Abstract
INTRODUCTION
Posterior Nutcracker syndrome (NCS) is a rare anomaly in which the left renal vein passes behind the aorta which compresses it against the vertebral column, restricting the venous drainage of the left kidney.
PRESENTATION OF CASE
A 46 year-old lady presented with intermittent painless hematuria for 6 years. Urinalysis showed microscopic hematuria. An abdominal CT scan showed left renal vein duplication with the retroaortic branch trapped between the vertebral column and the aorta at the level of the aortic bifurcation, suggestive of posterior NCS. There were multiple small cortical cysts, sand-like stones in the left kidney and duplication of both right and left renal arteries.
DISCUSSION
Posterior NCS in a patient with a duplicated left renal vein may not show all the clinical features of a typical NCS as the elevated pressure due to compression is dissipated through the pre-aortic branch of the duplicated renal vein. CT Angiography can be helpful in such a patient with multiple abnormalities. Management can range from simple surveillance to nephrectomy depending on the symptoms and renocaval pressure gradient.
CONCLUSION
Although posterior NCS is a rare anomaly of the left renal vein, it should be considered in the differential diagnosis of haematuria.
Keywords: Computerized tomography angiography (CTA), Hematuria, Posterior nutcracker syndrome, Renal vein duplication
1. Introduction
Nutcracker syndrome (NCS) is uncommonly diagnosed as a cause of hematuria. It is called as anterior NCS if left renal vein (LRV) is entrapped between aorta and superior mesenteric artery. Posterior NCS occurs when the persistent posterior branch of fetal periaortic vascular ring gets compressed between aorta and vertebral body. Due to this compression a renocaval pressure gradient is created which is the root cause of all the symptoms seen in NCS. The flow in the renal vein depends on the degree and the stage of syndrome. Well developed collateral veins dissipate the high pressure gradient and diminish the blood flow volume of the LRV, resulting in the absence of distended LRV and LRV hypertension.1 The pre aortic branch of the duplicated LRV act as collateral vein which dissipate the high pressure in the retroaortic LRV and the symptoms may be limited to microscopic hematuria as in our case. Ultrasonography is a good primary imaging study however, CT scan best defines renal stones, renal masses and may show abnormal renal vessels or abdominal masses causing extrinsic compression. The management of NCS has evolved in last four decades and there are several available options, from close expectant surveillance to complex open surgical procedures.
2. Presentation of case
A 46-year lady presented with recurrent painless hematuria for last 6 years. She was diagnosed as systemic hypertension two years back and was taking Irbesartan 150 mg/day orally along with some herbal medicines for hematuria. On examination, her vital signs and systemic examinations were within normal limit. Urinalysis showed presence of blood 3+ and red blood cell 5.1/high power field. Urinary red cell morphology by phase contrast microscopy showed 70% dysmorphic cells. Serum electrolytes and renal function tests were normal. An abdominal ultrasonogram (USG) showed fatty liver and sand like left renal stones. CT scan of abdomen showed left renal vein duplication with the retroaortic LRV trapped between the vertebral body and the aorta suggestive of posterior NCS (Figs. 1–3). The preaortic LRV showed no abnormality. There were multiple small cortical cysts, sand like stones in the left kidney and duplication of both right and left renal arteries. She was advised for color Doppler study but she refused for further investigations and wished to continue the herbal medications as she was only having intermittent painless hematuria.
Fig. 1.
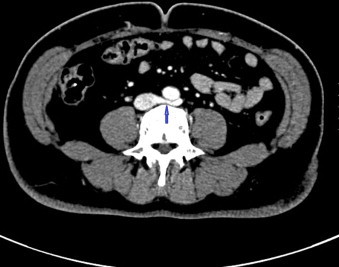
Left retroaortic renal vein entrapped between aorta and vertebra.
Fig. 2.
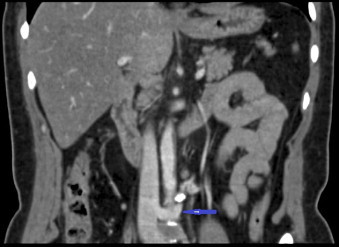
CT scan – Coronal view showing entrapped retroaortic left renal vein.
Fig. 3.
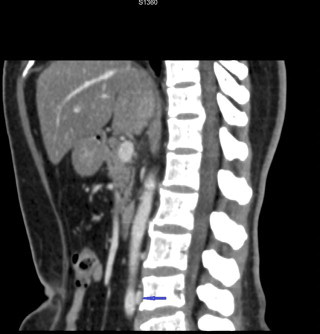
Sagittal view showing entrapped retroaortic left renal vein between aorta and vertebral body.
3. Discussion
Compression of LRV between the superior mesenteric artery and the aorta was first described by Grant in 1937 who found the anatomy analogous to a nut in a nutcracker.2 This description prompted the Belgian physician De Schepper to name this phenomenon as “Nutcracker Syndrome”.3 The first patient with NCS was described in 1950 although the compression was documented by venography only 2 decades later.2 Patient's age can range from childhood to the seventh decade of life but most symptomatic patients are in their second or third decade of life: there may be a second peak of NCS in middle-aged women.4
The clinical manifestation of NCS ranges a wide variety depending upon the renal venous pressure and associated other conditions such as presence of the preaortic branch of duplicated renal vein like in our case or formation of collaterals. The patient may be asymptomatic or may have only microscopic hematuria as seen in our case. Other patients may present with left flank pain, left sided varicocele, pelvic congestion syndrome, orthostatic disturbances, gastrointestinal symptoms and arterial hypertension depending on the level of renal venous pressure and consequent enlargement of intra renal or collateral vessels such as gonadal veins, ureteral veins, left inferior phrenic vein, capsular vein, left suprarenal vein and ascending lumbar vein. It has been seen in literatures that though NCS is an uncommon cause of hematuria, hematuria is the commonest presentation of NCS. The pressure gradient between the LRV and the vena cava rises due to compression leading to rupture of the thin walled septum between the small veins and the collecting system in the renal fornix causing hematuria. It has been postulated that hematuria may be the result of communication between dilated venous sinuses and adjacent renal calices.5 The normal renocaval pressure gradient shows high variability in healthy subjects ranging from 1.3 to 10 cm H2O and in NCS it may vary from 4.9 to 14 cm H2O. Nishimura regarded pressure gradient of 3 mm Hg or more as indicative of renal hypertension3 however pressure gradients have been reported to vary with position and hydration, as well as the degree of collateralization.2 The differential diagnosis includes Henoch Schonlein purpura, systemic lupus erythematosus, renal hemangioma, retrocaval ureter, renal endometriosis, renal stones and renal cyst. Though in our case both renal cysts and renal stones were present, they are unlikely causes of hematuria as 6 years is a long time for the persistence of sand like stones in the kidney to cause hematuria and as far as renal cyst is concerned, they were small cortical cysts so less likely that they will bleed in the collecting system.
Clinically the diagnosis of NCS is based on the assessment of the pathologic anatomy and physiology. A non-invasive imaging technique like CTA is the most appropriate and single imaging modality in a patient with multiple abnormalities like ours. The additional effect of three dimensional volume rendered CT integrates all of the necessary information previously obtained by conventional CT, angiography, venography and pyelography into single pre operative test allowing better visualization of disease and its relation to adjacent structures which eventually allow better operative planning if required.
The management of NCS depends upon the clinical presentation and the severity of the left renal vein hypertension. The option ranges from simple surveillance to nephrectomy. The correlation between imaging evidence of left renal vein compression and clinical symptoms remains challenging, and therefore intervention should be considered only when symptoms are severe or persistent, including severe unrelenting pain, severe hematuria, renal insufficiency, and failure to respond to conservative treatment after 24 months.4 Open surgical procedures employed to rectify the problem include the transposition of LRV, renal autotransplantation and gonadocaval bypass.6 But in a patient with posterior NCS or with chronic LRV thrombosis, anterior transposition of the renal vein alone may not be sufficient, and an interposition graft or implantation of the gonadal vein to the inferior vena cava may also be required.2 The other option for treatment is intra- or extra vascular stents or intrapelvic chemical cauterization. Patients with microscopic hematuria or intermittent short periods of painless gross hematuria, insignificant pain and a normal hemogram may be followed up closely without treatment.
4. Conclusion
Although posterior NCS is a rare anomaly of renal vein, it can be a cause of hematuria. The clinical presentation varies according to the severity of the renocaval pressure gradient but the presence of well developed collaterals or presence of the preaortic renal vein can limit the severity of this disease. CT scan is a simple, appropriate and optimum choice of imaging modality to diagnose posterior NCS as well as to find out associated other abnormalities with NCS. Conservative treatment is recommended for a patient with mild hematuria and those with serious impairment or severe symptoms may benefit from a surgical or intravascular intervention.
Conflicts of interest
The authors report that there are no conflicts of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
All authors have equal contribution to this article.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Takebayashi S., Ueki T., Ikeda N., Fujikawa Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns in retrograde left renal venography. AJR. 1999;172:39–43. doi: 10.2214/ajr.172.1.9888735. [DOI] [PubMed] [Google Scholar]
- 2.Reed N.R., Kalra M., Bower T.C., Vrtiska T.J., Ricotta J.J., Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vascular Surg. 2008;49(2):386–393. doi: 10.1016/j.jvs.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed K., Sampath R., Khan M.S. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg. 2006;31:410–416. doi: 10.1016/j.ejvs.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 4.Kurklinsky A.K., Rooke T.W. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552–559. doi: 10.4065/mcp.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buschi A.J., Harrison R.B., Brenbridge A.N.A.G., Williamson B.R.J., Gentry R.R., Cole R. Distented left renal vein; CT/sonographic normal variant. AJR. 1980;135:339–342. doi: 10.2214/ajr.135.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Xu D., Liu Y., Gao Y., Zhang L., Wang J., Jiangping C. Management of renal nutcracker syndrome by retroperitoneal laparoscopic nephrectomy with ex vivo autograft repair and autotrasplantation: a case report and review of the literature. J Med Case Rep. 2009;3:82. doi: 10.1186/1752-1947-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]


