Abstract
Photodynamic therapy (PDT) is a technique developed to treat the ever-increasing global incidence of cancer. This technique utilises singlet oxygen (1O2) generation via a laser excited photosensitiser (PS) to kill cancer cells. However, prolonged sensitivity to intensive light (6–8 weeks for lung cancer), relatively low tissue penetration by activating light (630 nm up to 4 mm), and the cost of PS administration can limit progressive PDT applications. The development of quantum-dot laser diodes emitting in the highest absorption region (1268 nm) of triplet oxygen (3O2) presents the possibility of inducing apoptosis in tumour cells through direct 3O2 → 1O2 transition. Here we demonstrate that a single laser pulse triggers dose-dependent 1O2 generation in both normal keratinocytes and tumour cells and show that tumour cells yield the highest 1O2 far beyond the initial laser pulse exposure. Our modelling and experimental results support the development of direct infrared (IR) laser-induced tumour treatment as a promising approach in tumour PDT.
The application of laser technology to diagnostic and therapeutic regimens across different medical fields has become widespread, encompassing diverse specialities ranging from ophthalmology to oncology. Since Diamond et al. in 1972 first successfully killed glioma cells in culture and in gliomas subcutaneously transplanted in rats with haematoporphyrin and visible light exposure photodynamic therapy has been used for the treatment of various cancers. The technique is based on the photodynamic effect (PDE) inducing damage (DNA, membranes, etc.) in a photosensitised cell in the presence of light and oxygen1,2,3. Briefly, photodynamic therapy uses a selectively localised light-sensitive drug (PS) that can absorb light and directly generate radicals (type I reaction) and activating molecular oxygen (type II reaction) to produce reactive oxygen species (ROS) in sufficient quantity to kill tumour cells. However, the PS is absorbed both by healthy tissues and by the tumour leading to in some cases prolong sensitivity of patients to intensive light. Due to this side effect, and the low tissue penetration by activating light (630 nm, up to 4 mm4,5,6), the low specificity of PSs to cancer types, and the cost of PS administration (for oesophageal carcinoma with post PDT period of 4–6 days £4370–6000)7, there is a need for further research in PDT methods. Therefore there is strong interest in the development of cancer phototherapy without the need for a PS8. Recent development of quantum-dot (QD) laser diodes (LDs) emitting in the near infrared (NIR) spectral range offers such an opportunity to develop direct laser therapy of cancer. The QD-LD emission wavelength centred at around 1268 nm coincides well with the near IR absorption band9 of oxygen molecule10. One particular opportunity involves activation of the apoptotic response through direct molecular oxygen photoexcitation. To date the idea of 1O2 activation has not attracted much attention because direct 3O2 → 1O2 transition in molecular oxygen is prohibited on the basis of spin-orbital selection rules. However new experimental development in solvent effect on the spin forbidden transitions of molecular oxygen have redrawn the selection rules governing the intermolecular enhancement11,12. The enhancement of 3O2 → 1O2 transition has been attributed to the major intensity contribution from O2−O2 bi-molecular collisions, which mix electron orbital states by an intermolecular exchange interaction, introducing some allowed characters into previously forbidden transitions. Furthermore, the action spectra in the range from 310 to 860 nm demonstrated for low-intensity laser therapy in a number of cell cultures13 suggest that transformation of cell metabolism in response to low power laser is consistent with absorption bands of molecular oxygen. 1O2 formation by direct photoexcitation with 1265 nm in pigment-free aerobic systems14 and in condensed phase at 77 K with 1064 nm15 have also been demonstrated. Recently it was shown that 1270 nm laser could induce cancer cell death in PS-free medium16. This pioneering research by Anquez et al. has clearly demonstrated cancer cells growth suppression induced by extensive 1270 nm laser irradiation16. However, direct monitoring of ROS (e.g., 1O2 or O−2) in the cell and cellular mechanisms of this laser effect remain unclear16. Therefore, we aimed to study whether 1268 nm irradiation by quantum dot laser can directly transform triplet oxygen to its active form singlet O2 in organic solution and in different cell lines without PSs also modelling laser-induced oxidative stress mechanisms and addressing the question whether this light wavelength exposure can kill cancer cells. To dissect the cellular mechanism of direct ROS generation by 1268 nm laser irradiation we developed a kinetic model of a redox homeostasis alteration and oxidative stress under pulse laser exposure. The main aim of the modelling is to elucidate a liminal nature of laser-induced oxidative stress followed by apoptosis signal17. As it has been shown in the modelling of radiation-induced oxidative stress in bacterium18, the threshold effect can be due to antioxidant cellular system and oxidative stress is reached when this protective system becomes saturated and overwhelmed under irradiation. To consider this mechanism in the model we took into account the laser-induced perturbation of the protective antioxidant cellular system which is assumed to be overwhelmed in cancer cells due to a constant endogenous oxidative stress19. Given these conditions we show by computational simulation that at PS-free medium laser irradiation is capable of triggering prolonged cellular oxidative stress (accumulation of ROS) by the impulse perturbation of redox homeostasis in cancer cells.
Results
Singlet oxygen photo-generation in organic solution in the present of molecular oxygen
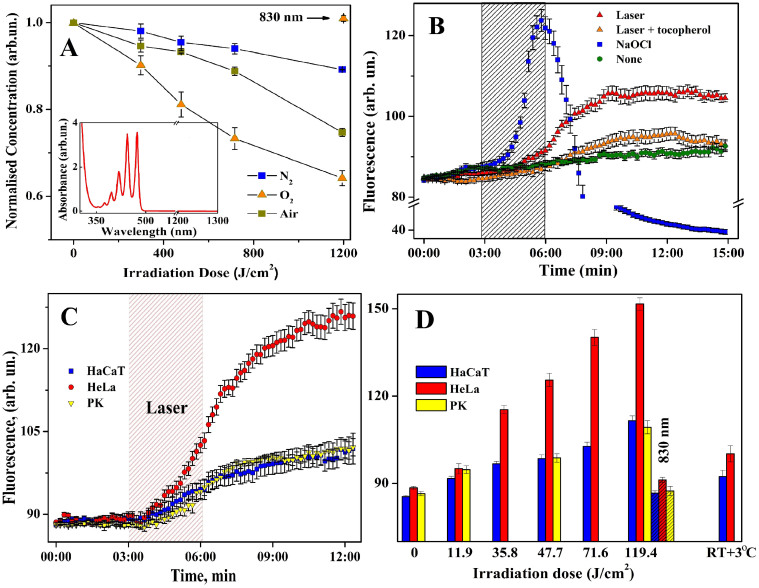
To investigate the direct photo-activation of molecular oxygen we used a well-known scavenger of singlet oxygen (naphthacene in carbon tetrachloride) as a substrate for the photo-oxygenation assessments20. The solvent concentration was 200 μM so that the solvent quenching was low and the singlet oxygen quenching would be the dominant process7. The difference in absorption at a given wavelength before and after laser irradiation was used as a measure of the 1O2 formation ratio. The interaction of naphthacene in carbon tetrachloride with 1O2 is known to be purely chemical, accompanied by formation of endoperoxides and loss of absorbance in the visible spectral range. The absorption spectrum of naphthacene shows no resolvable absorption at 1268 nm (Fig. 1A, insert) negating the possibility that the laser pulse can directly bleach naphthacene. The 20-min 1268 nm irradiation led to appreciable bleaching of the air-saturated solutions with a twofold increase in the bleaching rate in oxygen-purified solutions at an irradiation dose of 1200 J/cm2. Control 830 nm LD pulse induced no detectable changes (within a measurement error of 2%) of naphthacene absorption even in oxygen-enriched solutions. The absorption spectra of a control sample before and after 830-nm laser irradiation and a negative control as a measure of photobleaching under normal room lighting conditions are shown in Fig. 1A. Oxidation of naphthacene was determined by the 1O2 generation after direct 1268 nm laser 3O2 photoexcitation suggesting that similar photo-oxidation reactions might be detected in living cellular systems.
Figure 1. Laser-induced singlet oxygen generation in CCl4 and different cell lines.
(A) Naphthacene photobleaching in oxygen-, air-, and nitrogen-saturated CCl4. (n = 3, mean ± SD). Insert: Absorption spectrum of naphthacene. (B) Effect of 1268 nm irradiation of 47.7 J/cm2 along on DHOE fluorescence in HaCaT cells, and with 10 μM α-tocopherol, or 100 μM NaOCl only, background fluorescence (none). Means of n = 3, 20–30 cells per each ± SE. (C) Kinetics of laser-induced DHOE fluorescence in HaCaT, HeLa cells, and primary keratinocytes and (D) dose-dependency of DHOE fluorescence in all deferent cell lines taken at 12th min of recording. 830 nm laser irradiation (slash line pattern) taken as a negative control. Mean of n = 5 (20–30 cells per each) ± SE.
Laser-induced 1O2 production in living cells
The feasibility of oxygen photo-activation in the absence of PSs in true living cell systems remains uncertain. However the results of low-intensity laser therapy21, modification of red blood cell membrane proteins22, and cancer cell growth suppression in PS free-conditions16 by photo-excitation in the near infrared spectral range suggests that direct photo-oxidation in media containing molecular oxygen is apparent. Since the feasibility of oxygen photo-activation in the absence of PSs in true bio-systems is still unclear we chose dihydroethidium (DHE), which is specifically oxidized to dihydroxyethidium (DHOE) fluorescing at 585 nm23 by the superoxide anion (O2−, the first by-product of 1O2 reduction10 and ROS precursor in the cell23), to monitor singlet oxygen in immortalised epidermal keratinocytes (HaCaT)24 before, during, and after laser pulse irradiation. Experiments using HaCaT cells showed a significant difference between non-irradiated cells and those irradiated by a 1268 nm laser pulse of 47.7 J/cm2 causing an increase in DHOE (oxidized DHE) fluorescence with a lag-phase of 40–60 seconds reaching a steady-state level after 8 min and continued post termination of the laser pulse (Fig. 1B–D). At the same time, a strong donor of O2− NaOCl (100 μM) induced a dramatic increase in DHOE fluorescence. The pre-incubation of HaCaT cells for 10 min with α-tocopherol (a nonspecific ROS scavenger25,26) diminished the laser-induced fluorescence to near background levels. This suggests that the 1268 nm laser irradiation photo-oxidizes molecular oxygen inside the cell.
Next, we investigated primary keratinocytes (PK) and HeLa cells. As shown in figure 1C the 1268 nm laser pulse triggered O2−-dependent fluorescence in all three cell types with the most dramatic effect observed in HeLa cells and no difference between HaCaT and PK. The near IR laser-induced fluorescence demonstrated strong dose dependency without reaching saturation for the time, especially in HeLa cells (Fig. 1D). The increased sensitivity of HeLa cells could be explained by their malignant origin resulting in a high metabolic state leading to a weaker free radical defence system19 compared with noncancerous cells27. A LD emitting at 830 nm was employed as a temperature control as nearly identical heating was evident compared with 1268 nm LD. The 830 nm irradiation (119.4 J/cm2) having no absorption by O2 shows no effect on O2−-dependent fluorescence in any cell types (Fig. 1D) underpins the hypothesis that only irradiation absorbed by oxygen (1268 nm, etc.) can induce ROS production through singlet oxygen photoactivation. This observation together with temperature control experiments (Fig. 1D and S2, Supplementary Info) rules out suggestion that 1268 nm laser can generate extensive heat which induces significant amount of 1O2 in the cell. Most intriguing was the observation of a continued increase in ROS level inside the cells even after the laser had ceased, most prominent in HeLa cancer cells.
Cytosolic free calcium level and ion channel activity under laser pulses
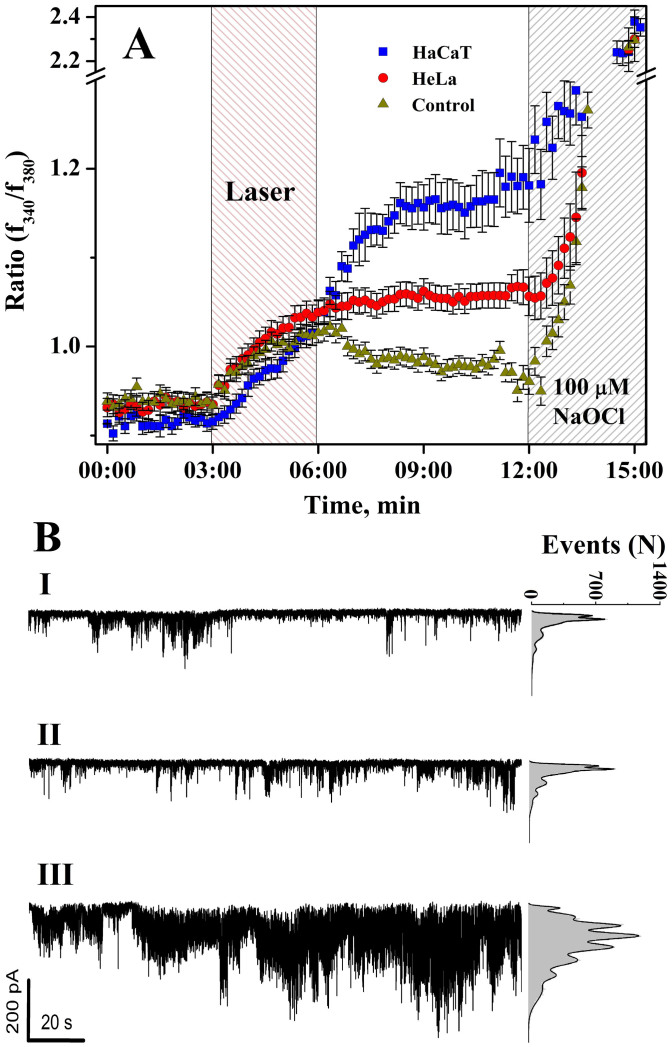
Products of oxidative stress (ROS, NO, organic radicals, etc.) are recognized as powerful regulatory messengers in cell signalling which very often affect cell calcium homeostasis28. In turn calcium homeostasis disruption can contribute to oxidative stress29. From our results, we anticipated that laser-induced 1O2 production could reflect on cytosolic calcium concentration ([Ca2+]cyt). Therefore single cell ratiometric Ca2+ imaging was employed to estimate calcium response of the HaCaT and HeLa cell lines after 1268 nm irradiation of 47.7 J/cm2 (Fig. 2A). Imaging showed an apparent increase in the fluorescence ratio by more than 1.2 times for HaCaT and HeLa cells, registered immediately after the laser was on. Following the cessation of irradiation, [Ca2+]cyt was measured for at least 7 min and found to continue rising in HaCaT cells whilst in contrast to the laser-induced singlet oxygen response, HeLa cells demonstrated plateau (discussed below). Further application of NaOCl (100 μM) induced a typical oxidative-stress-like calcium response in all cell lines (Fig. 2A). The LD emitting at 830 nm also temporally increased calcium fluorescence in both cell types falling to basic levels after the pulse was terminated.
Figure 2.
Effect of irradiations on (A) calcium ratio in HaCaT (1268 nm), HeLa (1268 nm) cells, and both cell lines with 830 nm (control). Mean of n = 4, 10–15 cells per each ± SE; (B) Single channel currents recorded at −100 mV holding voltage at cell-attached configuration before (I), during (II), and after (III) 1268 nm laser irradiation of 47.7 J/cm2. (6 cells). Right-hand segment: opened channel events point-amplitude.
To answer where IR irradiation-induced calcium originates from external or internal calcium stores we monitored single channel activities (Fig. 2B, traces) on the plasma membrane of HaCaT cells with a patch clamp in a cell-attached configuration before (I), during (II) and after (III) an IR pulse. The analysis of single-channel activity demonstrated a typical pattern (current amplitude 5–25 pA and dwell open time around 1–3 ms) associated with non-excited low-voltage activated Ca2+ channels30. The significant increase in channel activity by more than an order of magnitude was observed at the end of laser pulse (3 min) indicating that plasma membrane Ca2+ channels were activated by ROS-induced calcium release from internal stores (Fig. 2A; 2B, III). Pre-incubation with α–tocopherol (10 μM) for 10 minutes decreased general channel activity and fully prevented IR-induced channel activation (Fig. S1) pointing to ROS as the initial origin of the laser effect on channel activity.
Modelling laser-induced oxidative stress in the cell
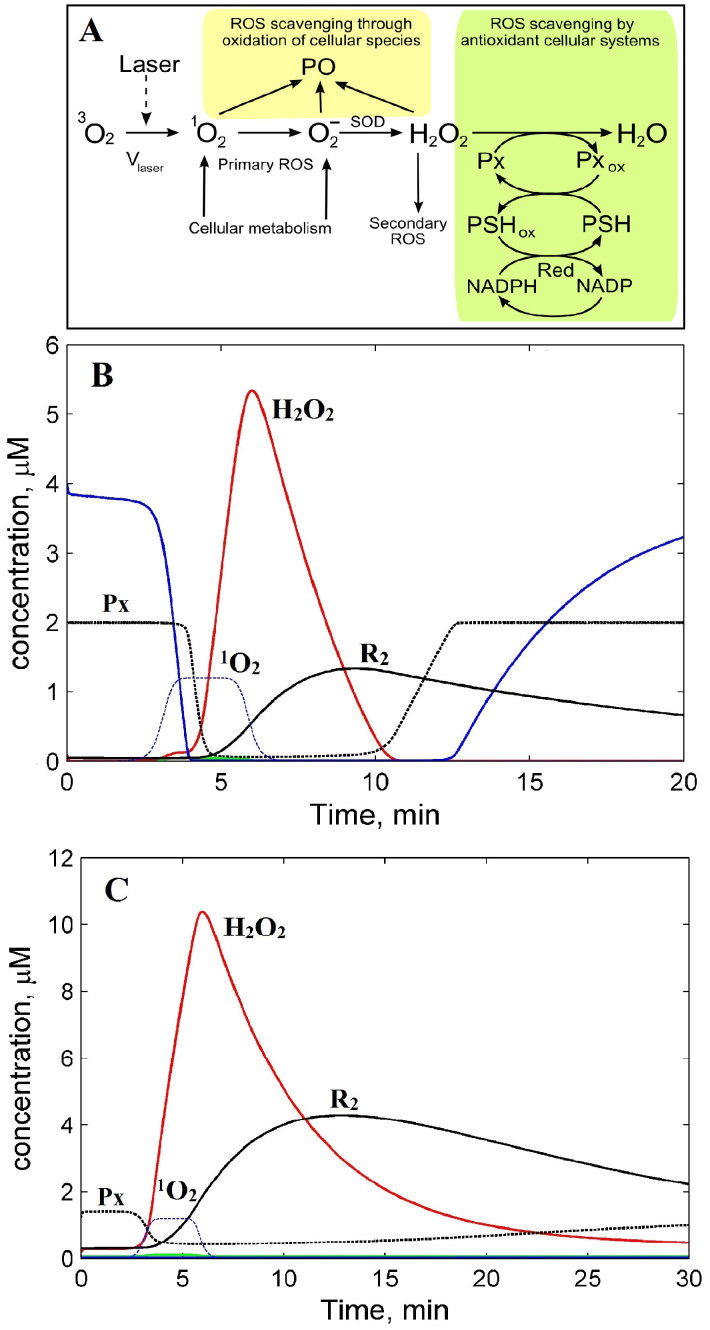
For comprehensive analysis of our results, a kinetic model of redox homeostasis and its imbalance by laser-induced ROS generation in normal and cancerous cells was developed (Fig. 3 and 4A).
Figure 3. A model of cell redox homeostasis and its imbalance by laser-induced ROS generation.
(A) Scheme of cellular ROS production and scavenging. (B) Kinetics of ROS in normal and (C) cancerous cells. H2O2 ( ); reduced PSH (
); reduced PSH ( ); primary ROS (1O2 and O2−), R1, (
); primary ROS (1O2 and O2−), R1, ( ); reduced thioredoxin peroxidase, Px (
); reduced thioredoxin peroxidase, Px ( ); sum of primary and secondary ROS, R2 (
); sum of primary and secondary ROS, R2 ( ); rate of 1O2 generation by 3 min laser pulse only (
); rate of 1O2 generation by 3 min laser pulse only ( ).
).
Figure 4.
Kinetics of ROS production in noncancerous cells at the different rates of singlet oxygen generation (A): Vlaser,1 ( ) and Vlaser,2 > Vlaser,1 (
) and Vlaser,2 > Vlaser,1 ( ) and in cancer cells at Vlaser,1 (
) and in cancer cells at Vlaser,1 ( ). Laser-induced singlet oxygen production rate used for modelling (
). Laser-induced singlet oxygen production rate used for modelling ( ). Experimental data of ROS production at 47.7 J/cm2 (
). Experimental data of ROS production at 47.7 J/cm2 ( ) and 71.6 J/cm2 (
) and 71.6 J/cm2 ( ) in HaCaT and 47.7 J/cm2 in HeLa (
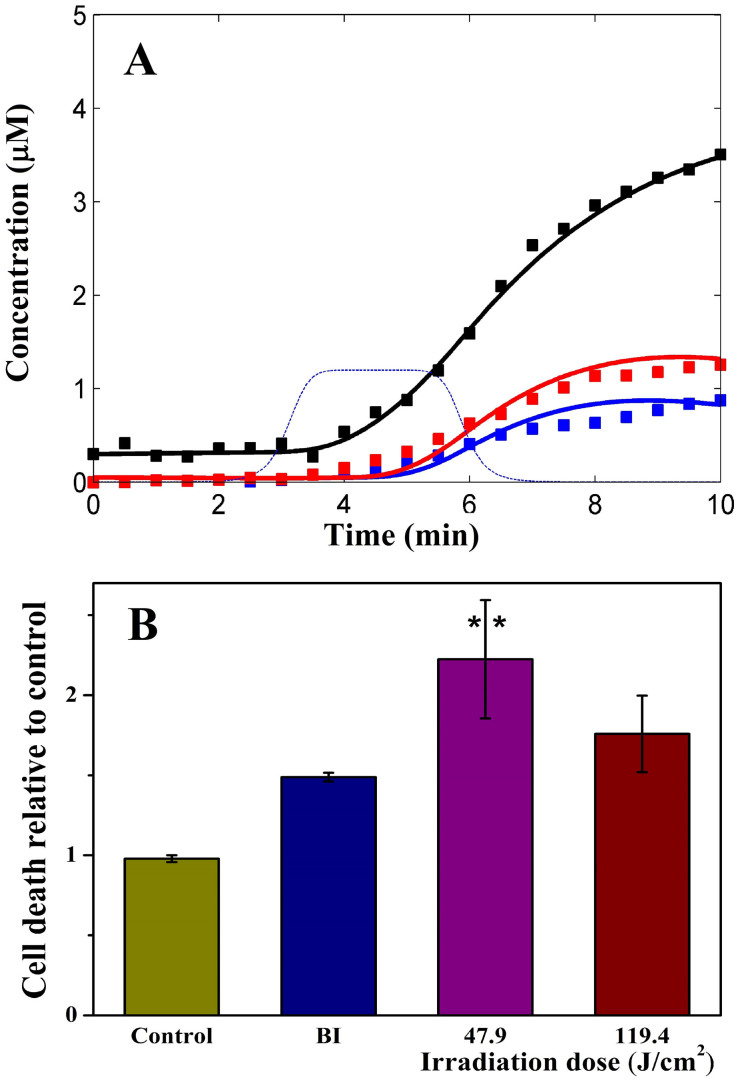
) in HaCaT and 47.7 J/cm2 in HeLa ( ) cells; and (B) HeLa cell death rate measured by an enzymatic assay of LDH release. BI 2536 (a PLK1 inhibitor) was used as a positive control. Graph shows a mean ± SE of one experiment, (n = 3).
) cells; and (B) HeLa cell death rate measured by an enzymatic assay of LDH release. BI 2536 (a PLK1 inhibitor) was used as a positive control. Graph shows a mean ± SE of one experiment, (n = 3).
The model reproduces a low basal level of primary ROS, H2O2, and secondary oxygen radicals in the absence of laser irradiation, up to 3 min before laser impulse (Fig. 4A, green, red, and black plots respectively). The model results mimic experimental data of H2O2 low concentration (0.01 μM4) in normal functioning cells. Such low level of ROS in the model is achieved due to the ROS scavenging processes considered (see Methods).
In the model, a 3 minute laser pulse (Fig. 3B, blue dashed line) caused the generation of 1O2 (the precursor of various ROS). As the lifetime of 1O2 is short in the cell (~5 μs31), it quickly decays through oxidation of cellular species and formation of radicals, in particular O2−10. O2− dismutates into H2O2 being a source of secondary oxygen radicals as a result of Fenton reactions32. The model predicts a rapid growth of H2O2 after the laser is applied and an immediate stop after the laser is removed (Fig. 3B, red line). Such H2O2 accumulation has been observed in water during exposure to 1264 nm laser irradiation33. To model the ideal conditions of H2O2 scavenging we introduced the enzymatic antioxidant system (including Px, PSH, Red, see Methods) which causes the fall of H2O2 concentration to basal levels during 5 min after the laser is removed. Due to scavenging of excessive H2O2 the concentration of both reduced enzymes of antioxidant system decrease from steady state concentrations by factor ten (Fig. 3B, blue and dashed black lines). During this time, H2O2 generates a secondary oxygen radical pool, R2 (Fig. 3B, black line). To estimate the kinetic parameters of the model (Table S1 and S2), the total kinetics of primary and secondary radicals generated by laser pulse was fitted against the experimental data on radicals generated by different laser irradiation doses Vlaser in HaCaT cells (Fig. 4A).
To simulate laser-induced ROS production in cancer cells we changed some parameters taking into account certain features of malignancy such as an initially high primary ROS production rate, Vo4,34,35,36; increased concentration of endogenous H2O2 and key antioxidant enzymes such as thioredoxin37 (Trx), thioredoxin reductase (TR) by up to 6.5 times34,37,38,39 and thioredoxin peroxidase (Tpx) up to 9 times38 due to high cytosolic expression of these enzymes in cancer cells. The best fitting of the theoretical ROS kinetics against HeLa cancer cell experimental data was obtained at 2-fold for Px, PSH and 4-fold for reductase (Red) concentrations, and H2O2 production rate Vo up to 7 μM/min4. Other model parameters were left unchanged (Fig. 4A and Tables S1 and S2).
Laser-triggered cancer cell death
To provide evidence that the 1268 nm laser can trigger apoptosis in the cell40 we carried out a preliminary experiment to assay cell death which suggested that 1268 nm laser irradiation of 47.7 and 119.4 J/cm2 is capable of killing HeLa cancer cells (Fig. 4B). The possibility of laser induced death as a result of cell overheating was ruled out due to temperature control experiments for cell death (Δt ≤ 6–7.5°C) measurements (Fig. 4B). The fact that 47.7 J/cm2 (200 mW) laser dose caused higher HeLa cell death rate than 119.4 J/cm2 irradiation dose (Fig. 4B) could be due to faster depletion of triplet oxygen in the cells during higher power 500 mW (119.4 J/cm2) laser pulse. These data and Anquez et al. results point to the fact that prolonged but low power 1268 nm laser irradiation is preferable for effective cancer cell killing rather than short-pulsed but powerful laser treatment.
Discussion
Soon after its inception nearly forty years ago, photodynamic therapy became popular as a promising approach to cure malignant tumours8. However, clinical practice revealed significant limitations in the use of this method mainly due to the post-treatment hypersensitivity of patients to intensive light and the low depth of tissue penetration by active light irradiation at 630 nm. The idea of direct phototransformation of triplet to singlet O2 with 1270 nm laser irradiation originated from the observed absorption spectrum previously thought to be spin-forbidden11 and readily used to detect singlet O2 forms of luminescence at this wavelength15. This was originally shown in pigment-free aerobic systems14, protein and cell suspensions22. However these data were generated using irradiation doses which exceed safety margins for cancer therapy. Our experiments in anoxia solution with a wider range of irradiation doses starting from 240 J/cm2 clearly demonstrated that QD LD emitting around 1268 nm can directly generate singlet oxygen in CCl4 solution only in the presence of molecular oxygen (Fig. 1A). This led us to suppose that, once triggered by the laser, singlet oxygen might then lead to further ROS production within the cell, which could potentially trigger apoptosis in cancer cells without the need for PSs14.
Indeed HaCaT cells loaded with DHE demonstrated after 3 min that a 1268 nm laser pulse increased DHOE fluorescence in the cytoplasm (Fig. 1B). The fact that the laser pulse transited 3O2 to 1O2 only in O2 containing solution (Fig. 1A) and that α-tocopherol abolished the laser-induced DHOE fluorescence in the cell, identifies singlet O2 as the origin of the effects and demonstrates for the first time this mechanism in a true cellular system (Fig. 1B). Most intriguing is the observation that cancer cells (HeLa) demonstrate hypersensitivity to 1268 nm LD irradiation compared with normal keratinocytes (Fig. 1C, D). These data also suggest that 1268 nm LD can be used to directly photoactivate molecular oxygen in vivo. Furthermore, the dosage of radiation based on the kinetics model data (see Fig. 3 and 4A) could be selected to strike a balance between therapeutic efficacy and undesirable damage to cells. The observation that laser-induced 1O2 generation shows significant delay in reaching steady-state levels of ROS after laser cessation prompted the idea that the laser excitation overcame the cellular ROS defence system particularly in the cancer cells34,41.
It is common knowledge that apoptosis can be triggered by many different stimuli, including cytokines, oxidative stress, and calcium release from the endoplasmic reticulum. Calcium release from the ER synchronizes the massive leak of cytochrome c from the mitochondria orchestrating apoptosis34,42. Oxidative stress itself can lead to massive disturbance of cell calcium homeostasis43 and contra versa44 demonstrating in some cases a tight ROS-Ca2+ feedback loop28. The significantly higher [Ca2+]cyt response to 1268 nm LD irradiation observed in HaCaT cells compared with HeLa cells (Fig. 2A) can be attributed to a general high reactivity of keratinocytes to ROS described in45. These results suggest that the observed increase in [Ca2+]cyt, Ca2+ channel activity and 1O2-production in the cell is likely to be associated with direct molecular oxygen photoactivation by 1268 nm irradiation. Cancerous HeLa cells have a higher general metabolic activity and demonstrate the highest 1O2 production in response to the laser pulse compared to non-cancerous cells which have a much weaker calcium response postulated as a means of protection46,47. Significant but reversible calcium response of both cell lines to control irradiation of 830 nm could be through activation of cytochrome c oxidase which can weakly absorb in the IR bandwidth leading to ROS-induced leak of calcium to the cytoplasm from mitochondria48 which is shortly terminated in both cancer and normal cells.
The high complexity of regulation within the redox system prompted us to create a kinetics model of cell redox homeostasis and its imbalance caused by the laser to explain our experimental results. First of all the results of modelling laser-induced ROS kinetics determined significant differences between normal and cancer cells (Fig. 3BC and 4A). An initial high rate of ROS production in cancer cells determines a higher background level of H2O2 and oxygen radicals (both around 0.2 μM) compared with normal cells 0.01 μM (Fig. 3BC, red and black plots). However H2O2 concentration is still under the threshold value of 1 μM after which apoptosis can trigger4. Secondly, the steady state concentration of reduced enzymes of antioxidant system, Px (Tpx and Trx) and PSH (Gpx and glutathione (GSH)) calculated in the model for cancer cells is low (0.02 μM) because the scavenging system is thought to be depleted by a higher rate of H2O2 production due to excessive metabolism. At the same rate of laser-induced generation of 1O2 (Vlaser,o) in both cell types, the depletion of ROS scavenging enzymes in the cytosol of cancer cells assumes to disrupt the antioxidant system bringing the H2O2 concentration up to 10.5 μM which is two-fold higher than its level in normal cells (Fig. 3BC; red plots). Additionally this leads to a longer time for restoration of background concentration of H2O2, reductase Px, and oxygen radicals (R2) in cancer cells after the laser is switched off (Figs. 3BC; red, dashed black, and solid black lines). The computational modelling suggests that the prolonged O2− generation after the initial laser pulse is determined by the size of the secondary pool of ROS generated through excessive H2O2 produced from the initial laser-generated 1O2 (Fig. 3B). Prolonged retention of a high level of H2O2 results in depletion of the ROS scavenging enzyme systems followed by a slow restoration after laser irradiation (Fig. 3BC). The model thus reproduces the following features of the laser-generated oxygen radicals observed in the experiments. Firstly, the model closely describes the experimental delay of radical generation observed at the start of laser pulse (Fig. 3C). The delay obtained in the model happens due to deferred generation of secondary radicals which significantly contribute to the total ROS levels comparing with fast laser-triggered primary radicals (Fig. 3B, black and green plots respectively). A bare contribution of primary radicals (1O2 and O2−) to the total ROS yield comes in part from fast conversion of 1O2 to O2− that dismutates to longer living H2O2. Secondly, the model reproduces the experimental observations that ROS production lasts longer beyond the laser pulse (Fig. 1B, C, and 3B, C). Such a significant level of H2O2 shown by the model after laser irradiation is a result of slower degradation due to a depletion of the ROS scavenging system (level of the reduced antioxidant enzymes, Px and PSH) and high H2O2 concentrations (Fig. 3B, red line). The model shows that ROS concentrations gradually decrease after 10 min because of the restoration of the scavenging system (an increase in Px and PSH) (Fig. 3B, blue and black dashed lines). Full restoration of ROS and the scavenging system to background levels perturbed by laser irradiation is predicted to be 30 min after laser irradiation is stopped.
To model the relatively high ROS generated in the cancer cells (Fig. 1C, D) we have changed the model parameters to consider a higher rate of general ROS production (Vo) and higher expression of the main antioxidant enzymes37,38 in cancer cells. The ROS kinetics computed with these parameters closely mimicked a high ROS accumulation in cancer compared with noncancerous cells (Fig. 4A, black plot) which is in agreement with our experimental data (Fig. 1C–D). We conclude that the higher laser-induced ROS levels observed in cancer cells might result from the depletion in cellular scavenging systems caused by a high general ROS production (Fig. 3BC) involving calcium homeostasis disruption and could be a trigger for apoptosis in cancer cells. Additional laser multi-impulse experiments for further model development will be needed to find optimal laser pulse frequency, duration and power for effective induction of oxidative stress in cancer cells leaving normal cells unharmed.
Finally, our experimental and computational results explaining high ROS levels in cancer cells together with the fact that 1268 nm laser can induce cytotoxicity in HeLa cells may in near future propose a new therapeutic approach based on direct laser photoactivation of molecular oxygen in the tumour without the need for exogenous drugs gain opportunity to develop PS-free cancer phototherapy.
Methods
Laser characteristics
A fibre coupled InGaAs/InAs quantum dot laser diodes (Innolume GmbH) in continuous wave regime were used as an irradiation sources. They have an emission spectrum centred at 1268 and 830 nm.
Measuring of singlet oxygen in organic solution
1O2 levels in carbon tetrachloride (Fluka) were measured as described in20 with the naphthacene (Sigma-Aldrich) with PerkinElmer Lambda-900 UV/VIS/NIR spectrometer. A control sample (3 ml) was photo-bleached under normal room lighting. Dose dependent 1O2 formation ratio was measured after 20 min at atmospheric pressure and room temperature (23°C, RT).
Cell lines culture
Cell lines were cultivated as described in27 and49. Prior to all experiments the confluent cells were disaggregated with a 0.1% tyrosine/EDTA for 3–10 min, transfer to modified phosphate-free Tyrode's medium (TM) (in mM: 140 NaCl, 3.6 KCl, 1.2 MgCl2, 1.8 CaCl2, and 10 HEPES, pH 7.4) and kept on ice. Prior to fluorescence and patch clamp experiments cells were placed for 20 min to medium perfused chamber (1 ml/min) where the temperature did not exceed RT by more than 3°C during LD pulse with highest power of 500 mW. The temperature in the perfused chamber and during following cancer cell death assay experiments was monitored under laser beam with TC-8 thermocouple Data Logger and PicoLog software (Pico Technology, USA).
Monitoring 1O2 level and [Ca2+]cyt in single cell
1O2 measurements in HaCaT, HeLa, and PK with DHE23 (5 μM, Invitrogen) were conducted with fluorescent microscope Axio Obersver A1 accomplished with AxoCam MRm camera and AxioVision 4.8.1 software (Zeiss). Fluorescence from cell nucleus area were acquired and normalized to background cell fluorescence before LD pulse.
Imaging of cytoplasm free calcium in HaCaT and HeLa was performed as described in_50 on the same microscope with fluorescent filter set (340 nm and 380 nm). Fura-2 (Molecular Probes) fluorescence from cell cytoplasm was acquired and fluorescence ratio was calculated with AxioVision software.
Single channel patch clamp recording
Single channel recordings of HaCaT cells were made with Axopatch 200B amplifier at cell-attached configuration with TM in pipette/chamber with holding potential of −100 mV. Data acquisition and single channel analysis were made with pCLAMP10.0 (Molecular Devices).
Cell death rate assay
Death rate assay of HeLa cells was performed 24 hours after treatments (irradiation, temperature control, and BI2536 application) using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer's instructions. This assay measures lactate dehydrogenase (LDH) activity in extracellular medium when this enzyme is released upon cell lysis. Cells were seeded at high density in 12-well plates at 0.5 × 106/well in DMEM supplemented with 10% FBS. The following day, media were changed for 0.5 ml of raw DMEM at room temperature (RT, 21°C for 119.4 J/cm2 and 23°C for 47.9 J/cm2) and cells were irradiated 3 min with 1268 nm laser either 47.9 J/cm2 or 119.4 J/cm2 doses. Temperature controls corresponding to the variation in temperature over RT due to irradiation (Δt = 6°C for 47.9 J/cm2 and 7.5°C for 119.4 J/cm2) were made by heating cells for 3 min in a water bath set to 29.3°C for 47.9 J/cm2 and 28.5°C for 119.4 J/cm2. The highest temperature reached during irradiation was 29.3°C. Raw DMEM was changed for DMEM supplemented with 10% FBS after irradiation and the cells were placed back in incubators. A positive control of cell death was performed by treating cells for 24 hours with 5 μM PLK1 inhibitor BI2536.
Computational method
A scheme of the model is given in Fig. 3A. The model takes into account the following processes: (i) endogenous generation of primary ROS (O2−, 1O2) and laser-produced 1O2, denoted by the variable R1; (ii) their transformation into H2O2 by superoxide dismutase (SOD); (iii) generation of a secondary pool of ROS from H2O2 through the Fenton reactions (R2); and (iv) scavenging H2O2 and its by-products by the cellular antioxidant system. Considering that the cellular antioxidant system involves many enzymes utilising H2O2 we phenomenologically modelled this system. The enzymatic sub-model of the H2O2 degradation is based on the redox cascade reactions which correspond to the key antioxidant cellular systems: thioredoxin peroxidase/thioredoxin/thioredoxin reductase (Tpx/Trx/TR) and glutathione peroxidase/glutathione/glutathione reductase (Gpx/GSS/GR) systems32. This sub-model is represented in Fig. 3A by the reactions and enzymes which we denoted by Px (peroxidases Tpx and Gpx degrading H2O2), PSH (GSH and Trx, protein containing thiol groups), and Red (reductases TR and GR redusing GSS and Trx). Note, in the model we did not consider genetic feedback regulation of the antioxidant enzyme expression induced by oxidative stress through activation of different signalling pathways such as NF-κB, Nrf2, HIF, and others17. However, we took into account an increase in the expression level of the antioxidant proteins (Px and PSH) in cancer cells in comparison with normal ones34,37,38,39.
The model was calibrated based on both the experimental data on kinetic parameters available from literature and our experimental kinetics data on ROS generation by laser at two irradiation doses in HaCaT and HeLa cells. Detailed description of the model and a set of model parameters obtained are given in Supplementary Information. The total concentration of primary R1 and secondary R2 radicals generated by laser irradiation was the readout of the model which is compared with experimental radical kinetics in our experiment. This phenomenological model was used to describe laser-induced change in cellular redox homeostasis leading to depletion of enzymatic antioxidant system and ROS accumulation in cells.
Author Contributions
E.U.R. initiated and supervised the project. S.G.S. has performed main part of fluorescence and patch clamp experiment as well as data analysis. S.A.Z. has carried out photochemistry experiments with naphthacene, partly involved in fluorescence experiments. A.G. proposed the oxidative stress kinetic model. C.P. and A.S. perform all experiments on cell death rate assay. All authors participated in discussion of the results and the manuscript writing.
Supplementary Material
Supplementary information
Acknowledgments
Authors thanks Dr P. Campbell and Prof W.H.I. McLean for laboratory support and Prof. A. Thompson for critical remarks to the manuscript and FP7 FAST-DOT and SICSA (Scottish Informatics and Computer Science Alliance), and Dr G. Malcolm from M-squared Ltd for partial financial support of this work.
References
- Harrod-Kim P. Tumor ablation with photodynamic therapy: Introduction to mechanism and clinical applications. J. Vascul and Interven Radiol 17, 1441–1448 (2006). [DOI] [PubMed] [Google Scholar]
- He P. J. et al. Enhanced apoptotic effect of combined modality of 9-hydroxypheophorbide alpha-mediated photodynamic therapy and carboplatin on AMC-HN-3 human head and neck cancer cells. Oncol Rep 21, 329–334 (2009). [PubMed] [Google Scholar]
- Bulina M. E. et al. A genetically encoded photosensitizer. Nat Biotech 24, 95–99 (2006). [DOI] [PubMed] [Google Scholar]
- Lee L. K., Whitehurst C., Pantelides M. L. & Moore J. V. In situ comparison of 665 nm and 633 nm wavelength light penetration in the human prostate gland. Photochem and Photobiol 62, 882–886 (1995). [DOI] [PubMed] [Google Scholar]
- Muller P. J. & Wilson B. C. An update on the penetration depth of 630 nm light in normal and malignant human brain tissue in vivo. Phys in Med and Biol 31, 1295 (1986). [DOI] [PubMed] [Google Scholar]
- Stolik S., Delgado J. A., Pérez A. & Anasagasti L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem and Photobiol B: Biol 57, 90–93 (2000). [DOI] [PubMed] [Google Scholar]
- Matheson I. B. C. & Lee J. Reaction of chemical acceptors with singlet oxygen produced by direct laser excitation. Chem Phys Lett 7, 475–476 (1970). [Google Scholar]
- Diamond I. et al. Photodymanic therapy of malignant tumors. Lancet 2, 1175–& (1972). [DOI] [PubMed] [Google Scholar]
- Zakharov S. D. & Ivanov A. V. Light-oxygen effect in cells and its potential applications in tumour therapy. Quant Elect 29, 1031–1053 (1999). [Google Scholar]
- Halliwell B. & Gutteridge G. M. Free radicals in biology and medicine (Oxford University Press, 2007). [Google Scholar]
- Long C. & Kearns D. R. Selection rules for the intermolecular enhancement of spin forbidden transitions in molecular oxygen. J. Chem. Phys. 59, 5729–5736 (1973). [Google Scholar]
- Matheson I. B. C. & Lee J. Comparison of the pressure dependences of the visible and infrared electronic absorption spectra of oxygen in gas and in Freon solution. Chem. Phys. Lett. 8, 173–176 (1971). [Google Scholar]
- Pavel S. Light therapy (with UVA-1) for SLE patients: is it a good or bad idea? Rheumatol 45, 653–655 (2006). [DOI] [PubMed] [Google Scholar]
- Krasnovsky A. A., Drozdova N. N., Ivanov A. V. & Ambartsumian R. V. Activation of Molecular Oxygen by Infrared Laser Radiation in Pigment-Free Aerobic Systems. Biochem (Moscow) 68, 963–966 (2003). [DOI] [PubMed] [Google Scholar]
- Jockusch S. et al. Singlet molecular oxygen by direct excitation. Photochem. Photobiol. Sci. 7, 235–239 (2008). [DOI] [PubMed] [Google Scholar]
- Anquez F. et al. Cancerous Cell Death from Sensitizer Free Photoactivation of Singlet Oxygen. Photochem and Photobiol 88, 167–174 (2012). [DOI] [PubMed] [Google Scholar]
- Trachootham D. et al. Redox Regulation of Cell Survival. Antiox & Redox Sign 10, 1343–1374 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuryak I. & Brenner D. J. A model of interactions between radiation-induced oxidative stress, protein and DNA damage in Deinococcus radiodurans. J Theor Biol 261, 305–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Alexandre J. & Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8, 579–591 (2009). [DOI] [PubMed] [Google Scholar]
- Bjarneson D. W. & Petersen N. O. The photochemistry of naphthacene in solution. J. Photochem and Photobiol A: Chem 63, 327–335 (1992). [Google Scholar]
- Waynant R. W. Lasers in medicine. (CRC Press, 2002). [Google Scholar]
- Zakharov S. D. et al. Structural rearrangements in the aqueous phase of cell suspensions and protein solutions induced by a light-oxygen effect. Quant Elect 33, 149–162 (2003). [Google Scholar]
- Peshavariya H. M., Dusting G. J. & Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Rad Res 41, 699–712 (2007). [DOI] [PubMed] [Google Scholar]
- Adams J. C. & Watt F. M. An unusual strain of human keratinocytes which do not stratify or undergo terminal differentiation in culture. J. Cell Biol 107, 1927–1938 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo S., Szabadkai G. & Rizzuto R. The Mitochondrial Antioxidants MitoE(2) and MitoQ(10) Increase Mitochondrial Ca2+ Load upon Cell Stimulation by Inhibiting Ca2+ Efflux from the Organelle. Mitoch and Oxid Stress in Neurodegen Disord 1147, 264–274 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C., Deeva I., Mikhal'chik E. & Korkina L. Beneficial effects of pro-/antioxidant-based nutraceuticals in the skin rejuvenation techniques. Cell and Mol Biol 53, 94–101 (2007). [PubMed] [Google Scholar]
- Shim J.-H. et al. E7-expressing HaCaT keratinocyte cells are resistant to oxidative stress-induced cell death via the induction of catalase. Proteomics 5, 2112–2122 (2005). [DOI] [PubMed] [Google Scholar]
- Singh D. K. et al. The Strength of Receptor Signaling Is Centrally Controlled through a Cooperative Loop between Ca2+ and an Oxidant Signal. Cell 121, 281–293 (2005). [DOI] [PubMed] [Google Scholar]
- Brookes P. S., Yoon Y., Robotham J. L., Anders M. W. & Sheu Sh.-Sh. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol - Cell Physiol 287, C817–C833 (2004). [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Low-voltage activated calcium channels: achievements and problems. Neurosci 92, 1157–1163 (1999). [DOI] [PubMed] [Google Scholar]
- Baier J. et al. Direct Detection of Singlet Oxygen Generated by UVA Irradiation in Human Cells and Skin. J. Invest Dermatol 127, 1498–1506 (2007). [DOI] [PubMed] [Google Scholar]
- Valko M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Intl J. Biochem & Cell Biol 39, 44–84 (2007). [DOI] [PubMed] [Google Scholar]
- Gudkov S. V. et al. Oxygen-Dependent Auto-Oscillations of Water Luminescence Triggered by the 1264 nm Radiation. J. Phys Chem B 115, 7693–7698 (2011). [DOI] [PubMed] [Google Scholar]
- Schumacker P. T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006). [DOI] [PubMed] [Google Scholar]
- Luo J., Solimini N. L. & Elledge S. J. Principles of Cancer Therapy: Oncogene and Non-oncogene Addiction. Cell 136, 823–837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski T. P. & Nathan C. F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res 51, 794–798 (1991). [PubMed] [Google Scholar]
- Biaglow J. E. & Miller R. A. The thioredoxin reductase/thioredoxin system - Novel redox targets for cancer therapy. Cancer Biol & Therapy 4, 6–13 (2005). [DOI] [PubMed] [Google Scholar]
- Cha M.-K., Suh K.-H. & Kim I.-H. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. & Clinic Cancer Res 28, 93–95 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam K. et al. Engineering and characterization of human manganese superoxide dismutase mutants with high activity and low product inhibition. FEBS J. 273, 4853–4861 (2006). [DOI] [PubMed] [Google Scholar]
- Ghavami S. et al. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J. Leukocyte Biol 76, 169–175 (2004). [DOI] [PubMed] [Google Scholar]
- Nordberg J. & Arnér E. S. J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol and Med 31, 1287–1312 (2001). [DOI] [PubMed] [Google Scholar]
- Mattson M. P. & Chan S. L. Calcium orchestrates apoptosis. Nat Cell Biol 5, 1041–1043 (2003). [DOI] [PubMed] [Google Scholar]
- Rosenstock T. R. et al. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J. Neurochem 88, 1220–1228 (2004). [DOI] [PubMed] [Google Scholar]
- Peng T.-I. & Jou M.-J. Oxidative stress caused by mitochondrial calcium overload. Ann. N-Y Acad Sci 1201, 183–188 (2010). [DOI] [PubMed] [Google Scholar]
- Hitoshi M., Yukiko I., Shoichi Y. & Yuri O. Reactive Oxygen Species in HaCaT Keratinocytes After UVB Irradiation Are Triggered by Intracellular Ca2+ Levels. J. Invest Dermat Symp Proc 14, 50–52 (2009). [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. Biol Chem 251, 7504–7507 (1976). [PubMed] [Google Scholar]
- Furuya Y. et al. The Role of Calcium, pH, and Cell Proliferation in the Programmed (Apoptotic) Death of Androgen-independent Prostatic Cancer Cells Induced by Thapsigargin. Cancer Res 54, 6167–6175 (1994). [PubMed] [Google Scholar]
- Karu T. I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem and Photobiol 84, 1091–1099 (2008). [DOI] [PubMed] [Google Scholar]
- Kovacs D. et al. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J. Dermatol Sci 54, 106–113 (2009). [DOI] [PubMed] [Google Scholar]
- Williams D. A. Quantitative intracellular calcium imaging with laser-scanning confocal microscopy. Cell Calcium 11, 589–597 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information