Abstract
People wearing mandibular two-implant overdentures (IOD) chew food with less difficulty than those wearing conventional complete dentures (CD). However, there is still controversy over whether or not this results in better dietary intake. In this randomized clinical trials (RCT), the amounts of total dietary fiber (TDF), macronutrients, 9 micronutrients, and energy in diets consumed by persons with IOD and CD were compared. Male and female edentate patients ≥ 65 yrs (n = 255) were randomly divided into 2 groups and assigned to receive a maxillary CD and either a mandibular IOD or a CD. One year following prosthesis delivery, 217 participants (CD = 114, IOD = 103) reported the food and quantities they consumed to a registered dietician through a standard 24-hour dietary recall method. The mean and median values of TDF, macro- and micronutrients, and energy consumed by both groups were calculated and compared analytically. No significant between-group differences were found (ps > .05). Despite quality-of-life benefits from IODs, this adequately powered study reveals no evidence of nutritional advantages for independently living medically healthy edentate elders wearing two-implant mandibular overdentures over those wearing conventional complete dentures in their dietary intake at one year following prosthesis delivery (International Clinical Trials ISRCTN24273915).
Keywords: nutrition/nutritional sciences, dental implant(s), edentulous/edentulism, geriatric dentistry, prostheses, removable prosthodontics.
Introduction
A large percentage of our aging population is edentulous (Morais et al., 2003; MacEntee, 2007). Studies have shown that edentate people have poorer nutrition than those with teeth (Sahyoun et al., 2003), as well as being more vulnerable to disease (Lowe et al., 2003). Furthermore, findings from several studies suggest that the number of teeth is a significant, independent risk indicator for mortality and that use of adequate dentures may reduce this risk (Abnet et al., 2005; Padilha et al., 2008). Thus, the importance of a mandibular two-implant overdenture (IOD) cannot be overemphasized in light of the scientific evidence supporting its use as a first-choice standard of care for elderly edentate individuals (Feine et al., 2002; Timmerman et al., 2004; Thomason et al., 2009). A panel of prosthodontic experts from US dental faculties supported treatment with IODs over conventional dentures (CDs) (Das et al., 2012), indicating their agreement with the McGill (Feine et al., 2002) and York Consensus Statements (Thomason et al., 2009), which were based on research demonstrating the superiority of mandibular IODs over CDs for many variables, including oral health–related quality of life (Awad et al., 2000; Jofre et al., 2013), patient satisfaction (Thomason et al., 2003; Heydecke et al., 2008), food avoidance (Gjengedal et al., 2012), chewing ability (de Oliveira and Frigerio, 2004), and food preparation (Awad et al., 2012). Although there is a long list of scientific reports and randomized clinical trials (RCT) on the advantages of IODs over CDs, few studies have investigated the effects of IODs on dietary intake. Those had limitations, such as the use of small sample sizes (Morais et al., 2003; de Oliveira and Frigerio, 2004; Muller et al., 2008; Gjengedal et al., 2012; Moynihan et al., 2012) or having been conducted in individuals with diabetes (Hamada et al., 2001). Thus, it is unknown if edentate individuals wearing mandibular IODs gain better nutrition through diet than those wearing CDs. Moreover, a look at the possible dietary differences between mandibular IODs and CDs becomes more essential in light of the increase in the elderly population, longer life expectancy, demand for better quality of life for the elderly, and evidence showing a relationship between an increased intake of some macro- and micronutrients and a reduced risk of some illnesses, such as cardiovascular disease, rheumatoid arthritis, pulmonary disease, and certain types of cancer (Hutton et al., 2002; Denny et al., 2003; Lowe et al., 2003; Abnet et al., 2005).
The main objective of this RCT was to assess the effects of mandibular IODs on the nutritional status of edentate elders. The primary hypothesis was that, at 6 and 12 mos post-treatment, patients who received mandibular IODs would have no significant difference in their blood serum concentration of homocysteine [tHcy] than those who received CDs. This was reported in a previous publication (Awad et al., 2012). The secondary objective, and the subject of this report was to determine whether those independently living edentate elders wearing new mandibular IODs and CDs consumed different daily amounts of micro- and macronutrients, as well as total dietary fiber (TDF) and energy. This outcome could indicate whether there was a difference between the groups in dietary intake that had not yet made a difference in blood nutrient values.
The following null hypotheses were tested:
There is no difference between the groups in the intake of TDF one year post-treatment.
There is no difference between the groups in the intake of energy (KCal) one year post-treatment.
There is no difference between the groups in the intake of macronutrients (proteins, fats, and carbohydrates) one year post-treatment.
There is no difference between the groups in the intake of 9 micronutrients (vitamins A, B6, B12, C, and D, as well as thiamin, riboflavin, folate, and niacin) one year post-treatment.
Materials & Methods
Study Design
Data reported in this paper were gathered from a randomized, controlled, parallel trial comparing the effects of 2 types of mandibular prosthetic treatment (IOD and CD) in independently living edentulous elders in Montreal, Canada. The study was conducted at the MUHC-Royal Victoria Hospital (RVH) Clinical Investigation Unit (Montreal, Canada). The data used in this analysis were obtained at baseline and 12 mos following prosthesis delivery, by a standard 24-hour dietary recall method.
The study protocol and informed consent documents were approved by the institutional review board at McGill University. All study participants gave their written informed consent. Participants were financially compensated for their transportation and parking costs.
Sample Size Estimation
The sample size estimation was carried out to maintain adequate power to assess the primary and secondary outcomes, including those described in this report (Awad et al., 2012). A total of 104 individuals per group would be required to ensure a power of 95%, with a two-sided test at a .05 level of significance for the primary outcome. We targeted our sample size for a somewhat higher traditional power than usual, to enhance power for secondary outcomes. In terms of secondary outcomes, this sample size provides 80% power with a type I error of .05 on all parameters, with a two-sided test. We also anticipated 18% loss to follow-up. Accordingly, we adjusted our targeted baseline sample size to 127 per group.
Recruitment
Recruitment and treatment allocation steps were described in a previous publication (Awad et al., 2012). In general, potential participants were recruited through French and English advertisements in local papers. Once eligibility and consent were confirmed, the research assistant obtained treatment assignment from an independent center using a computer-generated permuted block scheme. Various block sizes were used to preserve allocation concealment and reduce potential selection bias.
Participants
The sample population consisted of men and women (≥ 65 yrs) who had been edentate for a minimum of 5 yrs. Other inclusion criteria included adequate understanding of written and spoken English or French, as well as willingness and ability to understand the protocol and to give informed consent. Potential participants completed a medical history with questions about oral condition, general health, and use of medications. They also completed questionnaires on oral function to assess their ability to understand and use the measures to be employed in the study. They were then examined by two calibrated clinicians. Patients were excluded if they had insufficient bone for placement of 2 implants in the anterior mandible, acute or chronic symptoms of TMD, systemic or neurologic disease that contraindicated implant surgery, any neoplasia diagnosed < 5 yrs previously, a body mass index (BMI) < 20 or > 32 kg/m2, and/or current usage of dietary supplements, anti-neoplastic medication, phenytoin, or corticosteroids. Since memory must be intact to provide accurate 24-hour dietary recall information, potential participants were excluded if they scored 24 or less on the Mini-Mental State Evaluation.
The Intervention
Maxilla
Both the treatment and control groups received a maxillary CD fabricated in a standard manner.
Mandible: Treatment Group
Participants randomized to the experimental group received a mandibular IOD on 2 implants with ball attachments (ITI, Straumann-048.242/243, Waldenburg, Switzerland) in the canine region of the anterior mandible. After a three-month healing period, the participants received new mandibular IODs.
Mandible: Control Group
Participants received complete CDs.
Dietary Assessments
Three 24-hour dietary recalls were collected through telephone interviews at baseline and 12 mos after prosthesis delivery. Trained personnel used a structured template to collect information on dietary intake during the previous 24 hrs (Gray-Donald et al., 1995).
The interviews were conducted on 3 separate occasions, twice on weekdays and once on a weekend. Dietary intake values were used to calculate intake of TDF, macronutrients (proteins, fat, and carbohydrates), 9 of the micronutrients (vitamins A, B6, B12, C, and D, thiamin, riboflavin, folate, and niacin), and energy. Individual values were used to calculate mean daily intakes, as well as mean and median intakes of both groups. Participants were trained at baseline on food serving portions and how to calculate their daily food intake. To standardize computations and reduce individual computational errors, we analyzed the collected data using nutrient values from the Canadian Nutrient File with the help of specialized software from the McGill University Faculty of Dietetics and Human Nutrition (CANDAT, Godin London Inc., London, ON, Canada).
Statistical Analysis
Because of the skewed nature of the data, medians were estimated as the measure of central tendency. Wilcoxon rank sum tests were used for bivariate comparisons of the various independent variables, according to treatment received (JMP statistical software version 10; SAS Institute Inc., Cary, NC, USA). Quantile regression models were used to estimate the conditional median of the dependent variables (post-treatment values of the micronutrients) between treatments, adjusted for baseline values and gender, since they require no distribution assumption; these models are more robust to outlying observations of the predictor variables (Stata SE software version 10.1; StataCorp, College Station, TX, USA). Any p values ≤ .05 were considered statistically significant.
Results
Two hundred fifty-five (255) participants were enrolled and randomly assigned to receive a maxillary CD and either a mandibular CD (n = 128) or an IOD (n = 127). A total of 207 participants (n = 114 CD, n = 103 IOD) provided follow-up data at one year post-treatment and were included in this analysis (Fig.). Reasons for loss to follow-up included illness, fear of surgery, and/or lack of interest. At baseline, the groups were similar in age (CD mean, 69.7 ± SD 4.6; IOD mean, 70.5 ± SD 5.0) and gender distribution [CD, men N = 57 (44.5%), women N = 71 (55.5%); IOD, men N = 57 (44.9%), women N = 70 (55.1%); p = .95].
Figure.
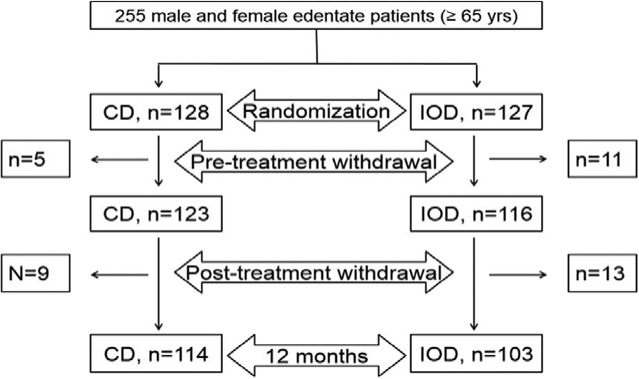
Flow chart of participants’ enrolment in the study (up to one year post-treatment).
The analysis failed to reject any of 4 null hypotheses (p > .36; Table 1), even with gender stratification (p > .17; Table 2). No significant inter-group difference was found in intake of TDF (p > .36; Table 1), energy (p > .58; Table 2), or macronutrients, i.e., proteins, fats, or carbohydrates (p > .41; Table 2) at baseline and one year post-treatment. There were also no significant differences between the two groups for any of the 9 examined micronutrients (vitamins A, B6, B12, C, D, and thiamin, riboflavin, folate, and niacin) (p > .13; Table 1).
Table 1.
Intergroup – Baseline and 12-month Average Means and Median Daily Intakes of TDF, Energy, Macronutrients, and 9 Micronutrients for IOD and CD Groups
| Baseline Results | 12-month Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Group | Mean (STD)* | Median (Q1, Q3)† | 95% CI‡ | p value§ | Mean (STD)* | Median (Q1, Q3)† | 95% CI‡ | p value§ |
| Fiber (TDF) (g) | IOD | 16.3 (7.7) |
14.7 (11.6, 18.7) |
15.9 (7.3) |
14.5 (11.1, 19.0) |
||||
| CD | 15.7 (7.1) |
14.9 (10.9, 19.0) |
(-1.3, 1.8) | .81 | 16.8 (7.7) |
15.4 (11.5, 19.9) |
(-2.4, 1.0) | .36 | |
| Energy (KCal) | IOD | 1,540.2 (541.7) |
1,432.7 (1,150.7, 1,909.3) |
1,474.4 (431.7) |
1,469.7 (1,201.3, 1,633.0) |
||||
| CD | 1,557.4 (554.2) |
1,478 (1,147.5, 1,877.4) |
(-160.0, 114.0) | .72 | 1,505.7 (482.0) |
1,403.0 (1,193.7, 1,741.1) |
(-123.3, 108.7) | .96 | |
| Macronutrients | |||||||||
| PROT (g) | IOD | 67.7 (22.3) |
66.1 (50.4, 79.4) |
64.7 (22.5) |
61.8 (49.2, 77.6) |
||||
| CD | 67.1 (24.8) |
62.8 (52.7, 79.0) |
(-4.2, 7.5) | .55 | 65.0 (22.7) |
61.4 (49.7, 78.1) |
(-5.7, 5.2) | .94 | |
| FAT (g) | IOD | 56.8 (23.6) |
51.6 (38.9, 70.1) |
56.0 (20.0) |
54.4 (41.1, 67.5) |
||||
| CD | 59.3 (27.4) |
53.3 (41.4, 72.3) |
(-8.0, 3.9) | .57 | 55.3 (19.5) |
53.0 (41.8, 66.3) |
(-4.2, 6.2) | .64 | |
| CARB (g) | IOD | 189.2 (80.5) |
166.7 (135.2, 235.0) |
177.1 (59.6) |
173.9 (133.6, 198.4) |
||||
| CD | 185.6 (69.2) |
174.6 (136.3, 233.8) |
(-20.4, 16.4) | .84 | 187.1 (83.5) |
174.6 (141.4, 213.0) |
(-18.1, 10.0) | .64 | |
| Micronutrients | |||||||||
| VIT A (μg) | IOD | 675.8 (922.6) |
533.3 (342.3, 733.3) |
661.1 (672.7) |
525.7 (385.7, 769.0) |
||||
| CD | 830.5 (1,016.6) |
562.2 (371.3, 860.1) |
(-118.3, 38.7) | .38 | 713.4 (717.3) |
572.7 (357.9, 797.4) |
(-96.7, 52.7) | .63 | |
| VIT D (μg) | IOD | 4.7 (4.1) |
3.1 (2.2, 6.1) |
4.1 (3.6) |
3.3 (2.0, 5.0) |
||||
| CD | 3.8 (3.2) |
3.1 (1.9, 4.7) |
(-0.1, 1.0) | .13 | 4.2 (4.9) |
3.1 (1.8, 5.0) |
(-0.3, 0.7) | .41 | |
| VIT C (mg) | IOD | 108.5 (75.6) |
90.5 (58.2, 141.2) |
98.5 (71.7) |
91.2 (47.9, 131.0) |
||||
| CD | 100.6 (64.8) |
86.1 (53.9, 130.8) |
(-10.6, 19.9) | .53 | 104.3 (65.3) |
89.0 (61.0, 143.1) |
(-22.8, 8.9) | .36 | |
| THIA (mg) | IOD | 1.4 (0.7) |
1.3 (0.9, 1.8) |
1.5 (0.7) |
1.3 (1.0, 1.8) |
||||
| CD | 1.4 (0.5) |
1.3 (1.1, 1.8) |
(-0.2, 0.1) | .69 | 1.4 (0.6) |
1.3 (1.1, 1.6) |
(-0.1, 0.1) | .98 | |
| RIBO (mg) | IOD | 2.0 (1.6) |
1.7 (1.4, 2.2) |
1.8 (0.6) |
1.7 (1.4, 2.1) |
||||
| CD | 1.8 (0.7) |
1.7 (1.4, 2.2) |
(-0.1, 0.2) | .69 | 1.9 (1.7) |
1.7 (1.4, 2.0) |
(-0.1, 0.2) | .68 | |
| Niacin (NEs) | IOD | 31.2 (10.5) |
30.5 (24.1, 38.2) |
30.8 (11.8) |
28.5 (23.6, 36.8) |
||||
| CD | 31.4 (12.0) |
28.3 (23.8, 37.6) |
(-2.3, 3.1) | .76 | 30.2 (11.1) |
28.6 (23.5, 35.3) |
(-2.3, 2.8) | .85 | |
| VIT B6 (mg) | IOD | 1.5 (0.6) |
1.4 (1.1, 1.8) |
1.6 (1.1) |
1.4 (1.1, 1.7) |
||||
| CD | 1.5 (0.6) |
1.4 (1.1, 1.8) |
(-0.1, 0.2) | .53 | 1.5 (0.6) |
1.4 (1.2, 1.7) |
(-0.2, 0.1) | .47 | |
| VIT B12 (μg) | IOD | 3.9 (2.9) |
3.3 (2.3, 4.7) |
4.4 (5.6) |
2.8 (2.0, 4.1) |
||||
| CD | 5.4 (7.8) |
2.8 (2.0, 5.1) |
(-0.3, 0.6) | .44 | 3.8 (3.4) |
3.1 (2.2, 4.3) |
(-0.5, 0.3) | .66 | |
| DFE (Folate) (μg) | IOD | 355.4 (148.5) |
308.3 (262.0, 442.7) |
362.9 (193.3) |
340.0 (252.7, 419.0) |
||||
| CD | 349.5 (135.2) |
326.7 (243.8, 433.1) |
(-33.3, 36.0) | .88 | 340.4 (116.8) |
315.2 (255.7, 413.7) |
(-21.7, 42.3) | .56 | |
Mean (Standard Deviation).
Median and quartiles (25th [Q1] and 75th [Q3]).
95% confidence interval for the median difference was obtained by non-parametric comparisons for each pair by the Wilcoxon method.
p values were obtained from the Wilcoxon rank-sum test.
Table 2.
Intergroup – 12-month Average Means and Median Daily Intakes of TDF, Energy, Macronutrients, and 9 Micronutrients after Stratification by Gender for IOD and CD Groups
| Variables | Male Results | Female Results | ||||||
|---|---|---|---|---|---|---|---|---|
| CD Group | IOD Group | CD Group | IOD Group | |||||
| Median (Q1, Q3) * | Median (Q1, Q3) * | 95% CI† | p value‡ | Median (Q1, Q3) * | Median (Q1, Q3) * | 95% CI† | p value‡ | |
| Fiber (TDF) (g) | 16.0 (12.5, 21.4) |
16.1 (11.5, 21.1) |
(-3.2, 2.1) | .63 | 14.7 (10.3, 19.7) |
13.9 (10.3, 17.9) |
(-2.9, 1.4) | .45 |
| Energy (KCal) | 1,452.3 (1,220.8, 1,987.7) |
1,527.7 (1,339.0, 1,828.3) |
(-152.0, 223.0) | .58 | 1,357.0 (1,105.8, 1,648.7) |
1,335.7 (1,080.3, 1,581.6) |
(-173.7, 104.3) | .61 |
| Macronutrients | ||||||||
| PROT (g) | 64.9 (53.1, 81.3) |
66.6 (53.2, 89.9) |
(-5.8, 11.8) | .57 | 57.5 (47.4, 74.9) |
59.2 (45.4, 68.0) |
(-8.9, 5.1) | .65 |
| FAT (g) | 58.2 (42.2, 74.4) |
59.0 (47.8, 72.0) |
(-7.3, 9.7) | .78 | 51.4 (39.5, 58.3) |
50.4 (37.9, 63.2) |
(-5.7, 7.2) | .79 |
| CARB (g) | 186.0 (152.0, 222.9) |
188.3 (158.7, 226.0) |
(-21.8, 26.4) | .82 | 166.7 (124.3, 188.9) |
161.3 (125.0, 182.4) |
(-25.6, 9.0) | .41 |
| Micronutrients | ||||||||
| VIT A (μg) | 606.0 (359.5, 875.7) |
574.0 (388.7, 814.0) |
(-131.5, 116.0) | .97 | 542.5 (357.5, 709.0) |
434.2 (369.8, 704.5) |
(-138.3, 52.3) | .42 |
| VIT D (μg) | 3.3 (2.0, 5.3) |
4.0 (2.5, 5.8) |
(-0.4, 1.5) | .27 | 2.8 (1.7, 4.8) |
3.1 (1.9, 4.7) |
(-0.6, 0.7) | .93 |
| VIT C (mg) | 83.9 (62.1, 137.6) |
88.0 (42.0, 131.0) |
(-26.0, 17.8) | .64 | 107.3 (59.0, 148.1) |
93.1 (49.7, 134.0) |
(-33.1, 12.6) | .37 |
| THIA (mg) | 1.4 (1.1, 1.7) |
1.5 (1.1, 2.3) |
(-0.1, 0.4) | .22 | 1.3 (1.1, 1.5) |
1.1 (0.9, 1.6) |
(-0.2, 0.1) | .32 |
| RIBO (mg) | 1.8 (1.5, 2.1) |
1.9 (1.6, 2.3) |
(-0.1, 0.3) | .17 | 1.5 (1.3, 2.0) |
1.6 (1.4, 1.8) |
(-0.2, 0.1) | .81 |
| Niacin (NEs) | 30.4 (24.7, 37.2) |
31.0 (25.1, 41.4) |
(-2.8, 5.1) | .61 | 27.2 (21.9, 33.8) |
27.3 (20.5, 33.6) |
(-3.6, 3.0) | .95 |
| VIT B6 (mg) | 1.4 (1.2, 1.8) |
1.4 (1.1, 1.9) |
(-0.2, 0.2) | .93 | 1.4 (1.1, 1.7) |
1.3 (1.0, 1.6) |
(-0.2, 0.1) | .30 |
| VIT B12 (μg) | 3.4 (2.3, 4.7) |
3.5 (2.3, 5.3) |
(-0.7, 0.7) | .94 | 2.7 (2.0, 3.9) |
2.7 (1.8, 3.4) |
(-0.7, 0.3) | .59 |
| DFE (Folate) (μg) | 337.3 (283.3, 471.7) |
378.7 (290.7, 457.7) |
(-38.3, 70.3) | .64 | 309.0 (242.3, 379.2) |
316.7 (249.0, 396.6) |
(-30.0, 44.3) | .71 |
Median and quartiles (25th [Q1] and 75th [Q3]).
95% confidence interval for the median difference was obtained by non-parametric comparisons for each pair by the Wilcoxon method.
p values were obtained from Wilcoxon rank-sum test.
A non-significant correlation was observed for the association between all dependent variables (TDF, energy, macro- and micronutrients) and treatment, adjusted for baseline values and gender (Table 3). However, a tendency for higher median dietary intake values of energy, 3 macronutrients (proteins, fats, and carbohydrates), vitamin D, thiamin, vitamin B6, vitamin B12, and folate among CD wearers was observed. Similarly, there was a tendency for higher median dietary intake values of TDF, vitamin A, vitamin C, riboflavin, and niacin among individuals with IODs. Based on this regression model, women had significantly higher median dietary intakes of thiamin and riboflavin and significantly lower intakes of energy and folate after adjustment for treatment received and baseline values. Moreover, baseline median intakes of energy, protein, thiamin, and riboflavin were significantly higher and baseline values for folate were significantly lower, after adjustment for treatment received and gender.
Table 3.
Association between Nutrients and Treatment Adjusted for Baseline Values, Gender*
| Variable | B† | 95% CI |
|---|---|---|
| Total Dietary Fiber (TDF) (g)
Implant Female Baseline |
1.0 -0.2 0.4 |
-0.57, 2.57 -1.83, 1.43 0.29, 0.51 |
| Energy (KCal)
Implant Female Baseline |
-46.2 -132.4 0.11 |
-160.26, 67.8 -258.6, -6.2** 0.01, 0.22** |
| PROT (g)
Implant Female Baseline |
-0.80 -4.8 0.21 |
-9.0, 7.4 -13.6, 3.9 0.01, 0.39** |
| FAT (g)
Implant Female Baseline |
-1.2 -6.3 0.08 |
-7.0, 4.5 -12.4, -0.22 -0.4, 0.2 |
| CARB (g)
Implant Female Baseline |
-0.16 -11.9 0.11 |
-11.2, 10.9 -24.1, 2.9 0.02, 0.19 |
| VIT A (μg)
Implant Female Baseline |
93.0 -100.71 0.03 |
-27.3, 213.3 -221.3, 19.9 -0.02, 0.08 |
| VIT D (μg)
Implant Female Baseline |
-0.2 -0.4 0.2 |
-0.85, 0.47 -1.07, 0.27 -0.11, 0.3 |
| VIT C (mg)
Implant Female Baseline |
10.67 11.9 0.3 |
-12.02, 33.4 -10.7, 34.6 0.14, 0.46 |
| THIA (mg)
Implant Female Baseline |
-0.05 -0.22 1.6 |
-0.22, 0.10 -0.41, -0.05** 1.13, 2.06** |
| RIBO (mg)
Implant Female Baseline |
0.004 1.0 0.003 |
-0.03, 0.2 0.30, 1.7** 0.002, 0.07** |
| Niacin (NEs)
Implant Female Baseline |
0.22 -2.22 0.11 |
-4.3, 3.92 -6.8, 2.3 -0.09, 0.31 |
| VIT B6 (mg)
Implant Female Baseline |
-0.001 -0.006 0.05 |
-0.91, 0.92 -0.94, 0.96 -0.24, 1.25 |
| VIT B12 (μg)
Implant Female Baseline |
-0.06 0.03 0.05 |
-1.83, 0.62 -1.52, 0.94 -0.06, 0.15 |
| DFE (Folate) (μg)
Implant Female Baseline |
-14.64 -45.24 0.23 |
-50.6, 21.3 83.0, 7.5** 0.10, 0.36** |
Analysis was based on quantile regression.
Unstandardized regression coefficient.
p < .05.
Discussion
To our knowledge, this is the first RCT with a sample size adequate for the study of differences in dietary intake between IOD and CD wearers. The results demonstrate that, one year after delivery of new prostheses, dietary intake of medically healthy elders receiving mandibular two-implant overdentures is not statistically different from that of those treated with conventional complete dentures. The two groups had similar intakes of TDF, energy, macronutrients (proteins, fats, and carbohydrates), and 9 micronutrients.
Although our results support findings from previous studies (Sebring et al., 1995; Hamada et al., 2001; Morais et al., 2003; Gjengedal et al., 2012), several limitations of this type of nutritional comparison should be mentioned. The bias due to reporting error inherent in the 24-hour dietary recall method may be greater than the inter-group difference (Subar et al., 1990). Moreover, a self-report of dietary intake could be biased by social desirability (Hebert et al., 1995). However, given the relatively large sample size used in this study and based on the average value of 3 recalls, these 24-hour recall data are likely to be appropriate.
Furthermore, our results also raise a number of questions: First, why are the dietary intakes so similar in both groups, despite the fact that IOD wearers report superiority in their masticatory performance and ability to chew a greater variety foods (de Oliveira and Frigerio, 2004; Van Kampen et al., 2004; Muller et al., 2008)?
The fact that this study revealed similar dietary intake in both groups suggests that either the same foods were eaten, but prepared differently, or that different foods with similar nutritional profiles were consumed. We believe that the reported differences in the way in which patients in both groups prepared their foods (Awad et al., 2012) helped to produce similar dietary intake effects, e.g., a person who bites an apple will take in the same nutrients as someone who uses a knife to cut the apple into pieces or drinks pure apple juice. Furthermore, our study participants were medically healthy, independently living elders who demonstrated a good nutritional state at baseline. They also had above-average educational levels and incomes. Thus, they were likely health-conscious and capable of choosing and preparing foods appropriately to compensate for difficulties in chewing. Therefore, the chance of gaining nutritional benefit in this population may be limited.
Second, what are the possible causes of the few significant differences found by the regression model for gender, adjusted for treatment and baseline values, and for baseline values, adjusted for treatment received and gender? Those significant differences by gender are expected, because males and females do differ in nutrition and caloric intake (Baker and Wardle, 2003). Furthermore, significant differences between baseline and post-treatment values are also expected because it is possible that, regardless of treatment received, both groups modified their diets from baseline because of receiving new prostheses.
Third, what are the implications of these similarities, and what could have been done to improve the chance of finding any potential differences? We believe that changing dietary habits, including the types of foods consumed, may be a multifactorial adaptation process that takes much longer than a year for a measurable effect (Schlettwein-Gsell, 1992). Additionally, dietary counseling can improve fruit and vegetable intake in an edentulous individual (Bradbury et al., 2006). Our study participants were not given specific individual dietary counseling before or during the study and, consequently, could have needed more time to adapt their eating habits to the new treatment. Thus, an RCT in which participants are followed for a longer period of time and in which they are given specific individual dietary counseling might maximize the possibility of dietary improvement (Moynihan et al., 2012).
In conclusion, although there is much evidence supporting the adoption of two-implant mandibular overdenture (IOD) treatment as the standard of care for edentate patients, this evidence does not include an improvement in dietary intake at one year for medically healthy independent edentate elders when given no specific dietary counseling.
Acknowledgments
The authors gratefully acknowledge the assistance of Prof. Jose Correa, all participants, Nicolas Drolet, the study dietitians, and Health Canada.
Footnotes
This study was funded by the Canadian Institutes of Health Research (CIHR Industry Grant # UCR-36052) and Straumann Canada Ltd.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. (2005). Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol 34:467-474 [DOI] [PubMed] [Google Scholar]
- Awad MA, Locker D, Korner-Bitensky N, Feine J. (2000). Measuring the effect of intra-oral implant rehabilitation on health-related quality of life in a randomized controlled clinical trial. J Dent Res 79:1659-1663 [DOI] [PubMed] [Google Scholar]
- Awad MA, Morais JA, Wollin S, Khalil A, Gray-Donald K, Feine JS. (2012). Implant overdentures and nutrition: a randomized controlled trial. J Dent Res 91:39-46 [DOI] [PubMed] [Google Scholar]
- Baker AH, Wardle J. (2003). Sex differences in fruit and vegetable intake in older adults. Appetite 40:269-275 [DOI] [PubMed] [Google Scholar]
- Bradbury J, Thomason J, Jepson N, Walls A, Allen P, Moynihan P. (2006). Nutrition counseling increases fruit and vegetable intake in the edentulous. J Dent Res 85:463-468 [DOI] [PubMed] [Google Scholar]
- Das KP, Jahangiri L, Katz RV. (2012). The first-choice standard of care for an edentulous mandible: a Delphi method survey of academic prosthodontists in the United States. J Am Dent Assoc 143:881-889 [DOI] [PubMed] [Google Scholar]
- de Oliveira TR, Frigerio ML. (2004). Association between nutrition and the prosthetic condition in edentulous elderly. Gerodontology 21:205-208 [DOI] [PubMed] [Google Scholar]
- Denny SI, Thompson RL, Margetts BM. (2003). Dietary factors in the pathogenesis of asthma and chronic obstructive pulmonary disease. Curr Allergy Asthma Rep 3:130-136 [DOI] [PubMed] [Google Scholar]
- Feine JS, Carlsson GE, Awad MA, Chehade A, Duncan WJ, Gizani S, et al. (2002). The McGill consensus statement on overdentures. Mandibular two-implant overdentures as first choice standard of care for edentulous patients. Montreal, Quebec, May 24-25, 2002. Int J Oral Maxillofac Implants 17:601-602 [PubMed] [Google Scholar]
- Gjengedal H, Dahl L, Lavik A, Trovik TA, Berg E, Boe OE, et al. (2012). Randomized clinical trial comparing dietary intake in patients with implant-retained overdentures and conventionally relined denture. Int J Prosthodont 25:340-347 [PubMed] [Google Scholar]
- Gray-Donald K, Payette H, Boutier V. (1995). Randomized clinical trial of nutritional supplementation shows little effect on functional status among free-living frail elderly. J Nutr 125:2965-2971 [DOI] [PubMed] [Google Scholar]
- Hamada MO, Garrett NR, Roumanas ED, Kapur KK, Freymiller E, Han T, et al. (2001). A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part IV: Comparisons of dietary intake. J Prosthet Dent 85:53-60 [DOI] [PubMed] [Google Scholar]
- Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. (1995). Social desirability bias in dietary self-report May compromise the validity of dietary intake measures. Int J Epidemiol 24:389-398 [DOI] [PubMed] [Google Scholar]
- Heydecke G, Thomason JM, Awad MA, Lund JP, Feine JS. (2008). Do mandibular implant overdentures and conventional complete dentures meet the expectations of edentulous patients? Quintessence Int 39:803-809 [PubMed] [Google Scholar]
- Hutton B, Feine J, Morais J. (2002). Is there an association between edentulism and nutritional state? J Can Dent Assoc 68:182-187 [PubMed] [Google Scholar]
- Jofre J, Castiglioni X, Lobos CA. (2013). Influence of minimally invasive implant-retained overdenture on patients’ quality of life: a randomized clinical trial. Clin Oral Implants Res 24:1173-1177 [DOI] [PubMed] [Google Scholar]
- Lowe G, Woodward M, Rumley A, Morrison C, Tunstall-Pedoe H, Stephen K. (2003). Total tooth loss and prevalent cardiovascular disease in men and women: possible roles of citrus fruit consumption, vitamin C, and inflammatory and thrombotic variables. J Clin Epidemiol 56:694-700 [DOI] [PubMed] [Google Scholar]
- MacEntee MI. (2007). The prevalence of edentulism and diseases related to dentures—a literature review. J Oral Rehabil 12:195-207 [DOI] [PubMed] [Google Scholar]
- Morais J, Heydecke G, Pawliuk J, Lund J, Feine JS. (2003). The effects of mandibular two-implant overdentures on nutrition in elderly edentulous individuals. J Dent Res 82:53-58 [DOI] [PubMed] [Google Scholar]
- Moynihan PJ, Elfeky A, Ellis JS, Seal CJ, Hyland RM, Thomason JM. (2012). Do implant-supported dentures facilitate efficacy of eating more healthily? J Dent 40:843-850 [DOI] [PubMed] [Google Scholar]
- Muller K, Morais J, Feine J. (2008). Nutritional and anthropometric analysis of edentulous patients wearing implant overdentures or conventional dentures. Braz Dent J 19:145-150 [DOI] [PubMed] [Google Scholar]
- Padilha DM, Hilgert JB, Hugo FN, Bós AJ, Ferrucci L. (2008). Number of teeth and mortality risk in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 63:739-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun NR, Lin CL, Krall E. (2003). Nutritional status of the older adult is associated with dentition status. J Am Diet Assoc 103:61-66 [DOI] [PubMed] [Google Scholar]
- Schlettwein-Gsell D. (1992). Nutrition and the quality of life: a measure for the outcome of nutritional intervention. Am J Clin Nutr 55(6 Suppl):1263S-1266S [DOI] [PubMed] [Google Scholar]
- Sebring NG, Guckes AD, Li SH, McCarthy GR. (1995). Nutritional adequacy of reported intake of edentulous subjects treated with new conventional or implant-supported mandibular dentures. J Prosthet Dent 74:358-363 [DOI] [PubMed] [Google Scholar]
- Subar AF, Harlan LC, Mattson ME. (1990). Food and nutrient intake differences between smokers and non-smokers in the US. Am J Public Health 80:1323-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason JM, Lund JP, Chehade A, Feine JS. (2003). Patient satisfaction with mandibular implant overdentures and conventional dentures 6 months after delivery. Int J Prosthodont 16:467-473 [PubMed] [Google Scholar]
- Thomason JM, Feine J, Exley C, Moynihan P, Müller F, Naert I, et al. (2009). Mandibular two implant-supported overdentures as the first choice standard of care for edentulous patients—the York Consensus Statement. Br Dent J 207:185-186 [DOI] [PubMed] [Google Scholar]
- Timmerman R, Stoker G, Wismeijer D, Oosterveld P, Vermeeren J, Van Waas M. (2004). An eight-year follow-up to a randomized clinical trial of participant satisfaction with three types of mandibular implant-retained overdentures. J Dent Res 83:630-633 [DOI] [PubMed] [Google Scholar]
- Van Kampen F, Van Der Bilt A, Cune M, Fontijn-Tekamp F, Bosman F. (2004). Masticatory function with implant-supported overdentures. J Dent Res 83:708-711 [DOI] [PubMed] [Google Scholar]


