosto A, Nicita G. Effectiveness of early pelvic floor rehabilitation treatment for post-prostatectomy incontinence. Eur Urol. 2005;48:734–738. doi: 10.1016/j.eururo.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Grise P, Thurman S. Urinary incontinence following treatment of localized prostate cancer. Cancer Control. 2001;8:532–539. doi: 10.1177/107327480100800608. [DOI] [PubMed] [Google Scholar]
- Herrington L. EMG Biofeedback: What can it actually show. Physiotherapy. 1996;82:581–583. [Google Scholar]
- Holm LV, Hansen DG, Larsen PV, Johansen C, Vedsted P, Bergholdt SH, Kragstrup J, Sondergaard J. Social inequality in cancer rehabilitation: A population-based cohort study. Acta Oncol. 2013;52:410–422. doi: 10.3109/0284186X.2012.745014. [DOI] [PubMed] [Google Scholar]
- Johansen C. Rehabilitation of cancer patients—research perspectives. Acta Oncol. 2007;46:441–445. doi: 10.1080/02841860701316057. [DOI] [PubMed] [Google Scholar]
- King DW, Lashley R. A quantifiable alternative to double data entry. Control Clin.Trials. 2000;21:94–102. doi: 10.1016/s0197-2456(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Messelink B, Benson T, Berghmans B, Bo K, Corcos J, Fowler C, Laycock J, Lim PH, Van Lunsen R, A Nijeholt GL, Pemberton J, Wang A, Watier A, Van Kerrebroeck P. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374–380. doi: 10.1002/nau.20144. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58:196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan UO.2012. EPIC Prostate Cancer Index Composite—sample size - http://www.med.umich.edu/urology/research/epic.html .
- Miller DC, Sanda MG, Dunn RL, Montie JE, Pimentel H, Sandler HM, Mclaughlin WP, Wei JT. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing, Preparing People to Change Addictive Behavior. The Guildford Press: NY, USA; 2002. [Google Scholar]
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- Rehabiliteringsforum . Hvidbog om rehabiliteringsbegrebet. Marselisborg Centret: Denmark; 2004. [Google Scholar]
- Ribeiro LH, Prota C, Gomes CM, De Bessa J, Jr., Boldarine MP, Dall'oglio MF, Bruschini H, Srougi M. Long-term effect of early postoperative pelvic floor biofeedback on continence in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. J Urol. 2010;184:1034–1039. doi: 10.1016/j.juro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wo Shah WJ. Quality of life and satisfaction with outcome among prostate-cancer survivors. New Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355:98–102. doi: 10.1016/S0140-6736(99)03473-X. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, Lund JA, Tasdemir I, Hoyer M, Wiklund F, Fossa SD. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- Williams MB, Hernandez J, Thompson I. Luteinizing hormone-releasing hormone agonist effects on skeletal muscle: how hormonal therapy in prostate cancer affects muscular strength. J Urol. 2005;173:1067–1071. doi: 10.1097/01.ju.0000143193.81585.5c. [DOI] [PubMed] [Google Scholar]
- World Health Organization, W.H.O 2012RE: World Health Organization WHO. Definition of rehabilitation http://www.who.int/topics/rehabilitation .
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]