Abstract
INTRODUCTION
Most gastroenterological surgeries, even pancreatic surgery, can now be performed laparoscopically. However, the management of concomitant abdominal aortic aneurysm (AAA) and intra-abdominal malignancy is controversial. The performance of endovascular repair (EVAR) for AAA has been increasing; however, there is no report of laparoscopic pancreaticoduodenectomy after EVAR.
PRESENTATION OF CASE
A pancreatic tumor was detected during follow-up after EVAR for AAA. The enlarging tumor was diagnosed as an intraductal papillary mucinous tumor with a nodule. Laparoscopic pancreaticoduodenectomy was safely performed. After laparoscopic dissection around the pancreas head, an additional incision was made in the upper abdomen, and pancreatic reconstruction was performed through the incision. In spite of grade B pancreatic fistulae, the patient recovered with medical therapy. The pathological diagnosis was intraductal papillary mucinous adenoma with small foci of carcinoma in situ. The patient has been well with neither recurrence of the tumor nor any cardiovascular events for 18 months.
DISCUSSION
The management of concomitant malignancy and AAA is challenging, especially in patients with a pancreatic tumor. The reasons for the rarity of treatment include prognosis, anatomical vicinity, and postoperative complications. EVAR reduces retroperitoneal adhesions. A laparoscopic approach provides a small operative field and decreases mutual interference with AAA. Moreover, reconstruction is performed through an upper abdominal incision apart from the AAA. Hand-sewing provides more reliable stability of the anastomosis.
CONCLUSION
The increasing frequency of performance of EVAR for AAA and subsequent computed tomography may help to detect malignancy. Laparoscopic surgery appears to be a valid approach to malignancy after EVAR.
Keywords: Laparosocopic surgery, Pancreaticoduodenectomy, Abdominal aortic aneurysm, Endovascular repair, Intraductal mucinous neoplasm, Pancreatic cancer
1. Introduction
Most gastroenterological surgeries, even pancreatic surgery, can now be performed laparoscopically. Accordingly, the number of reports of laparoscopic pancreaticoduodenectomy (Lap PD) has gradually increased.1,2 Although the feasibility and safety of Lap PD have been established in institutes particularly experienced in the skilled performance of this technique (hereafter referred to as “experienced institutes”), the benefit of Lap PD beyond conventional surgery has not yet been shown.3
The management of concomitant abdominal aortic aneurysm (AAA) and intra-abdominal malignancy is controversial. Three issues must be considered in the development of a treatment technique in such cases. The first is mutual interference because of operative fields that are in close vicinity to one another, resulting in adhesions or collateral injuries. The second is prognosis; some malignancies have a very poor prognosis. The last is postoperative complications, especially intra-abdominal abscessation with graft infection. In particular, the pancreas is the organ that is most resistant to resolution of these issues because of its anatomical proximity to the aorta and severity of pancreatic fistulae as a postoperative complication. Hence, reports of AAA therapy and pancreatic surgery are rare.4,5
The performance of minimally invasive therapy has recently increased. The feasibility and safety of endovascular repair (EVAR) for AAA have been established.6,7 In addition, laparoscopic colectomy and EVAR for AAA were successfully performed in a patient.8 Similarly, Lap PD and EVAR for AAA could have some benefits for patients. However, to the best of our knowledge, concomitant treatment by Lap PD and EVAR has never been reported.
2. Presentation of case
A 70-year-old Japanese man was referred from vascular surgery for investigation of a pancreatic tumor, which was identified as a cystic tumor of the pancreas head by computed tomography (CT). Within 1.5 years, the tumor had grown from 16 to 31 mm. We suspected an intraductal papillary mucinous neoplasm (IPMN). He had a previous history of percutaneous coronary intervention for acute myocardial infarction when he was 66 years old and aortic stent grafting for an AAA when he was 68 years old. The AAA was located on the infrarenal aorta with a thrombus of 52 mm (Fig. 1). EVAR was performed using a Zenith AAA endovascular graft (Cook Inc., Bloomington, IN).
Fig. 1.
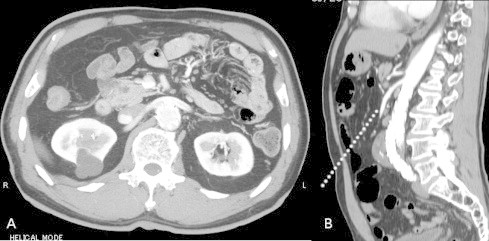
(A) Enhanced abdominal computed tomography scan of the portal vein phase showing a cystic tumor of 31-mm diameter in the pancreas head without dilatation of the main pancreatic duct. The intra-aortic endovascular stent is shown in the same slice. (B) Sagittal plane showing an infrarenal abdominal aortic aneurysm and the endovascular stent. An additional dotted line shows the laparoscopic axis from the umbilicus, which is apart from the aortic aneurysm.
The height, weight, and body mass index of the patient were 163 cm, 67.3 kg, and 25.3, respectively. A CT scan showed a cystic tumor of 31-mm diameter in the pancreas head without dilatation of the main pancreatic duct (Fig. 1). The aortic stent was observed behind the pancreas head. Endoscopic retrograde pancreatography showed a normal main pancreatic duct (Fig. 2), but contrast-enhanced endoscopic sonography revealed a nodule among the cyst mucus (Fig. 2). Thus, we diagnosed the enlarging tumor as a branched-type IPMN with a nodule and planned to perform a resection.
Fig. 2.
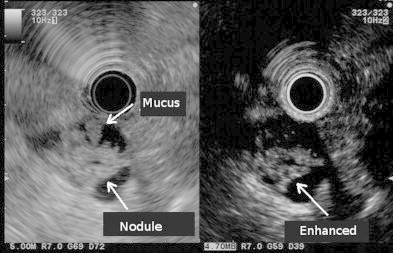
Contrast-enhanced endoscopic sonography showing a nodule in the cyst mucus. The nodule can be clearly observed within the mucus in the cyst.
To avoid disturbing the AAA or stimulating a residual AAA, we intended to perform Lap PD. Open laparoscopy was performed at the umbilicus, and an additional five ports were placed (Fig. 3). The patient had severe visceral steatosis, and the abdominal cavity was filled with omental fat. We cautiously performed a subtotal stomach-preserving PD. For mobilization around the ligament of Treitz and the fourth portion of the duodenum, an additional port was placed at the middle of the inferior abdomen (Fig. 3). During this procedure, neither duodenal adhesion to the aorta nor other inflammatory changes due to the previously placed stent graft were observed. No operative manipulations were affected by the caudal side of the AAA. After mobilization, an upper-middle incision of 15 cm was made, and the pancreas head was excised and removed.
Fig. 3.
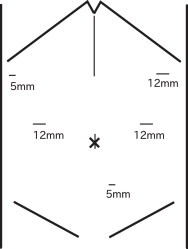
Picture showing the abdomen of the patient. The thin lines indicate the locations of the incisions. The short lower line indicates the additional incision made for dissection of the ligament of Treitz. The upper-middle line indicates the incision made for reconstruction.
Reconstruction was performed by a modified Child's procedure through the abdominal incision. A pancreato-jejunal anastomosis was created by hand-suturing between the pancreatic duct and the jejunal mucosa. Although the patient required medical therapy for pancreatic fistulae (grade B according to the International Study Group on Pancreatic Fistula [ISGPF]9), his postoperative recovery was uneventful.
The pathological diagnosis was intraductal papillary mucinous adenoma with small foci of carcinoma in situ (Fig. 4). The patient has been well for 18 months with no recurrence or cardiovascular complications.
Fig. 4.
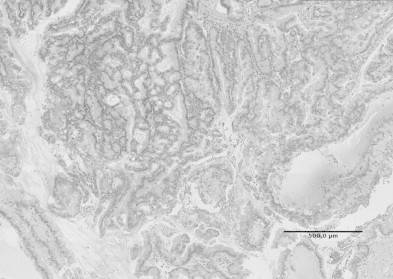
The pathological diagnosis was intraductal papillary mucinous adenoma with small foci of carcinoma in situ.
3. Discussion
Lap PD after EVAR for AAA was safely performed with both rigorous preoperative planning and a meticulous operation. Although Lap PD is one of the most complicated procedures in laparoscopic surgery, its safety and feasibility have been reported in experienced institutions.1–3 Pancreatic cancer, as a representation of pancreas head tumors, has a poor prognosis. Thus, the indications for Lap PD in patients with pancreatic cancer are very limited. This case involved an IPMN, and the patient was thus a good candidate for Lap PD.
Treatment for concomitant AAA and malignancies, especially pancreas tumors, is controversial. Therefore, reports of treatment of concomitant AAA and pancreas tumors are very rare.10,11 There are three main reasons for this rarity. The first is that pancreatic cancer has a poor prognosis. There are few operative indications for this type of neoplasia, and reported cases have shown a poor prognosis.4,10 When pancreatic cancer and AAA are simultaneously present, pancreatectomy is first recommended, including determination of the stage of the cancer.10 Second, mutual interference between the two conditions is undeniable because of the proximity of the pancreas to the aorta. Deiparine advocated division of the retroperitoneal dissection procedure: right-sided dissection for PD and left-sided dissection for abdominal aortic bypass.10 The last is the severity of the postoperative complications after pancreatectomy.
In the present case, laparoscopic dissection of the pancreas head was safely performed without interference of the residual AAA because the axis of the laparoscopic procedure was located apart from the AAA (Fig. 1). Moreover, laparoscopic procedures require smaller operative fields using magnified visualization. These are benefits of the laparoscopic approach for patients with AAA.
After the resection, an upper abdominal incision was made and reconstruction of the pancreas stump was performed through the incision. This reconstruction involved Wirsung anastomosis, which represents the usual manner of standard PD in our institute. Total Lap PD has been reported in experienced institutes1,2; however, other reconstruction methods and no reconstruction have also been reported.3 The most important factor to prevent postoperative complications is the quality of reconstruction. Thus, we performed reconstruction by hand as usual because laparoscopic reconstruction had not replaced hand sewing at that time in our institute. The additional upper incision is adequately located apart from the AAA; therefore, the reconstruction procedure is safely performed without interference by the AAA. The ISGPF grade B pancreatic fistulae healed with medical therapy and without graft infection.
4. Conclusion
We herein reported the performance of Lap PD after EVAR for AAA. The pancreatic tumor was detected during follow-up of the AAA after EVAR. Increased performance of EVAR for AAA and following CT may help to detect malignancy. Laparoscopic surgery is a valid approach to malignancy after EVAR.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Masahiko Kawaguchi contributed to the writing of this paper. Norihiko Ishikawa, Mari Shimada, and Hideki Moriyama performed the Lap PD with the corresponding author. Yuji Nishida and Hiroshi Ohtake performed EVAR for AAA. Go Watanabe performed the critical revision and final proof of the article.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Gagner M., Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726–730. doi: 10.1007/s00534-009-0142-2. [DOI] [PubMed] [Google Scholar]
- 2.Palanivelu C., Rajan P.S., Rangarajan M., Vaithiswaran V., Senthilnathan P., Parthasarathi R. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg. 2009;16:731–740. doi: 10.1007/s00534-009-0157-8. [DOI] [PubMed] [Google Scholar]
- 3.Corcione F., Pirozzi F., Cuccurullo D., Piccolboni D., Caracino V., Galante F. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc. 2013;27:2131–2136. doi: 10.1007/s00464-012-2728-z. [DOI] [PubMed] [Google Scholar]
- 4.Jibawi A., Ahmed I., El-Sakka K., Yusuf S.W. Management of concomitant cancer and abdominal aortic aneurysm. Cardiol Res Pract. 2011;2011:516146. doi: 10.4061/2011/516146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konno H., Kaneko H., Hachiya T., Maruo Y., Tanaka T., Suzuki S. Surgical management for a malignancy of the digestive organs accompanied with an abdominal aortic aneurysm. Surg Today. 1998;28:988–991. doi: 10.1007/s005950050269. [DOI] [PubMed] [Google Scholar]
- 6.Prinssen M., Verhoeven E.L.G., Buth J., Cuypers P.W.M., van Sambeek M.R.H.M., Balm R. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 7.Ohrlander T., Dencker M., Dias N.V., Gottsäter A., Acosta S. Cardiovascular predictors for long-term mortality after EVAR for AAA. Vasc Med. 2011;16:422–427. doi: 10.1177/1358863X11425713. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H., Unno N., Nakamura T., Kurachi K., Yamamoto N., Inuzuka K. Two-stage surgery for endovascular repair and laparoscopic colectomy for a patient with abdominal aortic aneurysm and concomitant colon cancer: report of a case. Ann Vasc Dis. 2009;2:47–60. doi: 10.3400/avd.AVDcr08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Deiparine M.K., Prian G.W., Koep L.J. Abdominal aortic aneurysm repair after pancreaticoduodenectomy. J Vasc Surg. 1995;21:537–539. doi: 10.1016/s0741-5214(95)70299-7. [DOI] [PubMed] [Google Scholar]
- 11.Sheen A.J., Baguneid M., Ellenbogen S., Walker M.G., Siriwardena A.K. Sequential endovascular repair and pancreaticoduodenectomy for abdominal aortic aneurysm copresenting with periampullary cancer. Ann Vasc Surg. 2006;20:114–116. doi: 10.1007/s10016-005-9406-8. [DOI] [PubMed] [Google Scholar]


