Abstract
Objective
Although hyperactivity would seem to increase energy expenditure, attention deficit/hyperactivity disorder (ADHD) appears to increase the risk for being overweight. The present study examined the Body Mass Index (BMI) in children with ADHD and its relationship with age, gender, ADHD and comorbid symptom severity, inhibitory control, developmental coordination disorder (DCD), sleep duration and methylphenidate use.
Method
Participants were 372 Dutch children with ADHD combined type aged 5–17 years participating in the International Multicenter ADHD Genetics (IMAGE) study. We categorized BMI according to international age- and gender-specific reference values and calculated BMI-standard deviation scores (BMI-SDS). The control population was matched for age, gender and ethnicity and originated from the same birth cohort as the ADHD group. Inhibitory control was measured by the computerized Stop-signal task. Prevalence differences of underweight, overweight and obesity between groups were expressed in odds ratio’s. We used linear regression analyses with gender, age, parent- and teacher-rated ADHD and comorbid scores, inhibitory control, sleep duration, motor coordination and methylphenidate use to predict BMI-SDS.
Results
Boys with ADHD 10–17 and girls 10–12 years of age were more likely to be overweight than children in the general Dutch population. Younger girls and female teenagers, however, seemed to be at lower risk for being overweight. Higher oppositional behavior and social communication problems related to higher BMI-SDS scores, whereas more stereotyped behaviors related to lower BMI-SDS scores. We found no effects of the other examined associated risk factors on BMI-SDS.
Conclusion
ADHD in boys is a risk factor for overweight. In girls with ADHD, the prevalence of overweight is age-dependent and most pronounced in girls 10–12 years of age. They have a fourfold risk of being obese. Higher oppositional and social communication problems pose an increased risk for overweight, whereas sleep duration, motor coordination problems and methylphenidate use do not.
Keywords: ADHD, BMI, DCD, sleep, methylphenidate
Although attention deficit/hyperactivity disorder (ADHD) by definition includes hyperactivity, this hyperactivity does not necessarily mean high physical activity. In clinical practice many children with ADHD are overweight and several recent studies show that children with a clinical diagnosis of ADHD are indeed heavier than the average child.1–3 This relationship between ADHD and overweight has also been observed in the general population. Children with ADHD were found to be overweight about twice as often as their typically functioning peers. Conversely, children with overweight were twice as likely to exhibit elevated rates of ADHD symptoms than their average-weight counterparts.4, 5 Other studies, however, did not confirm this ADHD-overweight association. In a general population study in the US, the prevalence of overweight was equal in children with ADHD and same-age peers.6 Further, a recent study even found evidence of lower weights in children with ADHD, compared to controls.7
Several of these studies were subject to methodological problems. Some did not define their clinical samples exhaustively or based the ADHD diagnoses solely on self- or parent-reports, others did not measure weight and height accurately or consistently and others again included children taking medications for ADHD with known potential for weight gain side effects such as risperidone or clonidine. However, the most likely explanation for the inconsistent findings may be the different definitions of overweight and obesity and the use of dated reference groups. A proper reference population with respect to year of measurement and ethnicity is extremely important because of the worldwide rapid increase of Body Mass Index (BMI) in the general childhood population (with particularly fast increased in certain ethnic groups) during the 1990s (i.e. strong cohort effects).8 Comparison of ADHD samples to dated reference populations is likely to overestimate the prevalence of overweight and obesity in ADHD by not taking into account these cohort effects. Another vital issue is the lack of agreement on the definition of overweight and obesity. In adults, the BMI is widely used and cut-offs of 25 and 30 kg/m2 are recognized internationally as a definition of overweight and obesity, respectively. In children, this is more complicated, since BMI changes substantially with age. Defining age- and gender-specific BMI-z-scores is helpful, however, cut-off scores are not universally agreed upon. In the Unites States, the 85th and 95th percentiles of BMI have been recommended as cut-off points for overweight and obesity, respectively. Use of such descriptive cut-off points is inherently problematic, however, as they may shift with changes in the population. Given the strong increase in overweight and obesity, children below these cut-off points may also be overweight and obese. Therefore, Cole and others defined normative, international gender- and age-specific BMI cut-offs for overweight and obesity based on growth data from six countries: Brazil, Great Britain, Hong Kong, The Netherlands, Singapore and the United States.9 Subjects include were 97.876 males and 94.851 females from birth to 25 years of age. It could be a better comparison group than the usual US determined cut-offs at 85 and 95 percentiles, since for each of the surveys centile curves were drawn that at age 18 years passed through the 25 and 30 BMI adult cut-offs. Although not without their shortcomings (e.g. about the validity of these cut-off points for all ethnicities), a large advantage of such international cut-offs is the increased comparability of findings across studies.
An obvious additional gap in our knowledge about the possible association between ADHD and overweight are the potential mechanisms underlying such an association. Our study aims to investigate these possible underlying mechanisms, which has not extensively been done before. Given the core symptoms of ADHD, i.e. hyperactivity, impulsivity and poor attention, this disorder is by definition characterized by a high degree of impulsiveness, which may lead to uncontrolled eating. ADHD has indeed been associated with eating disorders in girls and women with ADHD.10 Children with ADHD also have an elevated sensitivity to immediate reward, even when a delayed reward is more appealing.11 The availability of or exposure to easy (fast) food, for instance, may then more readily trigger snacking or impulse eating. Also, heightened distractibility and reduced planning and organizational abilities can underlie chaotic eating patterns, raising the odds of becoming overweight. Moreover, ADHD has been linked to an increased risk of addiction.12 Teenagers not receiving treatment for their ADHD tend to smoke or use cannabis and cocaine more often than treated peers or healthy controls. It is possible that this population is then also at greater risk for food addictions. Furthermore, motor coordination problems may play a substantial role, since about one third of the ADHD population also has motor issues in terms of developmental coordination disorder (DCD).13 It is known that motor coordination has a reverse (or negative) correlation with BMI, i.e. high motor proficiency is associated with lower BMIs.14 As children with DCD are less physically active, this subgroup may be especially overweight. Additionally or alternatively, overweight in ADHD may also be related to difficulties with sleeping, which are often present in children with ADHD.15 It has recently been demonstrated that young children that do not get enough sleep are at increased risk of becoming overweight; each hour a child sleeps less raises the BMI by a factor of 0.5.16 The obesogenic effect of reduced sleep is thought to be mediated by both behavioural and hormonal factors. There is simply more time to eat, and sleep deprivation reduces leptin and raises ghrelin levels, which increases appetite. A lack of sleep may additionally cause fatigue during the day, reducing activity levels. Finally, there may be a relationship between medication use and BMI. Methylphenidate, the most widely used drug for the treatment of ADHD, is known to reduce appetite, potentially causing weight loss.17 A large-scale population study in the USA indeed found that unmedicated children with ADHD had an elevated risk of becoming overweight (OR 1.5), whereas the children taking medication were more likely to be underweight (OR 1.6).5 A very recent population study likewise showed that the risk of obesity was highest in unmedicated girls.3 However, an Israeli study found no effects of medication on BMI.7
In sum, previous studies on ADHD and overweight are difficult to compare with each other due to the use of different definitions for ADHD, overweight and different reference groups. To date, the relationship between ADHD and overweight is not fully understood. The present study therefore addressed two crucial issues: is overweight more common in children with a well-defined, robust ADHD diagnosis using an appropriate control population matched for year of measurement and ethnicity, and do gender and age play a role? And if such an association is observed, what are the potential underlying mechanisms? We had the opportunity to examine the role of several of these potential mechanisms underlying overweight and ADHD and tested the following four hypotheses:
Prevalence of overweight and obesity is higher in children with ADHD compared to children in the general population.
BMI and Inhibitory Control are related in children with ADHD.
Overweight can be predicted by comorbid motor problems (DCD) or comorbid psychiatric problems (oppositional defiant behaviour, anxiety or autism spectrum disorder traits).
Sleep duration and use of methylphenidate effects BMI in children with ADHD.
Methods
Participants
The children participating in our study were recruited between 2004 and 2006 as part of the International Multicenter ADHD Genetics (IMAGE) study from paediatric and child psychiatric services, and through advertisements in the magazine and on the website of the Dutch Parents Association of Children with ADHD.18, 19 The sample was obtained from the Dutch culture, more specifically from Caucasian parents. Immigrant Dutch children were excluded from the study in order to avoid heterogeneity because of the in origin genetic design of the study. All had earlier been seen by a paediatrician, neurologist or child psychiatrist. Before inclusion, eligible children were reassessed for ADHD. Apart from fulfilling the DSM-IV criteria for combined subtype ADHD, they needed to be of Caucasian descent, be 5 to 18 years old, live at home, and attend primary or high school. Exclusion criteria were IQ<70, known genetic syndromes (Down, Turner, Fragile-X), autism, neurological conditions such as current or past brain disorders or epilepsy. An additional exclusion criterion for the current study was the current use of obesogenic pharmacological agents and/or medication known to affect sleep, such as clonidine, pipamperon, risperidone, pimozide and melatonine. This lead to the exclusion of 14 (3.6%) out of the original 386 participants and resulted in a total sample of 372 children. A minority of the remaining children (13/372, 3.5%) had also used these types of medication previously but did not do so at the time of assessment. The study was approved by regional ethics review boards and written informed consent was obtained from the parent(s) and from the child (if over 12 years of age).
Instruments
ADHD
A first assessment confirming previous ADHD-combined type (ADHD-C) diagnoses was by means of Conners’ parent and teacher rating scales (long form, revised) 20, the parent and teacher Strengths and Difficulties Questionnaires (SDQ) and the Social Communication Questionnaire (SCQ) 21. T-scores ≥ 63 on the Conners ADHD subscales, inattentive (L), hyperactive-impulsive (M) and total scores (N), and scores > 90th percentile on the SDQ-hyperactivity scale were considered clinically significant. Children with clinical scores on any of the questionnaires were invited to the hospital for further assessment, during which visit the parents completed the Parental Account of Children’s Symptoms (PACS) interview, a semi-structured, standardized, investigator-based interview 22. A standardized algorithm was applied to the PACS to derive each of the 18 DSM-IV ADHD symptoms, providing operational definitions for each behavioural symptom. These were combined with items that were scored 2 (‘pretty much true’) or 3 (‘very much true’) on the teacher-rated Conners ADHD subscales (L, M and N) to generate the total number of hyperactive-impulsive and inattentive symptoms of the DSM-IV symptom list. Parent and teacher T-scores on the separate scales of Inattention and Hyperactivity-Impulsivity of the Conners were used as continuous measures of ADHD symptom severity. Data were available for all children.
Body Mass Index
Body weight and height were measured by professionals in the hospital. The children were clothed but wore no shoes or coats. Weight was measured to 0.1 kg using an electronic flat scale (Seca 877, Optimed), height was determined to the nearest 5 mm using a wall-mounted tape measure (Seca 20, Optimed). BMI was calculated as weight in kilograms divided by squared height in meters. The children were classified as underweight, overweight (including obesity) or obese, based on internationally accepted Cole’s cut-off points, corresponding to adult BMI thresholds of 17.5, 25 and 30, respectively 9, 23 We calculated BMI standard deviation scores (z-scores) using Growth Analyser 3.5 software (Dutch Growth Foundation, Rotterdam, the Netherlands). The control population was a Dutch sample of 90.071 children, collected by 11 community health services, during 2002–2004. The children were matched for age, gender and ethnicity and originated from the same birth cohort as the ADHD group24.
Inhibitory control
Inhibitory control was measured using the computerized Stop-signal task (described in detail in Rommelse et al.).25 Subjects were presented two types of trials: go-trials and stop-trials. Go-trials consisted of the presentation of a go-stimulus (drawing of a plane) that was either pointing to the right or to the left. Children were instructed to press a response button that corresponded to the direction of the stimulus as quickly and as accurately as possible. Stop-trials were identical to the go-stimulus, but, in addition, a stop-signal was presented. Children were required to withhold their response to the stop-signal. The delay between the go- and stop-signal was dynamically varied so that the child successfully inhibited 50% of the stop-trials and unsuccessfully inhibited the other 50%. At this point, the go-process and stop-process are of equal duration, which makes it possible to estimate the latency of the stop-process: the Stop Signal Reaction Time (SSRT) (Logan, 1994). The SSRT was used as a measure reflecting inhibitory control. Data was available for 232 (62.4%) of the 372 children.
Motor problems
Parents completed the Developmental Coordination Disorder Questionnaire (DCDQ) to help uncover any motor deficits. Originally developed in Canada by Wilson and colleagues, the 17-item inventory identifies children with motor problems in daily life and was translated and validated in The Netherlands. Its internal consistency is high (alpha=.88).26 Parents were asked to compare their child’s coordination skills with those of other same-age children using a 5-point scale, ranging from “Not at all like this child,” to “Extremely like this child”. The summary score can range from 17 to 85, with lower scores reflecting poorer performance. Data was available for 330 (88.7%) of the 372 children.
Comorbid psychiatric problems
Conner’s indices from parents and teacher (specifically: Oppositional, Anxious/shy, Perfectionism, Social problems, Psychosomatic (parents only) and Emotional Lability) and the three separate scales from the SCQ (Social interaction, Communication, and Stereotyped behavior) were used to operationalize comorbid traits.
Sleep and methylphenidate
In the knowledge that methylphenidate potentially gives a reduction in appetite and sleep disruption, parents completed a 36-item questionnaire specifically designed for this study to assess the children’s development, motor milestones, sleep habits and bedtime resistance, infections, hospital admissions, use of psychostimulantia and associated healthcare consumption (e.g. physiotherapy). To help dichotomize the children into pertinent groups, i.e. short vs. normal sleepers and users of psychostimulantia vs. non-users, we posed the following questions: “Does your child generally sleep shorter than children of the same age?” and “Does your child use methylphenidate?”. We did not distinguish between immediate release and extended release preparations of methylphenidate. Also, we did not query other stimulant medication than methylphenidate since in The Netherlands methylphenidate is by far the mostly used and the only approved stimulant. If affirmative of methylphenidate use, parents were asked to indicate whether their child took this medication shorter than one year, between one and two years or longer than two years. Data on sleep was available for 351 (94.4%), data on methylphenidate use for 363 (94.1%) of the 372 children. Two-hundred-seventy-two (74.9%) of the 363 children took methylphenidate.
Statistics
Data were stratified by age to form three age groups: 5–9 years (N=109), 10–12 years (N=156) and 13–17 years (N=121). These age groups were chosen based on developmental considerations (representing early childhood, middle childhood and adolescence, respectively) and sample size (providing sufficient group sizes for further analyses on prevalence estimates). Using both Cole’s cut-off points and p5, p85, p95 percentile cut-off points based on BMI-standard deviation scores (BMI-SDS), we calculated the prevalence of underweight, overweight and obesity in our ADHD sample (measured in 2004) and compared it with the prevalence rates of abnormal weight in a Dutch population sample measured in 2003.23 This is a good comparison group since it was measured in the same time period and was matched for age, gender and ethnicity. Odds ratios with confidence intervals (LCL=lower control limit; UCL=upper control limit) and exact p-values were used to compare the ADHD group with the population values of underweight, overweight and obesity per age group. In order to explore whether ADHD symptom severity, inhibitory control problems, motor problems, comorbid psychiatric problems, psychostimulant use and sleep duration were related to weight, linear regression analyses were used with BMI-SDS as the dependent variable. Some predictor variables were recoded to facilitate interpretation: higher scores indicated more ADHD symptoms, more motor, comorbid psychiatric and sleep problems, a worse stop signal reaction time and the use of methylphenidate. Analyses were conducted for each separate predictor with the main effect of the predictor, sex and age and the two- and three-way interactions between the predictor and sex and age to examine moderating effects. A p-value <0.05 was considered statistically significant. All statistical analyses were carried out with SPSS (version 18.0; IBM Corporation, Somers, NY, USA).
Results
Underweight, overweight and obesity
Groups stratified for age and sex were compared for ADHD symptom severity, motor and comorbid psychiatric problems, inhibitory control, methylphenidate use, and sleep problems (Table 1). In addition to some age effects, substantial sex differences emerged for ADHD symptom severity, comorbid psychiatric symptom severity and treatment with methylphenidate. Compared to boys, girls had higher inattention and hyperactivity-impulsivity scores, more oppositional behaviour, were more anxious and psychosomatic, and had more social problems and communication problems according to parents and/or teachers, yet were less often treated with methylphenidate. No gender differences regarding sleep duration, motor problems or inhibitory control were found.
Table 1.
Participant characteristics stratified by sex and age.
| Boys | Girls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5–9 years N=86 |
10–12 years N=124 |
13–17 years N=96 |
t/χ2 | 5–9 years N=21 |
10–12 years N=25 |
13–17 years N=20 |
t/χ2 | t/χ2 | |
| Parent Inattention T-score (M, SD) | 67.8 (8.0) | 68.1 (7.7) | 70.0 (8.7) | ns | 77.2 (9.3) | 82.7 (7.7) | 79.1 (10.6) | ns | ♂<♀ |
| Parent Hyperactivity-Impulsivity T-score (M, SD) | 74.6 (9.6) | 76.8 (10.1) | 79.5 (10.3) | ns | 82.1 (6.6) | 85.5 (6.9) | 81.1 (12.7) | ns | ♂<♀ |
| Teacher Inattention T-score (M, SD) | 62.0 (7.7) | 64.6 (7.3) | 68.3 (9.9) | 5–9 = 10–12 < 13–17 | 67.7 (11.1) | 69.6 (11.3) | 70.4 (16.3) | ns | ♂<♀ |
| Teacher Hyperactivity-Impulsivity T-score (M, SD) | 67.2 (9.0) | 68.6 (9.8) | 72.9 (12.1) | 5–9 = 10–12 < 13–17 | 72.1 (11.5) | 77.9 (13.7) | 73.2 (19.2) | ns | ♂<♀ |
| Developmental Coordination Disorder Total score (M, SD) | 50.7 (10.7) | 50.7 (13.1) | 53.4 (13.6) | ns | 54.2 (11.2) | 51.9 (12.6) | 53.8 (8.3) | ns | ♂=♀ |
| Stop Signal Reaction Time (M, SD) | 350.6 (109.7) | 308.3 (82.4) | 243.3 (46.6) | 5–9 > 10–12 > 13–17 | 321.1 (71.1) | 255.8 (88.9) | 241.4 (64.0) | ns | ♂=♀ |
| Short sleep (%) | 54.7% | 54.6% | 37.1% | 5–9 = 10–12 > 13–17 | 47.4% | 40.9% | 37.5% | ns | ♂=♀ |
| Use of methylphenidate (%) | 72.9% | 87.3% | 83.1% | 5–9 < 13–17 < 10–12 | 44.4% | 68.2% | 55.6% | ns | ♂>♀ |
Weight categories defined according to Cole’s cut-offs and according to percentile cut-offs can be found in Table 2. When Cole’s cut-off points were used, overweight appeared more common in boys with ADHD aged 10–12 and 13–17, and underweight less common in boys with ADHD of all age categories, providing the overall impression of increased weight in boys with ADHD, with two comparisons reaching significance. Findings for extreme overweight (obesity) were less clear, with obesity appearing less prevalent in the youngest (5–9) and oldest (13–17) boys with ADHD but more prevalent in the 10–12 year old boys, although none of these findings reached significance. For girls with ADHD, findings were clearly influenced by age, with young (5–9) and older (13–17) girls with ADHD having significantly less overweight and obesity compared to population girls, whereas 10–12 year old girls with ADHD were heavier than normal girls having significantly less underweight and more overweight and a more than four-fold risk of obesity.
Table 2.
Percentage underweight, overweight and obese children with ADHD in different age groups according to different cut-offs.
| UNDERWEIGHT | ||||
|---|---|---|---|---|
| P<51 | Cole’s cut-offs | |||
| ADHD | ADHD | Population2 | OR (LCL-UCL) | |
| Age 5–9 years | ||||
| Boys N=86 | 3.5 | 5.8 | 11.2 | 0.51 (0.18–0.84) |
| Girls N=21 | 0 | 9.5 | 11.2 | 0.85 (0.26–2.81) |
| Age 10–12 years | ||||
| Boys N=124 | 2.4 | 6.5 | 10.3 | 0.63 (0.37–1.07) |
| Girls N=25 | 0 | 4.0 | 11.6 | 0.34 (0.12–0.99) |
| Age 13–17 years | ||||
| Boys N=96 | 5.2 | 6.3 | 8.6 | 0.73 (0.38–1.41) |
| Girls N=20 | 0 | 10.0 | 9.2 | 1.09 (0.24–4.86) |
| OVERWEIGHT (INCLUDING OBESE) | ||||
| p≥851 | Cole’s cut-offs | |||
| ADHD | ADHD | Population3 | OR (LCL-UCL) | |
| Age 5–9 years | ||||
| Boys N=86 | 25.6 | 11.6 | 14.3 | 0.81 (0.48–1.37) |
| Girls N=21 | 19.0 | 4.8 | 18.7 | 0.26 (0.11–0.63) |
| Age 10–12 years | ||||
| Boys N=124 | 31.5 | 20.2 | 13.0 | 1.55 (1.01–2.38) |
| Girls N=25 | 40.0 | 28.0 | 15.0 | 1.87 (0.80–4.36) |
| Age 13–17 years | ||||
| Boys N=96 | 30.2 | 18.8 | 14.4 | 1.31 (0.81–2.12) |
| Girls N=20 | 5.0 | 0.0 | 16.6 | 0.11 (0.04–0.31)4 |
| OBESE | ||||
| p≥951 | Cole’s cut-offs | |||
| ADHD | ADHD | Population3 | OR (LCL-UCL) | |
| Age 5–9 years | ||||
| Boys N=86 | 12.8 | 1.2 | 2.6 | 0.46 (0.13–1.63) |
| Girls N=21 | 4.8 | 0.0 | 3.8 | 0.12 (0.02–0.99)4 |
| Age 10–12 years | ||||
| Boys N=124 | 21.0 | 3.2 | 2.3 | 1.39 (0.50–3.88) |
| Girls N=25 | 28.0 | 12.0 | 2.6 | 4.61 (0.58–36.55) |
| Age 13–17 years | ||||
| Boys N=96 | 18.8 | 1.0 | 2.6 | 0.38 (0.11–1.27) |
| Girls N=20 | 0.0 | 0.0 | 2.8 | 0.13 (0.01–1.40)4 |
Percentile cut-offs were based on BMI-SDS scores in comparison to the Dutch reference population of 1980 (Third National Growth Study, Fredriks 2000). These were not used to analyze whether an increased prevalence of abnormal weight was present in the ADHD sample measured in 2004, since these prevalence rates are confounded by a strong increase of overweight between 1980 and 2004 in the general population. However, these prevalence rates were based on the same Dutch population of 1980 which was also used by Cole et al. and indicate the large discrepancy in prevalence estimates of abnormal weight depending on the various cut-offs used.
Fourth National Growth Study in the Netherlands (1997)
Data reprinted with permission from the authors. Data concerns children (N=90.071) from urban and rural area’s measured between 2002 – 2004 in local community health services that routinely monitor the health of approximately 95% of all 0–19-year-old children living in The Netherlands37. Ethnicity was not recorded but was recorded during the Fourth National Growth Study in 1997. Under the assumption that ethnicity was similarly distributed in the 2002–2004 sample compared to the 1997 sample, a correction for ethnicity was made as follows: prevalence overweight 2002–2004 Caucasian sample = prevalence overweight 2002–2004 total sample – (prevalence overweight 1997 total sample - prevalence overweight 1997 Caucasian sample). A similar approach was taken for obesity and underweight (van Buuren, 2004).
Peto odds ratio with 95% confidence intervals using the null hypothesis to provide an estimate (Greenland 1990)
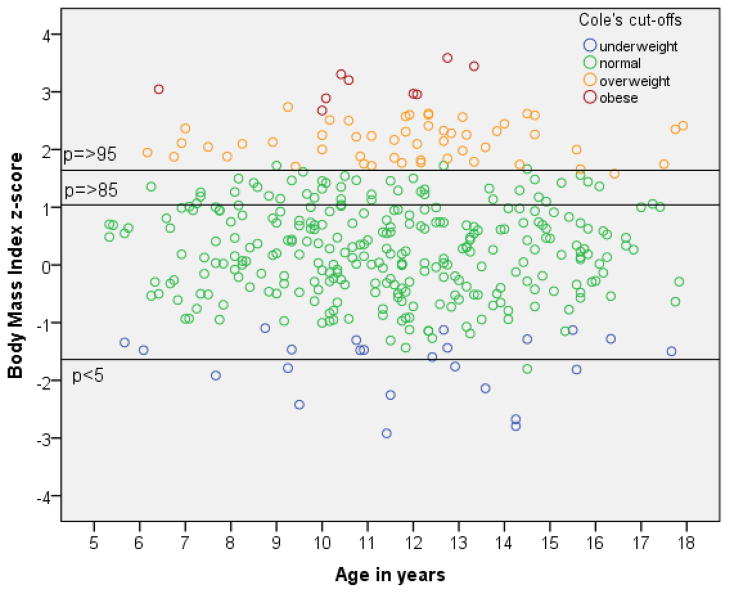
When different cut-off points were used (Table 2 and Figure 1), the findings were much more extreme. Applying the widely used percentile cut-offs to define underweight (p<5), overweight (p≥85) and obesity (p≥95), percentage-estimates of overweight and obese children with ADHD were substantially higher compared to using Cole’s cut-off points, resulting in many more significant comparisons (data not shown). The most strikingly, the prevalence of obesity in 5–9, 10–12 and 13–17 year old boys with ADHD were more than 10-times, 8-times and 18-times higher, respectively, if percentile versus Cole’s cut-off points were used.
Figure 1.
Comparison of underweight, normal weight, overweight and obesity using Cole’s cut-off points versus percentile cut-off points in 372 children with combined type ADHD.
Relationship between BMI-SDS and ADHD symptom severity, inhibitory control, medication use, motor problems, comorbid psychiatric problems, and sleep duration
In the linear regression analyses we found significant main effects (no interactions) for the following scales of the Conners and SCQ: Social problems parents t = 1.98, p = .05, β = 0.13; Oppositional teacher t = 2.95, p = .003, β = 0.24; Stereotyped behavior t = −2.96, p = .003, β = −0.18. Together they significantly predicted a small percentage (4.4%) of increased BMI-SDS, F = 5.58, p = .001, with higher social problems and more oppositional behavior relating to higher BMI scores, yet with higher stereotyped behaviors relating to lower BMI-SDS scores. No other significant main effects or two- or three-way interaction effects emerged, indicating that none of the other risk factors had a substantial impact on the BMI-SDS score. Only three trends (p<.10) emerged in the data, with teacher-rated inattention interacting somewhat with sex (t = 1.82, p = .07), but no main effect of teacher-rated inattention in boys (t = −0.81, p = .42) or girls (t = 0.01, p = .99). In addition, teacher-rated hyperactivity had a marginal main effect on BMI-SDS (t = −1.75, p = .08) and interacted with sex (t = 1.71, p = .09). Higher teacher-rated hyperactivity contributed marginally to a lower weight in boys (t = −1.79, p = .07), but not so in girls (t = 1.17, p = .25).
Discussion
The present study examined BMI in a well-defined sample of children with combined-type ADHD and its possible relationship with gender, age, severity of ADHD and comorbid psychiatric symptoms, inhibitory control, developmental coordination disorder, short sleep and methylphenidate use. We show that ADHD does not prevent children from developing overweight or even obesity. However, results vary greatly with age and gender. In boys, ADHD seems to be a general risk factor for overweight. They are more often overweight, especially from age 10 onwards. In girls, ADHD seems to be a risk factor for overweight and obesity in 10–12 years olds, particularly. In the study period, 13% of boys and 15% of girls 10 to 12 years old in the general population in The Netherlands were overweight 26 compared to 20% of the 10–12 year old boys with ADHD and 28% of the same-aged girls with ADHD, resulting in odds ratio’s for overweight of 1.55 and 1.87, respectively. For obesity, the numbers were even higher, with OR 1.39 in boys and OR 4.61 in girls. However, in young girls (5–9 years old) and in teenage girls (13–17 years old), ADHD seems to protect against overweight (OR 0.26 and 0.11, respectively).
We found only few relationships between BMI-SDS and the hypothesized risk factors: social problems and oppositional behaviours related to somewhat higher BMI-SDS scores, and stereotyped behaviours to somewhat lower BMI-SDS scores. Impulsivity scores did not relate to BMI-SDS, nor did inhibitory control. No effect of the other risk factors such as motor coordination problems, sleep duration and methylphenidate use on BMI-SDS was found.
The differences across the age and sex groups may be explained by the fact that the youngest age group is less overweight since they probably are under greater parental control. The 10–12 years olds are becoming more independent, may be getting pocket money and spending it on sweets and snacks. Teenage girls with ADHD showed less overweight than 10–12 years old girls with ADHD. A better self-regulation in this higher age group could theoretically play a role in this, although this is not supported by the Stop Signal Reaction Time task. Maybe the teenage girls have more body awareness compared to boys and younger children. However, the girls with ADHD in our sample may not be very representative of the general female ADHD population because they were more severely impaired than the boys on virtually all measures (Table 2), while at the same time they were treated less often with methylphenidate. Future studies specifically targeting female patients with ADHD should further clarify the relationship between ADHD and BMI in girls and possible underlying mechanisms.
We find it remarkable that we found only very few relationships with the examined associated risk factors. The finding that higher oppositional behaviours and communication problems related to somewhat higher BMI-SDS scores, may possibly be explained by a potentially lower socio-economic class in these children. A relationship between lower socioeconomic class and overweight is well established, but we could not elaborate on this since we had no reliable data on social class of the parents. The finding that stereotyped behaviors conversely related to BMI-SDS may potentially reflect the restrictive eating habits often observed in children with high autism traits. The Stop Signal Reaction Time as a measure of inhibitory control did not predict BMI. Theoretically, both ADHD and obesity may arise from common underlying neurobiological mechanisms, such as heightened impulsivity.27 We also did not observe a relationship between motor problems and BMI-SDS. A recent study found BMI-dependent motor performance decrements to be more pronounced with age.28 We did not replicate these findings. Although we also found relatively higher BMI values in the two older age groups in boys, the motor deficits did not become more severe compared to those we noted at younger ages. Furthermore, whereas in the literature the relationship between overweight and disturbed sleep patterns is well documented, and children suffering from sleep-disordered breathing are known to exhibit more ADHD-type symptoms 29, in our study sleep duration did not predict BMI. We also failed to find a correlation between BMI and methylphenidate use, even for those children that had been taking the drug for more than two years (data not shown). This finding is in sharp contrast to the findings of Waring et al., who even found evidence of underweight in medicated children, albeit that the authors did not provide information about the type of medication.5
It cannot be stressed enough, that different cut-off points lead to enormous changes in prevalence rates of overweight and obesity. We used Cole’s cut-off points to be able to compare our ADHD sample to other studies, in particular a Dutch cohort study matched for year of measurement and ethnicity. These cut-offs lead to relatively low estimates of overweight. When Cole cut-offs are expressed as percentiles, the overweight threshold corresponds to the 88th BMI percentile and obesity is thresholded at the 99th percentile, which are very conservative criteria. If we had been using percentile cut-offs at the 85th and 95th percentile to define overweight and obesity, the obesity prevalence would rise 10- to 18-fold (Figure 1). We chose a very conservative approach for our study in order to not overestimate overweight and obesity prevalence rates. Our results highlight the importance of choosing the most appropriate reference population regarding birth cohort when conducting studies of bodyweight and ADHD.
In addition to the large sample size of boys with ADHD, obvious strengths of our study are the robustness of the ADHD diagnosis and the accurate matching of our ADHD group to a control population regarding ethnicity and birth cohort. Weaknesses are the relatively small number of girls and the fact that we based our assessment of motor coordination, comorbid psychiatric problems and sleep problems solely on parental perception and/or teacher reports. Also, the study is cross-sectional in nature, implying that the direction of the association between excess weight and ADHD is unknown. Longitudinal studies are needed to shed more light on the development of overweight and obesity in ADHD.
Clinical implications
The finding of ADHD as a risk factor for overweight is highly relevant, as childhood obesity is a major social concern. It is not only associated with serious health consequences and reduced health-related quality of life, but also with various psychosocial problems, such as low self-esteem and depression. A recent prospective study monitoring BMI and mental health problems in young Australian children found that higher BMIs at age 4 to 5 years were predictive of poorer social functioning and emotional issues at age 8 to 9 years.30 Overweight children often become overweight adults, and the odds of developing type 2 diabetes and cardiovascular disease are higher in chronically overweight people. Notably, recent findings suggest that people who were obese as children but had attained normal weight in adulthood ran less risk of weight-related health problems, with a reported probability similar to that reported for adults who were of normal weight throughout their lives.31 So, prevention and treatment of overweight in children warrant very high priority. Since children with ADHD are an at-risk subgroup for overweight particularly when oppositional and social communication problems are present, it is time to apply this new knowledge to the treatment of ADHD. To date, assessment of side effects of medical treatment in the office focuses mainly on weight loss. Instead of being concerned about underweight in medicated children with ADHD, clinicians should be aware of the risk of overweight, also in this group of children. In sum, health workers should pay more attention to overweight in children with ADHD. Dietary patterns and physical exercise warrant more attention and deserve a place in the monitoring of children with ADHD.
Conclusion
ADHD in boys is a risk factor for overweight. In girls with ADHD the prevalence of overweight is age-dependent, with the highest risk during middle childhood (10–12 years). Oppositional behaviors and social communication problems may aggravate this, whereas sterotyped behaviors may be protective. Other associated risk factors such as short sleep, motor coordination problems and the use of methylphenidate theoretically play a role in some subgroups, but we could not find support for this in the current study. Our study has important implications for the clinical management of children with ADHD. Based on our findings, we make a strong plea for dietary patterns to be assessed and addressed more systematically in children with ADHD. Also health workers should pay more attention to sports and physical fitness in children with ADHD. Given the current surge in childhood obesity, it also is of great relevance to screen the obese childhood population for ADHD, and to address the ADHD-related symptoms, together with the children’s dietary, activity and sleep patterns.
Acknowledgments
This study was partly funded by NIH grants R01MH081803 and R01MH62873 to S.V. Faraone.
Dr. Stephen Faraone receives research support from the following sources: McNeil Pediatrics, Eli Lilly & Company, the National Institute of Mental Health, the National Institute of Child Health and Development and the National Institute of Neurological Diseases and Stroke. Dr. Stephen Faraone is a speaker for the following speaker’s bureaus: Eli Lilly & Company, McNeil Pediatrics, Cephalon, Novartis and Shire Laboratories. Dr. Stephen Faraone has had an advisory or consulting relationship with the following pharmaceutical companies: McNeil Pediatrics, Noven Pharmaceuticals, Shire Laboratories, Cephalon, Novartis and Eli Lilly & Company.
Biographies
Dr. Maras has been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, in the past 2 years. He is not an employee or a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties.
Jan K Buitelaar has been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, Organon/Shering Plough, UCB, Shire, Medice and Servier in the past 3 years. He is not an employee of any of these companies. He is not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties.
Footnotes
Conflict of interest: Dr. Fliers, Franke, Bul, Höhle and Rommelse report no biomedical financial interests or potential conflicts of interest.
Reference List
- 1.Cortese S, Angriman M, Maffeis C, et al. Attention-deficit/hyperactivity disorder (ADHD) and obesity: a systematic review of the literature. Crit Rev Food Sci Nutr. 2008 Jun;48(6):524–37. doi: 10.1080/10408390701540124. [DOI] [PubMed] [Google Scholar]
- 2.Ptacek R, Kuzelova H, Paclt I, et al. ADHD and growth: anthropometric changes in medicated and non-medicated ADHD boys. Med Sci Monit. 2009 Dec;15(12):CR595–CR599. [PubMed] [Google Scholar]
- 3.Kim J, Mutyala B, Agiovlasitis S, et al. Health behaviors and obesity among US children with attention deficit hyperactivity disorder by gender and medication use. Prev Med. 2011 Mar;52(3–4):218–22. doi: 10.1016/j.ypmed.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Erhart M, Herpertz-Dahlmann B, Wille N, et al. Examining the relationship between attention-deficit/hyperactivity disorder and overweight in children and adolescents. Eur Child Adolesc Psychiatry. 2012 Jan;21(1):39–49. doi: 10.1007/s00787-011-0230-0. [DOI] [PubMed] [Google Scholar]
- 5.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008 Jul;122(1):e1–e6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 6.Curtin C, Bandini LG, Perrin EC, et al. Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: a chart review. BMC Pediatr. 2005;5:48. doi: 10.1186/1471-2431-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubnov-Raz G, Perry A, Berger I. Body mass index of children with attention- deficit/hyperactivity disorder. J Child Neurol. 2011 Mar;26(3):302–8. doi: 10.1177/0883073810380051. [DOI] [PubMed] [Google Scholar]
- 8.de Wilde JA, van DP, Middelkoop BJ, Verkerk PH. Trends in overweight and obesity prevalence in Dutch, Turkish, Moroccan and Surinamese South Asian children in the Netherlands. Arch Dis Child. 2009 Oct;94(10):795–800. doi: 10.1136/adc.2009.163709. [DOI] [PubMed] [Google Scholar]
- 9.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000 May 6;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surman CB, Randall ET, Biederman J. Association between attention- deficit/hyperactivity disorder and bulimia nervosa: analysis of 4 case-control studies. J Clin Psychiatry. 2006 Mar;67(3):351–4. doi: 10.4088/jcp.v67n0303. [DOI] [PubMed] [Google Scholar]
- 11.Luman M, van Meel CS, Oosterlaan J, et al. Reward and Punishment Sensitivity in Children with ADHD: Validating the Sensitivity to Punishment and Sensitivity to Reward Questionnaire for Children (SPSRQ-C) J Abnorm Child Psychol. 2011 Jul 26; doi: 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. 2011 Jul;24(4):280–5. doi: 10.1097/YCO.0b013e328345c956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fliers E, Rommelse N, Vermeulen SH, et al. Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: effects of age and gender. J Neural Transm. 2008;115(2):211–20. doi: 10.1007/s00702-007-0827-0. [DOI] [PubMed] [Google Scholar]
- 14.Lopes VP, Stodden DF, Bianchi MM, et al. Correlation between BMI and motor coordination in children. J Sci Med Sport. 2012 Jan;15(1):38–43. doi: 10.1016/j.jsams.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Cortese S, Faraone SV, Konofal E, et al. Sleep in Children With Attention- Deficit/Hyperactivity Disorder: Meta-Analysis of Subjective and Objective Studies. J Am Acad Child Adolesc Psychiatry. 2009 Jul 21; doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 16.Carter PJ, Taylor BJ, Williams SM, et al. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008 Sep;47(9):994–1009. doi: 10.1097/CHI.ObO13e31817eOea7. [DOI] [PubMed] [Google Scholar]
- 18.Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006 Oct;11(10):934–53. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 19.Kuntsi J, Neale BM, Chen W, et al. The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct. 2006;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conners CK. Conners’Rating Scales-Revised: Technical manual. MHS; 2003. Sixth Printing. [Google Scholar]
- 21.Goodman R. The strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997 May;38(5):581–86. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor E, Schachar R, Thorley G, et al. Conduct disorder and hyperactivity: 1. Separation of hyperactivity and antisocial conduct in British child psychiatric patients. Br J Psychiatry. 1986;149:760–67. doi: 10.1192/bjp.149.6.760. [DOI] [PubMed] [Google Scholar]
- 23.Hirasing RA, Fredriks AM, van BS, Verloove-Vanhorick SP, et al. Increased prevalence of overweight and obesity in Dutch children, and the detection of overweight and obesity using international criteria and new reference diagrams. Ned Tijdschr Geneeskd. 2001 Jul 7;145(27):1303–8. [PubMed] [Google Scholar]
- 24.van den Hurk K, van Dommelen P, van Buuren S, et al. Prevalence of overweight and obesity in the Netherlands in 2003 compared to 1980 and 1997. Arch Dis Child. 2007 Nov;92(11):992–5. doi: 10.1136/adc.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rommelse NN, Altink ME, Oosterlaan J, et al. Support for an independent familial segregation of executive and intelligence endophenotypes in ADHD families. Psychol Med. 2008 Nov;38(11):1595–606. doi: 10.1017/S0033291708002869. [DOI] [PubMed] [Google Scholar]
- 26.Schoemaker MM, Smits-Engelsman BC, Jongmans MJ. Psychometric properties of the movement assessment battery for children-checklist as a screening instrument for children with a developmental co-ordination disorder. Br J Educ Psychol. 2003 Sep;73(Pt 3):425–41. doi: 10.1348/000709903322275911. [DOI] [PubMed] [Google Scholar]
- 27.Cortese S, Morcillo PC. Comorbidity between ADHD and obesity: exploring shared mechanisms and clinical implications. Postgrad Med. 2010 Sep;122(5):88–96. doi: 10.3810/pgm.2010.09.2205. [DOI] [PubMed] [Google Scholar]
- 28.D’Hondt E, Deforche B, Vaeyens R, et al. Gross motor coordination in relation to weight status and age in 5- to 12-year-old boys and girls: a cross-sectional study. Int J Pediatr Obes. 2011 Jun;6(2–2):e556–e564. doi: 10.3109/17477166.2010.500388. [DOI] [PubMed] [Google Scholar]
- 29.Cortese S, Maffeis C, Konofal E, et al. Parent reports of sleep/alertness problems and ADHD symptoms in a sample of obese adolescents. J Psychosom Res. 2007 Dec;63(6):587–90. doi: 10.1016/j.jpsychores.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer MG, Harchak T, Wake M, et al. Four-year prospective study of BMI and mental health problems in young children. Pediatrics. 2011 Oct;128(4):677–84. doi: 10.1542/peds.2010-3132. [DOI] [PubMed] [Google Scholar]
- 31.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011 Nov 17;365(20):1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]