Abstract
Background:
Persin is a plant toxin that displays synergistic cytotoxicity with tamoxifen in human breast cancer cell lines. Here, we examined the ability of persin to circumvent tamoxifen resistance and delineated the intracellular signalling pathways involved.
Methods:
The induction of apoptosis in tamoxifen-resistant and -sensitive breast cancer cells was measured by flow cytometry following treatment with persin±tamoxifen. Markers of endoplasmic reticulum stress (ERS) were analysed following treatment, and their causal role in mediating persin-induced apoptosis was determined using chemical inhibitors and RNA interference.
Results:
Cells that were resistant to an apoptotic concentration of tamoxifen maintained an apoptotic response to persin. Persin-induced apoptosis was associated with an increase in markers of ERS, that is, CHOP expression and XBP-1 splicing and was decreased by CHOP siRNA. The CASP-4 inhibitor Z-YVAD-FMK markedly inhibited persin-induced apoptosis in both tamoxifen-sensitive and -resistant cells.
Conclusion:
The cytotoxic effects of persin are CASP-4 dependent and mediated by CHOP-dependent and -independent ERS signalling cascades. Increased ERS signalling contributes to persin-induced reversal of tamoxifen resistance.
Keywords: tamoxifen, persin, breast cancer, apoptosis, endoplasmic reticulum stress
Breast cancer remains the most common cancer of women and the second most frequent cause of cancer death despite a major decline in breast cancer mortality in the past decade. However, despite the widespread clinical efficacy of endocrine therapies for the treatment of breast cancer, they are ineffective as therapy for oestrogen receptor (ER)-negative disease, and even among those patients with ER-positive disease that do initially respond, intrinsic or acquired therapeutic resistance remains a major obstacle to an effective cure (reviewed in Musgrove and Sutherland, 2009). Thus, there is a clear need for the development of novel therapeutic strategies that can enhance and broaden responsiveness, particularly in hormone refractory disease.
There is now accumulating evidence that aberrant apoptosis regulation has an important role in the development of endocrine resistance (Butt et al, 2007; Musgrove and Sutherland, 2009). More specifically, recent studies have highlighted the role of the endoplasmic reticulum in the initiation of a pathologically and therapeutically relevant apoptotic programme (Moenner et al, 2007; Liao et al, 2008) which, when disrupted, can be associated with anti-oestrogen resistance (Gomez et al, 2007). Imbalances in endoplasmic reticulum homeostasis triggered through cellular stresses such as hypoxia, low glucose or through the accumulation of misfolded proteins leads to the generation of an endoplasmic reticulum-mediated stress response and the triggering of an intrinsic apoptotic pathway, mediated, in part, through the generation of ceramide (Liao et al, 2008).
The endoplasmic reticulum stress (ERS) response has also been a recent focus from a cancer therapeutic perspective, given its emerging role as a protective mechanism against chemotherapy-induced cytotoxicity and as a potential initiating step in the development of chemoresistance (reviewed in Schönthal, 2009). Cancer cells often display elevated levels of ERS markers compared with their non-malignant counterparts, leading to the hypothesis that they exist in a state of chronic, low level ERS as an adaptive mechanism to enable growth and survival under adverse conditions, such as hypoxia and low nutrient environments. This differential ERS status of normal and malignant cells also provides an opportunity for therapeutic intervention as it may sensitise cancer cells to inducers of apoptosis.
Phytochemicals have provided a particularly abundant source of anti-cancer agents (Nishino et al, 2005), and we have previously described a novel plant toxin, persin, that has unique, in vivo actions in the mammary epithelium and potent apoptotic effects in human breast cancer cells in vitro (Butt et al, 2006). Persin is an avocado acetogenin, derived from the biosynthesis of long-chain fatty acids with close structural homology to the essential n-6 polyunsaturated fatty acid (PUFA), linoleic acid. Interestingly, conjugated linoleic acids (linoleic acid isomers) have anti-carcinogenic properties with particular efficacy against breast cancer (Ip et al, 2002). Furthermore, other PUFAs structurally similar to persin can attenuate steroid hormone receptor sensitivity to circulating oestrogens by direct modulation of receptor levels, and these data have formed the rationale for a promising phase II clinical trial of gamma linoleic acid and the anti-oestrogen, tamoxifen as primary therapy for breast cancer, which demonstrated a significant improvement in the overall clinical response (Kenny et al, 2000). Persin also exhibits potent synergistic cytotoxicity with the active metabolite of tamoxifen, 4-hydroxytamoxifen (4-OHT) in vitro in both ER-positive and ER-negative breast cancer cells, but not in phenotypically normal, breast epithelial cells (Roberts et al, 2007). This synergy is mediated via enhanced ceramide signalling—more specifically, persin generates de novo ceramide production that is further enhanced by 4-OHT's inhibition of ceramide glycosylation (Roberts et al, 2007). Thus, the generation of an ERS response mediated by ceramide signalling could represent an upstream initiating event in persin-induced cytotoxicity, which could, in turn, impact on its ability to modulate tamoxifen responsiveness.
Herein, we have further defined the mechanistic basis of persin-induced cytotoxicity and synergy with 4-OHT in human breast cancer cells, by determining its effects on ERS pathways in 4-OHT-sensitive and -resistant cell lines. We demonstrate that persin causes apoptosis in both 4-OHT-sensitive and -resistant cell lines. Persin treatment results in a robust ERS response with evidence that persin's ability to augment the pro-apoptotic response to 4-OHT is mediated through enhanced ERS signalling. These data not only define the intracellular pathways mediating persin's actions in breast cancer cells, but also highlight potential therapeutic strategies through which 4-OHT resistance may be overcome.
Materials and Methods
Purification and isolation of persin
The isolation and purification of persin from milled freeze-dried leaves of the avocado, P. americana, and monitoring of batch purity has previously been described (Oelrichs et al, 1995).
Cell lines and reagents
The human breast cancer cell lines MCF-7 (Michigan Cancer Foundation, Detroit, MI) and T-47D (EG&G Mason Research Institute, Worcester, MA, USA) were maintained in RPMI-1640 medium supplemented with 5% fetal calf serum (FCS), 10 μg ml−1 insulin, and 2 mM glutamine under standard conditions. The TAM-R cell line was derived from MCF-7 cells as previously reported (Knowlden et al, 2003) and was maintained in phenol red-free RPMI-1640 supplemented with 5% charcoal-stripped-steroid-depleted FCS, 4 mM glutamine, and 100 nM 4-OHT. MCF 5-21 (4-OHT sensitive) and MCF 5-23 (4-OHT resistant) sub-clones of MCF-7 cells (Hu et al, 1993) were maintained in 10% FCS, 10 μg ml−1 insulin, and 4 mM glutamine. 4-OHT was purchased from Sigma (St Louis, MO, USA), CASP-4 inhibitor, Z-YVAD-FMK and pan-caspase inhibitor, Z-VAD-FMK were purchased from R&D Systems (Minneapolis, MN, USA), and tunicamycin was purchased from Merck Pty. Ltd. (Kilsyth, VIC, Australia).
Measurement of apoptosis by flow cytometry
For M30 analysis, floating and attached cell populations were combined, fixed, and permeabilised in ice-cold methanol and then resuspended in PBS/0.5% bovine serum albumin with FITC-conjugated M30 CytoDEATH monoclonal antibody (Enzo Life Sciences, Farmingdale, NY, USA) before the M30-positive (apoptotic) population was determined by flow cytometry on BD FACSCantoII (Becton Dickinson, Mountain View, CA, USA).
Immunoblot analysis
Proteins from whole-cell lysates were resolved under reducing conditions on 12% SDS-polyacrylamide gels using standard methods. Resolved proteins were transferred onto polyvinylidene difluoride membranes and probed with antibodies against binding immunoglobulin protein (BiP) (Cell Signaling Technology, Danvers, MA, USA), CCAAT/enhancer binding protein homologous transcription factor/growth arrest and DNA damage-inducible gene 153 (CHOP/GADD153) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bim (Calbiochem, Darmstadt, Germany) and ERα (Santa Cruz Biotechnology) overnight at 4 °C. Immunoreactive protein bands were detected by the relevant anti-IgG antibodies conjugated with horseradish peroxidase followed by enhanced chemiluminescence (Pierce, Rockford, IL, USA). Blots were checked for equal loading by reprobing with anti-β-actin antibody (Sigma).
XBP-1 activation assay
Cells were incubated for 8, 16, or 24 h with tunicamycin, persin, or vehicle control, total RNA was isolated using the RNeasy kit (Qiagen, Limberg, The Netherlands) and 1 μg was reverse transcribed using the Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer's instructions. XBP-1 cDNA was amplified using human-specific primers (GeneWorks; forward: 5′-AAACAGAGTAGCAGCTCAGACTGC-3′ reverse: 5′-GTATCTCTAAGACTAGGGGCTTGGTA-3′) and Pfu DNA Polymerase (Promega), and the PCR product digested with PstI restriction enzyme (Promega) for 6 h. The digested PCR product was then separated using 2% agarose gel electrophoresis.
Caspase-4 inhibition
Cells were incubated for 24 h with persin, persin and the CASP-4-specific inhibitor Z-YVAD-FMK (20 μM), persin and the pan-caspase inhibitor Z-VAD-FMK (20 μM) or vehicle control. M30 analysis was used to determine apoptosis.
RNA interference
Small interfering RNA (siRNA) specific for CHOP (ON-TARGETplus SMART pool human DDIT3) and non-targeting controls (ON-TARGETplus siCONTROL) was purchased from Dharmacon Inc. (Lafayette, CO, USA). At seeding, cells were transfected with Lipofectamine 2000 (Invitrogen Life Technology Inc., Carlsbad, CA, USA) in the presence of siRNAs according to the manufacturer's protocol. Following overnight incubation, cells were treated with persin and/or 4-OHT for a further 24 h.
Statistical analysis
All data represent the mean of at least three independent experiments. Statistical analysis was carried out using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences between groups (control and treated) were evaluated by two-tailed, unpaired Student's t-test, or one-way ANOVA with Tukeys multiple comparison test (synergy analysis), and a P-value of<0.05 was considered as statistically significant.
Results
Response of TAM-R cells to persin
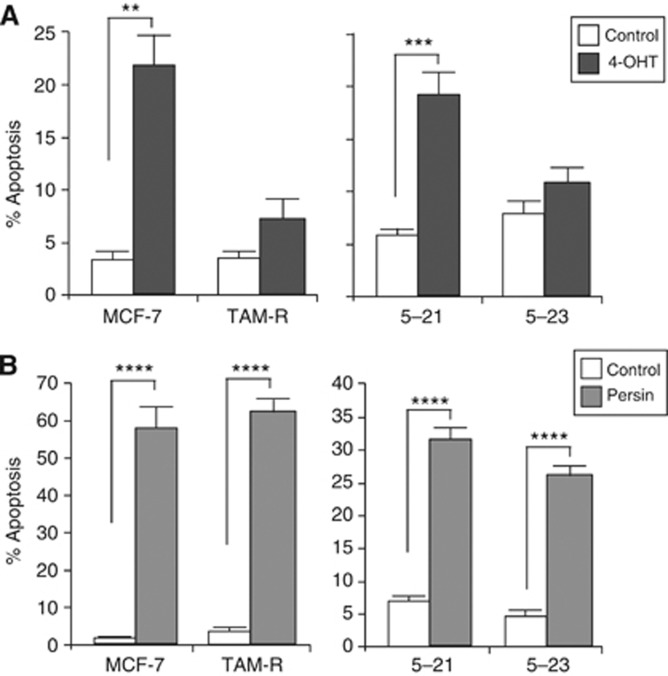
The two resistance models used in this study are the TAM-R acquired resistance model developed through continuous passage of MCF-7 cells for 6 months in the presence of 4-OHT (Knowlden et al, 2003), and a de novo resistance model consisting of 4-OHT-sensitive (MCF 5-21) and 4-OHT-resistant (MCF 5-23) sub-clones of MCF-7 derived by limiting dilution (Hu et al, 1993). It has previously been demonstrated that TAM-R and MCF 5-23 cells are resistant to cytostatic concentrations of 4-OHT, that is, <1 μM (Knowlden et al, 2003). Here, we have further characterised these models and shown that they are also resistant to an apoptotic concentration of 4-OHT (7.5 μM; Figure 1A) compared with their sensitive counterparts (Figure 1A).
Figure 1.
The response of 4-OHT-sensitive and -resistant breast cancer cells to both tamoxifen and persin-induced apoptosis. (A) MCF-7/TAM-R and MCF 5-21/23 cells were treated with an apoptotic concentration of 4-OHT (7.5 μM) or vehicle control, and the induction of apoptosis was analysed in attached and floating cells by M30-FITC positivity 24 h after treatment. Values are calculated from at least three independent experiments±s.e. (B) MCF-7/TAM-R and MCF 5-21/23 cells were treated with an apoptotic concentration of persin (10 μg ml−1) or vehicle control, and the induction of apoptosis was analysed in attached and floating cells 24 h after treatment. Values are calculated from at least three independent experiments±s.e. **P<0.005, ***P<0.001, ****P<0.0001 for treated cells vs untreated controls.
Having demonstrated a differential apoptotic response to 4-OHT, we determined whether resistant cells exhibited a broader apoptotic resistance by determining their response to an apoptotic concentration of persin. Figure 1B shows that in both of the cell models there was a significant increase in apoptosis (as determined by M30 positivity) following persin (10 μg ml−1) treatment, regardless of the sensitivity of the cells to 4-OHT suggesting that intact apoptotic pathways are maintained despite the development of 4-OHT resistance. However, while both TAM-R and MCF 5-21/23 cells displayed a similar differential response to 4-OHT (Figure 1A), the TAM-R cell model displayed a more pronounced apoptotic response to persin than the MCF 5-21/23 model (Figure 1B).
Co-treatment with persin reverses 4-OHT resistance in TAM-R and MCF 5-23 cells
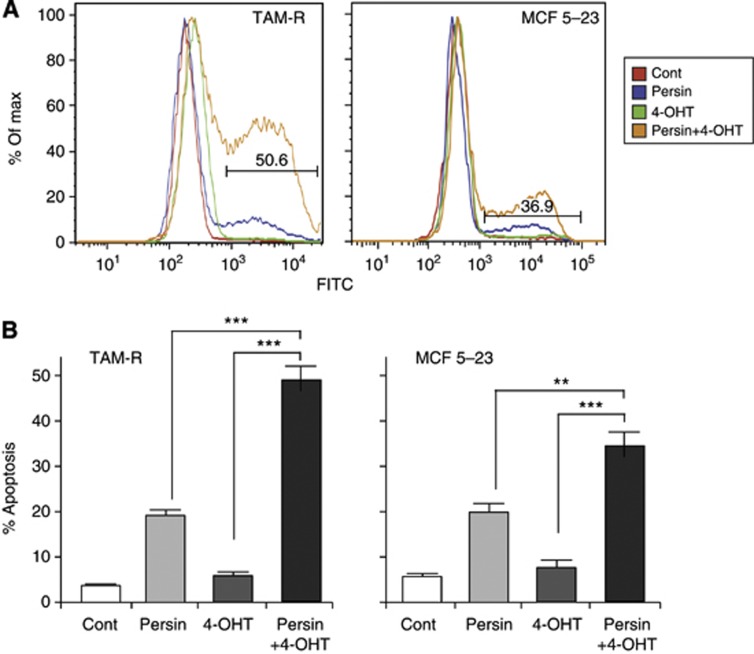
Our previous studies have demonstrated a cytotoxic synergy between persin and 4-OHT in human breast cancer cells (Roberts et al, 2007). We determined the effects of a lower, non-apoptotic concentration of persin on the apoptotic response to 4-OHT in both the 4-OHT resistant cell lines, TAM-R and MCF 5-23. Following treatment with persin (1 μg ml−1) and 4-OHT (7.5 μM) alone or in combination for 24 h, apoptosis was determined by flow cytometric analysis of cells stained using the M30 CytoDEATH antibody. Figure 2A is an overlay of representative histograms, showing a robust induction of apoptosis in cells receiving the combined treatment. The graphs in Figure 2B show data combined from at least three independent experiments and demonstrate the significantly augmented apoptotic response when persin was used in combination with 4-OHT compared with either single agent alone—an effect that was observed in both of the 4-OHT-resistant cell lines examined.
Figure 2.
Co-treatment with persin and 4-OHT reverses 4-OHT resistance. (A) TAM-R and MCF 5-23 cells were treated with a non-apoptotic concentration of persin (1 μg ml−1) or 4-OHT (7.5 μM) alone or in combination for 24 h, then attached and floating populations were analysed for M30-FITC positivity by flow cytometry. Overlays of representative histograms showing the M30-FITC-positive (apoptotic) fraction. (B) Bars, mean of M30-positive fractions from at least three independent experiments±s.e. **P<0.01, ***P<0.001, for combination treatment vs single treatment.
Persin-induced apoptosis is associated with an increase in markers of ERS
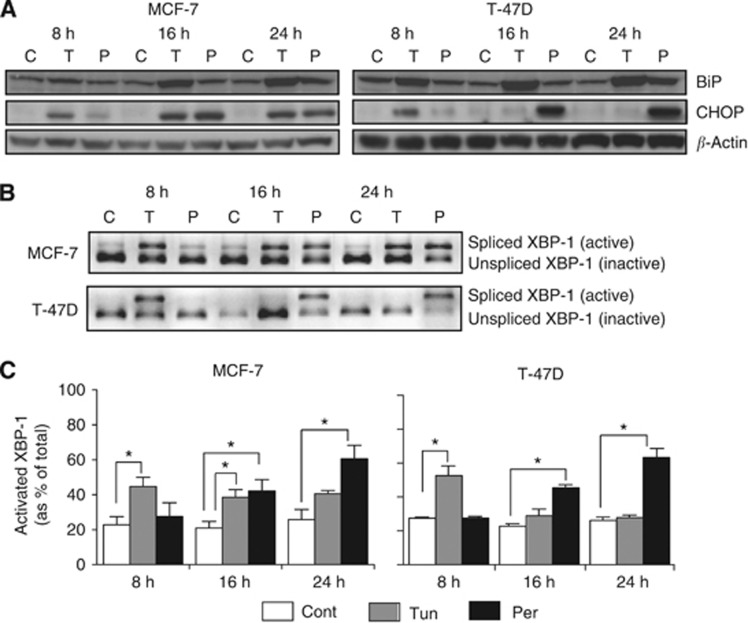
To further explore the mechanistic basis of persin-induced cytotoxicity, we examined components of ERS signalling pathways in two persin-sensitive human breast cancer cell lines, MCF-7 and T-47D, using the known ERS inducer, tunicamycin, as a positive control. Our previous studies have demonstrated a robust induction of apoptosis in both MCF-7 and T-47D cells following 24 h treatment with persin (Butt et al, 2006; Roberts et al, 2007). Here, we examined the effects of an apoptotic concentration of persin (10 μg ml−1) or tunicamycin (10 μg ml−1) on several markers of the ERS response—expression of BiP, CHOP/GADD153 (C/EBP homologous protein/DNA damage-inducible gene 153) and splicing of X-box-binding protein-1 (XBP-1).
Figure 3A demonstrates that treatment of breast cancer cells with persin (10 μg ml−1) results in an induction of BiP and CHOP to levels comparable to the known ERS inducer, tunicamycin. Persin treatment resulted in a significant induction of CHOP within 16 h and BiP by 24 h in both cell lines (Figure 3A).
Figure 3.
Persin-induced apoptosis is associated with the upregulation and activation of markers of endoplasmic reticulum stress. (A) MCF-7 and T-47D cells were treated with an apoptotic concentration of persin (P; 10 μg ml−1), the endoplasmic reticulum stress inducer, tunicamycin (T; 10 μg ml−1), or untreated (C) for 8, 16, or 24 h, and levels of BiP and CHOP were determined by immunoblotting. β-Actin was used as a loading control. Representative blots from three independent experiments are shown. (B) MCF-7 and T-47D cells were treated with tunicamycin (T; 10 μg ml−1), an apoptotic concentration of persin (P; 10 μg ml−1) or vehicle control (C) for 8, 16, or 24 h. RNA was then collected, cDNA produced, XBP-1 cDNA amplified using PCR, and the product digested with the restriction enzyme PstI to determine the proportion of undigested (active) to digested (inactive) XBP-1. Representative gels from at least three independent experiments are shown. (C) Densitometric analysis of undigested vs digested XBP-1 PCR product in MCF-7 and T-47D cells treated with tunicamycin (10 μg ml−1) or persin (10 μg ml−1) for 8, 16, or 24 h, and vehicle controls. Bars, mean of three independent experiments±s.e. *P<0.05 for treated cells vs untreated controls.
Splicing of XBP-1 mRNA is stimulated by ERS as part of the unfolded protein response (UPR). XBP-1 isoforms were identified and quantitated in breast cancer cells treated with persin (10 μg ml−1), tunicamycin (10 μg ml−1), or vehicle control (Figure 3B). Figure 3C demonstrates that splicing of XBP-1 in persin-treated cells followed a similar activation profile to CHOP induction—XBP-1 was significantly activated within 16 h of treatment and activation was sustained for up to 24 h. Tunicamycin activated XBP-1 within 8 h in both cell lines.
Interestingly, differential temporal dynamics were observed in the ERS response to persin compared with tunicamycin. Treatment with the latter initiated a more rapid response (within 8 h) from all ERS markers examined compared with persin, but the response to persin was more sustained, typically being maintained up to 24 h following treatment.
The role of ERS in persin-induced apoptosis
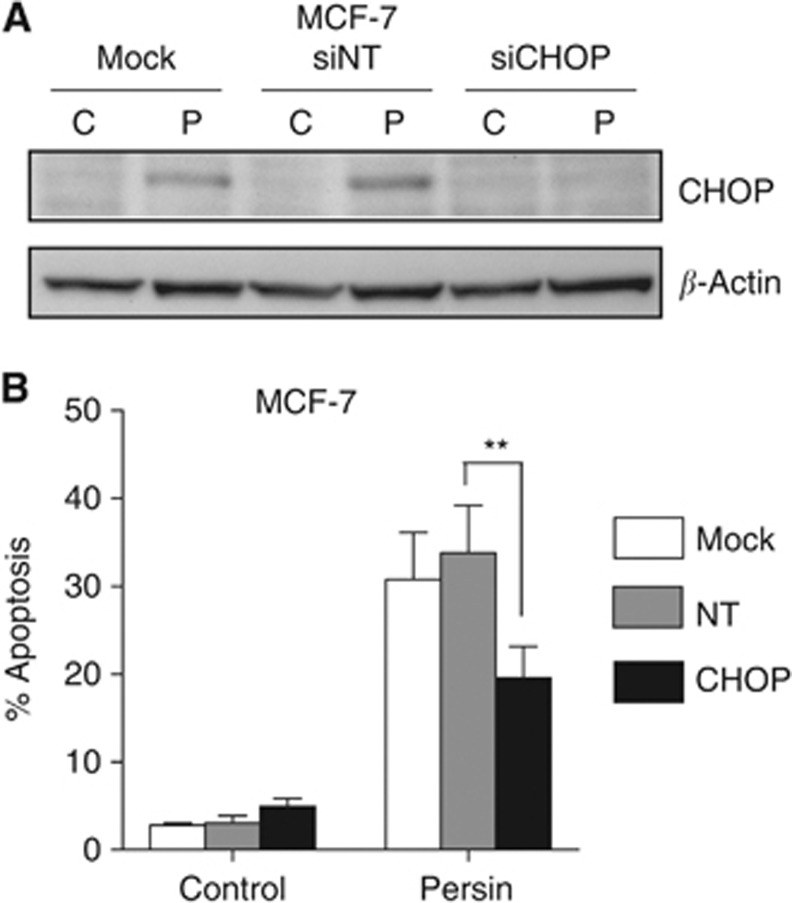
Having identified an increase in markers of ERS associated with persin treatment, we then determined the role of the ERS response in the induction of persin-induced apoptosis. Since CHOP induction is central to ERS response pathways (Kim et al, 2008), we examined the effects of attenuating CHOP expression using specific siRNA. The induction of CHOP protein expression following 24 h treatment with an apoptotic concentration of persin (10 μg ml−1) was typically reduced by ∼85% in the presence of CHOP siRNAs (Figure 4A). Figure 4B shows that this attenuation of CHOP expression significantly decreased persin-induced apoptosis in MCF-7 cells.
Figure 4.
The role of CHOP in the induction of persin-induced apoptosis. (A) MCF-7 cells were treated for 24 h with CHOP-specific siRNAs (siCHOP; 20 nM), non-targeting controls (siNT; 20 nM) or mock transfected and then treated for a further 24 h with an apoptotic concentration of persin (P; 10 μg ml−1) or vehicle control (C). Protein samples were prepared and analysed for CHOP expression by immunoblotting. Representative blots from four independent experiments are shown. β-Actin was used as a loading control. (B) Attached and floating populations were then analysed for M30-FITC positivity by flow cytometry. Bars, mean of M30-positive fractions from four independent experiments±s.e. **P<0.005 for CHOP siRNA treatment vs NT siRNA treatment.
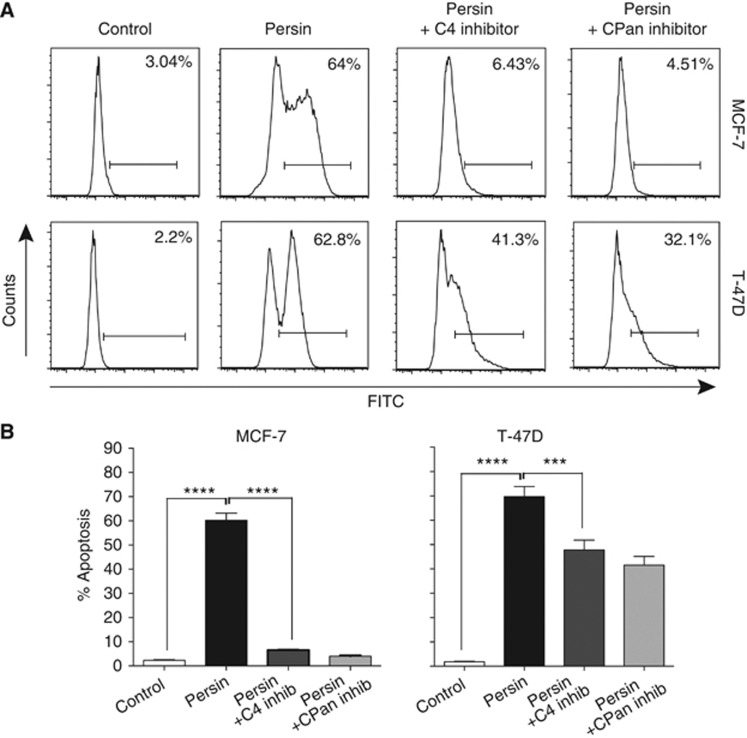
As CASP-4 has a role in mediating ERS-induced apoptosis (Kim et al, 2008), we next examined the role of CASP-4 activation in the apoptotic response to persin in MCF-7 and T-47D cells using the CASP-4-specific inhibitor, Z-YVAD-FMK. Cells were treated with an apoptotic concentration of persin (10 μg ml−1) in the presence or absence of Z-YVAD-FMK (20 μM) or the pan-caspase inhibitor, Z-VAD-FMK (20 μM) and apoptosis determined by M30-cytoDEATH antibody staining. Figure 5A shows representative histograms of apoptosis induction in both cell lines following single agent or combined treatment. Combined data from three independent experiments demonstrate a significant attenuation of persin-induced apoptosis in both cell lines following treatment with Z-YVAD-FMK at comparable levels to broad spectrum caspase inhibition (Figure 5B), suggesting that it is predominantly mediated through a CASP-4-dependent, ERS signalling cascade.
Figure 5.
Inhibition of caspase-4 reduces persin-induced apoptosis. (A) MCF-7 and T-47D cells were treated with an apoptotic concentration of persin (10 μg ml−1) alone or in combination with caspase-4 inhibitor (20 μM) or pan-caspase inhibitor (20 μM) for 24 h, and then attached and floating populations were analysed for M30-FITC positivity by flow cytometry. Representative histograms showing the M30-FITC-positive (apoptotic) fraction are shown. (B) Bars, mean of M30-positive fractions from three independent experiments±s.e. ***P<0.001; ****P<0.0001 for combination treatment vs single treatment.
Collectively, these results suggest that persin's apoptotic effects in breast cancer cells are mediated predominantly via CHOP-dependent ERS signalling cascades that induce apoptosis through CASP-4 activation.
Persin-induced apoptosis in 4-OHT-resistant cells is associated with markers of ERS
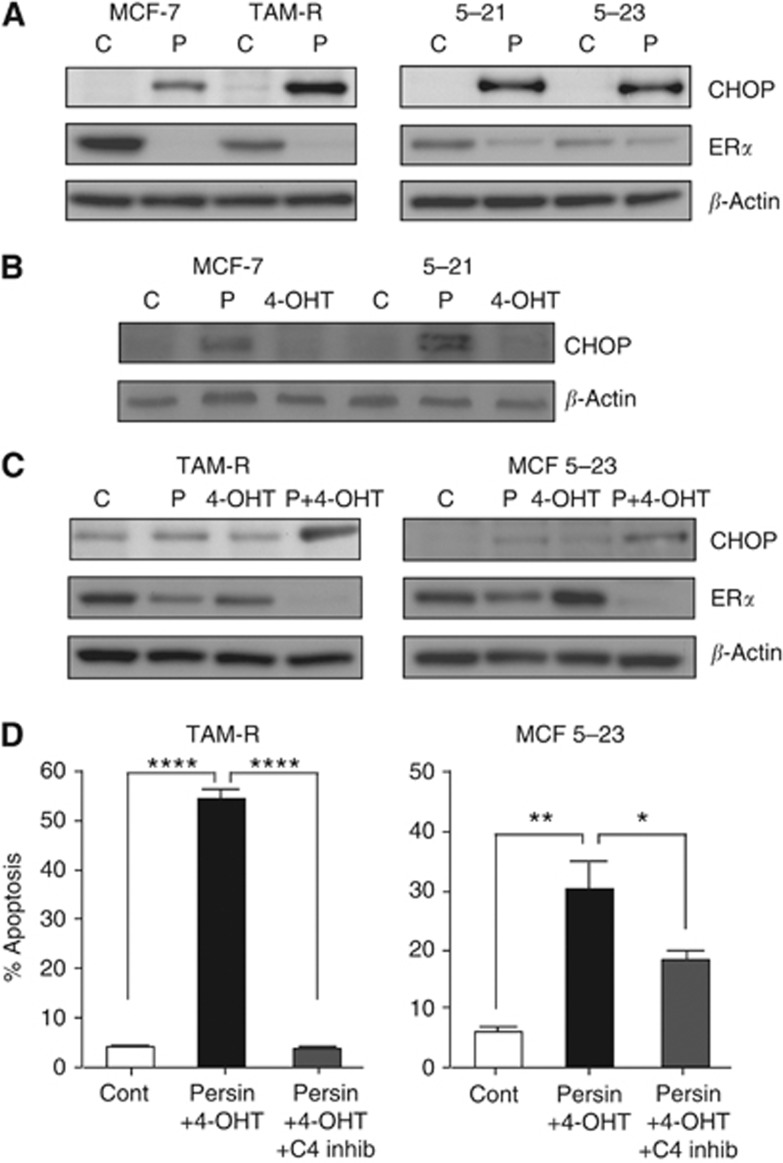
When 4-OHT-resistant cells were treated with an apoptotic concentration of persin (10 μg ml−1), they showed changes in ERS markers similar to the 4-OHT-sensitive MCF-7 and T-47D cells: treatment of TAM-R and MCF 5-23 cells with persin (10 μg ml−1) for 24 h resulted in an increase in CHOP expression (Figure 6A). Interestingly, we also observed a decrease in ERα expression in 4-OHT-resistant cells in response to persin (Figure 6A) as previously observed in persin-sensitive cells (Roberts et al, 2007). These effects on the ERS response appear to be specific for persin, as treatment of TAM-sensitive cells with apoptotic concentrations of 4-OHT did not result in any changes in CHOP expression compared with untreated controls (Figure 6B).
Figure 6.
Persin-induced apoptosis in 4-OHT-resistant cells is associated with changes in markers of endoplasmic reticulum stress. (A) Immunoblot analysis of CHOP and ERα expression in whole-cell lysates from cells treated with an apoptotic concentration of persin (P; 10 μg ml−1) or vehicle control (C) for 24 h. β-Actin was used as a loading control. Representative blots from three independent experiments are shown. (B) Immunoblot analysis of CHOP expression in whole-cell lysates from MCF-7 and MCF 5-21 cells treated with apoptotic concentrations of persin (P; 10 μg ml−1), 4-OHT (4-OHT; 7.5 μM) or vehicle control (C) for 24 h. β-Actin was used as a loading control. Representative blots from three independent experiments are shown. (C) Immunoblot analysis of CHOP and ERα expression in whole-cell lysates from TAM-R and MCF 5-23 cells treated with a non-apoptotic concentration of persin (P; 1 μg ml−1) or 4-OHT (T; 7.5 μM) alone or in combination (P+4-OHT) for 24 h or vehicle control (C) for 24 h. β-Actin was used as a loading control. Representative blots from three independent experiments are shown. (D) TAM-R and MCF 5-23 cells were treated with a non-apoptotic concentration of persin (1 μg ml−1) and 4-OHT (7.5 μM) alone or with caspase-4 inhibitor (20 μM) for 24 h, and then attached and floating populations were analysed for M30-FITC positivity by flow cytometry. Bars, mean of M30-positive fractions from three independent experiments±s.e. *P<0.05; **P<0.005; ****P<0.0001 for combination treatment with inhibitor vs no inhibitor treatment or vehicle controls.
Persin and 4-OHT proapoptotic synergy is mediated via an ERS response
We then proceeded to determine what role persin-induced ERS played in persin's ability to reverse 4-OHT resistance. When the 4-OHT-resistant cell lines TAM-R and MCF 5-23 were treated with a low non-apoptotic concentration of persin (1 μg ml−1) and an apoptotic concentration of 4-OHT (7.5 μM), CHOP protein expression was increased (Figure 6C), and again, a significant decrease in ERα was also observed. These changes are greater than the additive effect of the single agents alone.
We further delineated the molecular mechanisms driving the proapoptotic synergy between persin and 4-OHT by determining the cellular effects of inhibiting CASP-4 on the observed synergistic response. Figure 6D shows that treatment with the CASP-4 inhibitor significantly abrogated persin and 4-OHT-induced apoptosis. In TAM-R cells, apoptosis was reduced to levels observed in vehicle-treated control cells, whereas in MCF 5-23 cells, the apoptosis was reduced by ∼50%.
Discussion
As the mechanisms of mammalian cell death signalling become further delineated, the possibility of manipulating the intracellular apoptotic machinery for therapeutic benefit is being explored experimentally (Liu et al, 2011), with several apoptosis-inducing therapies showing early promise in the clinical trial setting (Reed, 2002). From a breast cancer perspective, while the role of apoptosis in influencing the endocrine response is still evolving (Butt et al, 2007; Musgrove and Sutherland, 2009), the possibility of combining endocrine therapies with those inducing apoptosis has significant potential to both maximise responsiveness and circumvent resistance. However, such advances are dependent upon a deeper understanding of the molecular determinants of 4-OHT responsiveness and the intracellular apoptotic signalling cascades that impinge upon them.
In our previous preclinical studies, we described the cytostatic and cytotoxic efficacy of the avocado-derived toxin, persin in human breast cancer cell lines (Butt et al, 2006), as well as demonstrating its ability to sensitise both ER-negative and ER-positive cells to the apoptotic effects of 4-OHT through modulations of de novo sphingolipid biosynthesis (Roberts et al, 2007). Given this ability to modulate the sensitivity to 4-OHT, we have extended our previous work to further dissect the interactions between persin-induced apoptosis and the cellular response to 4-OHT, thereby gaining greater insight into the pathways governing anti-oestrogen resistance.
This study demonstrates that while tamoxifen-resistant cells are resistant to an apoptotic concentration of 4-OHT, they retain a robust apoptotic response to persin—comparable to that observed in their 4-OHT-sensitive counterparts. This suggests that the block to 4-OHT-induced cell death in these cell models may occur either upstream in the apoptotic cascade relative to persin action, or via a parallel pathway. The former hypothesis is further supported by the ability of persin to circumvent 4-OHT resistance and restore sensitivity to 4-OHT-induced apoptosis.
These data provided the rationale for further exploration of the mechanistic basis of persin-induced cytotoxicity and its point of convergence with 4-OHT-mediated apoptotic signalling. Previously, we have shown that Bim, c-Jun NH2-terminal kinase (JNK), and ceramide are all upstream mediators of persin-induced apoptosis (Butt et al, 2006; Roberts et al, 2007). As all of these have been implicated in ERS pathways (Woodcock, 2006; Puthalakath et al, 2007), we further explored the effects of persin on the cellular ERS response.
We showed that persin elicits a robust ERS response in MCF-7 and T-47D breast cancer cells as determined by the induction/activation of ERS markers, BiP, CHOP, and XBP-1, to levels comparable to those following treatment with the known ERS inducer, tunicamycin. Furthermore, this increase in ERS signalling appears to be causal, at least in part, for persin-induced apoptosis as demonstrated by inhibition of CHOP and CASP-4—central mediators of the ERS response. However, persin-induced apoptosis was not completely ameliorated by knockdown of CHOP expression with siRNA, suggesting either some remaining functional CHOP expression following siRNA treatment, or that CHOP-independent pathways may also be involved in mediating persin's cytotoxicity.
CHOP induction is the point of convergence for three upstream ERS transducers localised on the endoplasmic reticulum membrane—PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and Ire1. These transducers are activated under ERS conditions when the unfolded protein sensor, BiP dissociates from them to bind to unfolded proteins in the endoplasmic reticulum (Oyadomari and Mori, 2004). CASP-4 is localised to the endoplasmic reticulum membrane and there are now several lines of evidence pointing to its central role as a mediator of ERS-induced apoptosis in cancer cells (Hitomi et al, 2004; Kim et al, 2008), including the abrogation of ERS-mediated apoptosis by CASP-4 inhibition in several cancer cell models (reviewed by Di Fazio et al, 2012).
Persin's effects on BiP suggest that it may act upstream of the ERS cascade, but whether this is via a direct interaction with BiP or indirectly through alternate intermediaries remains not yet clear. However, since de novo ceramide synthesis takes place in the ER, there may be a link between persin's known effects on sphingolipid metabolism (Roberts et al, 2007) and its induction of ERS-mediated apoptosis.
Previous work has linked key components of the ERS pathway to the endocrine response. For example, Gu et al (2002) reported that the development of anti-oestrogen resistance in vitro is associated with an increase in XBP-1 expression in human breast cancer cells, later demonstrating that XBP-1 is a key factor in mediating anti-oestrogen responsiveness through modulating apoptosis (Gomez et al, 2007). Clinical studies have also demonstrated an association between XBP-1 activation and clinical outcome in endocrine-treated breast cancers (Davies et al, 2008). More recent studies have shown modulations in CHOP (Tiwary et al, 2011) and BiP (Brüning et al, 2010) associated with the response to tamoxifen in vitro, including a reversal of tamoxifen resistance (Tiwary et al, 2011). Furthermore, Ariazi et al (2011) reported a CASP-4-dependent, ERS response was associated with oestrogen-induced apoptosis in in vitro models of acquired resistance.
However, despite these reports, the exact mechanisms involved remain unclear—including what might shift the tamoxifen-induced UPR from a survival to an apoptotic end point, and why tamoxifen-resistant cells appear particularly sensitive to ERS inducers. One possibility is that any further stress induction on the heightened state of ERS present in a high proportion of breast cancers (Fernandez et al, 2000; Scriven et al, 2009) tips the survival/apoptosis balance causing cytotoxicity. In addition, given that oestrogen stimulation can itself induce an UPR, the agonistic response to tamoxifen displayed by some tamoxifen-resistant cells may also contribute to increased ERS signalling and apoptosis.
Collectively, these studies and our recent data presented here provide a strong rationale for further exploring the role of ERS pathways in tamoxifen responsiveness in breast cancer, both as potential biomarkers of the endocrine response and as novel therapeutic targets to maximise responsiveness—particularly in endocrine refractory disease. Furthermore, the accumulating data on the overexpression of ERS signalling components, such as BiP and XBP-1, in a high proportion of breast cancers (Scriven et al, 2009) and their effects on therapeutic resistance and clinical outcomes (Lee et al, 2006; Davies et al, 2008; Tiwary et al, 2011), emphasise their clinical relevance in this regard. Thus, the further exploration of the mechanisms of action of persin or similar, more therapeutically amenable analogues (Brooke et al, 2011) may have clinical utility in this context.
Acknowledgments
This research was supported by Breast Cancer Australia (AJB), National Health and Medical Research Council (NHMRC) of Australia Program Grant 535903 (EAM and RLS) and Fellowships 427601 (RLS) and 427610 (TJB), Cancer Institute NSW CDF Fellowship (EAM), FRL Fellowship (AB) and Research Scholar Award (EJS), an Australian Postgraduate Award (EJS), the Australian Cancer Research Foundation (RLS), the R.T. Hall Trust (RLS), the Petre Foundation (RLS) and the Breast Cancer Campaign (JMG).
References
- Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, Tapia C, Kim HR, Yerrum S, Sharma CG, Nicolas E, Balagurunathan Y, Ross EA, Jordan VC. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. PNAS. 2011;108 (47:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke DG, Shelley EJ, Roberts CG, Denny WA, Sutherland RL, Butt AJ. Synthesis and in vitro evaluation of analogues of avocado-produced toxin (+)-(R)-persin in human breast cancer cells. Bioorg Med Chem. 2011;19 (23:7033–7043. doi: 10.1016/j.bmc.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Brüning A, Friese K, Burges A, Mylonas I. Tamoxifen enhances the cytotoxic effects of nelfinavir in breast cancer cells. Breast Cancer Res. 2010;12 (4:R45. doi: 10.1186/bcr2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AJ, Musgrove EA, Sutherland RL. Live or let die: oestrogen regulation of survival signalling in endocrine response. Breast Cancer Res. 2007;9 (5:306. doi: 10.1186/bcr1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AJ, Roberts CG, Seawright AA, Oelrichs PB, MacLeod JK, Liaw TYE, Kavallaris M, Somers-Edgar TJ, Lehrbach GM, Watts CK, Sutherland RL. A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol Cancer Ther. 2006;5 (9:2300–2309. doi: 10.1158/1535-7163.MCT-06-0170. [DOI] [PubMed] [Google Scholar]
- Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, Rudland PS, Sibson DR. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123 (1:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- Di Fazio P, Ocker M, Montalbano R. New drugs, old fashioned ways: ER stress induced cell death. Curr Pharm Biotechnol. 2012;13 (11:2228–2234. doi: 10.2174/138920112802501962. [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Tabbara SO, Jacobs LK, Manning FCR, Tsangaris TN, Schwartz AM, Kennedy KA, Patierno SR. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M, Clarke R. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21 (14:4013–4027. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, Liu A, Zhu Y, Davis N, Leonessa F, Brünner N, Wang Y, Clarke R. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780) Cancer Res. 2002;62 (12:3428–3437. [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165 (3:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XF, Veroni M, De Luise M, Wakeling A, Sutherland R, Watts CK, Zalcberg JR. Circumvention of tamoxifen resistance by the pure anti-estrogen ICI 182,780. Int J Cancer. 1993;55 (5:873–876. doi: 10.1002/ijc.2910550529. [DOI] [PubMed] [Google Scholar]
- Ip C, Dong Y, Ip MM, Banni S, Carta G, Angioni E, Murru E, Spada S, Melis MP, Saebo A. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer. 2002;43:52–58. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- Kenny FS, Pinder SE, Ellis IO, Gee JM, Nicholson RI, Bryce RP, Robertson JFR. Gamma linolenic acid with tamoxifen as primary therapy in breast cancer. Int J Cancer. 2000;85:643–648. doi: 10.1002/(sici)1097-0215(20000301)85:5<643::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7 (12:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- Liao P-C, Tan S-K, Lieu C-H, Jung H-K. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem. 2008;104:1509–1523. doi: 10.1002/jcb.21730. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Lin M, Yu JY, Liu B, Bao JK. Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 2011;300 (2:105–114. doi: 10.1016/j.canlet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67 (22:10631–10634. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9 (9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- Nishino H, Murakoshi M, Mou XY, Wada S, Masuda M, Ohsaka Y, Satomi Y, Jinno K. Cancer prevention by phytochemicals. Oncology. 2005;69:38–40. doi: 10.1159/000086631. [DOI] [PubMed] [Google Scholar]
- Oelrichs PB, Ng JC, Seawright AA, Ward A, Schaffeler L, MacLeod JK. Isolation and identification of a compound from avocado (Persea americana) leaves which causes necrosis of the acinar epithelium of the lactating mammary gland and the myocardium. Nat Toxins. 1995;3:344–349. doi: 10.1002/nt.2620030504. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11 (4:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129 (7:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1 (2:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- Roberts CG, Gurisik E, Biden TJ, Sutherland RL, Butt AJ. Synergistic cytotoxicity between tamoxifen and the plant toxin persin in human breast cancer cells is dependent on Bim expression and mediated by modulation of ceramide metabolism. Mol Cancer Ther. 2007;6 (10:2777–2785. doi: 10.1158/1535-7163.MCT-07-0374. [DOI] [PubMed] [Google Scholar]
- Schönthal AH. Endoplasmic reticulum stress and autophagy as targets for cancer therapy. Cancer Lett. 2009;275 (2:163–169. doi: 10.1016/j.canlet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br J Cancer. 2009;101 (10:1692–1698. doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary R, Yu W, Degraffenried LA, Sanders BG, Kline K. Targeting cholesterol-rich microdomains to circumvent tamoxifen-resistant breast cancer. Breast Cancer Res. 2011;13 (6:R120. doi: 10.1186/bcr3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. 2006;58 (8:462–466. doi: 10.1080/15216540600871118. [DOI] [PubMed] [Google Scholar]