Abstract
This review aimed at evaluating the effectiveness of reconstructive procedures for treating peri-implantitis. Searches of electronic databases and cross-referencing were performed for human comparative clinical trials with ≥10 implants for ≥12 months of follow-up, reporting radiographic defect fill and at least one of the following parameters: probing depth reduction, clinical attachment level gain, bleeding on probing reduction, and mucosal recession. The searches retrieved 430 citations. Only 1 randomized controlled trial was identified, which compared reconstructive therapy and open flap debridement. Case series studies were also included to evaluate the overall performance of the reconstructive procedures. Twelve studies were finally included. Meta-analysis revealed that the weighted mean radiographic defect fill was 2.17 mm (95% confidence interval [CI]: 1.46-2.87 mm), probing depth reduction was 2.97 mm (95% CI: 2.38-3.56 mm), clinical attachment level gain was 1.65 mm (95% CI: 1.17-2.13 mm), and bleeding on probing reduction was 45.8% (95% CI: 38.5%-53.3%). Great variability in reparative outcomes was found, attributed to patient factors, defect morphology, and reconstructive agents used. Currently, there is a lack of evidence for supporting additional benefit of reconstructive procedures to the other treatment modalities for managing peri-implantitis.
Keywords: dental/oral implants, osseointegration, peri-implant, guided bone regeneration, implantology, bone regeneration
Introduction
Peri-implantitis is defined as inflammation of peri-implant tissues accompanied with changes in the level of crestal bone and with the presence of bleeding on probing and/or suppuration, with or without concomitant deepening of peri-implant pockets (Lang and Berglundh, 2011). If not properly managed, it is a debilitating condition that results in loss of function and esthetics (Fransson et al., 2010). Recent studies and reviews (Zitzmann and Berglundh, 2008; Fransson et al., 2009; Albrektsson et al., 2012; Koldsland et al., 2010; Mir-Mari et al., 2012) reported that peri-implantitis occurred in 2.7% to 47.1% of implants. This wide range in prevalence rates can be attributed to the differences in study population, disease definition, and implant micro- and macrostructures. The number of implants affected by peri-implantitis is likely to increase as more implants are placed. Therefore, identifying an effective strategy for treating this disease is imperative.
Surgical methodologies are commonly applied to manage moderate and advanced peri-implantitis (Aljateeli et al., 2012). Resective techniques are used to treat shallow intrabony defects, while regenerative procedures are indicated for deep, crater-type defects (Schwarz et al., 2010). Regenerative procedures, applying the concept of guided bone regeneration, use of bone grafts, and membranes, are implemented to rebuild peri-implant supporting bone; however, since regeneration can be defined only under histology, the term reconstructive is used for this article instead. The implants, once treated, may be covered or left in a peri-mucosal position. Until now, reports comparing the clinical efficacy of various materials and techniques are limited, making selection of therapies empirical and surgeon preference oriented (Esposito et al., 2012).
The effectiveness of reconstructive procedures has been measured by a variety of radiographic and clinical parameters—among them, radiographic defect fill (RDF), probing depth (PD) reduction, clinical attachment level (CAL) gain, and reduction of bleeding on probing (BOP) (Roos-Jansaker et al., 2007a, 2007b; Schwarz et al., 2009; Aghazadeh et al., 2012). A successful procedure should result in resolution of inflammation, readhesion of peri-implant soft tissues, bone regeneration, and reosseointegration. This systematic review aimed to (1) assess the potential of reconstructive surgeries for providing better results in comparison to other surgical therapies, (2) investigate the overall radiologic and clinical outcomes of reconstructive surgeries, and (3) identify any procedure and material that could potentially yield superior results for reconstructive procedures.
Materials & Methods
Focused Question
Do reconstructive surgical procedures provide beneficial clinical outcomes in comparison with other surgical techniques (resective surgeries and open flap debridement) in the treatment of peri-implantitis? As an alternative focused question, what are the overall treatment outcomes of reconstructive procedures in treating peri-implantitis?
Selection Criteria
Initial searches aimed at identifying studies that compared at least 1 clinical and radiographic parameter between reconstructive therapies and other surgical modalities, such as resective or open flap debridement surgeries, for treating peri-implantitis, with a minimum sample size of 10 implants and at least 12 months of observation. Studies that had performed implantoplasty in combination with reconstructive approach were also included. Screw-shaped implants with either smooth or rough surface were included. Clinical and radiographic parameters of interest were RDF, PD reduction, CAL gain, BOP reduction, and recession of the mucosal margin.
Search Strategy
A health sciences librarian (MPM) performed database searches in Ovid MEDLINE, PubMed, EMBASE, and Dentistry and Oral Sciences Source (limits: 1990-2013, peer reviewed journals). Each search was structured in 2 parts, with the first covering the targeted disease, peri-implantitis, and the second, regeneration and its variants. Combinations of controlled terms (MeSH and EMTREE) and key words were used whenever possible. A pooled set of 15 sentinel articles, which were identified during preliminary searches by one of the authors (VK), was used as a tool to validate the searches.
For the search in the PubMed, the search terms were as follows, where mh represents the MeSH terms and tiab represents title and/or abstract:
(“peri-implantitis”[mh] OR “peri-implantitis”[ti] OR ((“dental implantation, endosseous”[mh] OR “dental implants”[mh]) AND (“peri implant”[tiab] OR “peri-implantitis”[tiab]))) AND (regeneration[tiab] OR regenerative[tiab] OR “guided tissue regeneration”[mh] OR surgery[ti] OR surgical[ti] OR “bone graft”[ti] OR “bone grafts”[ti]) AND English[la] NOT (letter[pt] OR comment[pt] OR editorial[pt]) NOT (“animals”[mh] NOT “humans”[mh])
For the search in EMBASE, the search terms were as follows:
‘periimplantitis’/exp OR ‘peri-implantitis’:ti OR (‘tooth implantation’/exp/mj OR ‘biodegradable implant’/exp OR ‘dental implant’:ab,ti OR ‘dental implants’:ab,ti AND (‘peri-implant’:ti OR ‘peri-implantitis’:ti)) AND (‘tissue regeneration’/exp OR ‘regeneration’:ab,ti OR ‘regenerative’:ab,ti OR surger*:ti OR surgical*:ti OR ‘bone graft’/exp OR ‘bone graft’:ti OR ‘bone grafts’:ti) AND [english]/lim NOT ([animals]/lim NOT [humans]/lim) NOT (‘letter’/exp OR ‘editorial’/exp OR note:it OR erratum:it) AND [1990-2012]/py
For Dentistry and Oral Sciences Source, the terms were as follows:
(DE “PERI-implantitis” OR TI “peri-implantitis” OR AB “peri-implantitis”) AND (DE “GUIDED tissue regeneration” OR DE “Bone Regeneration” OR DE “Regeneration” OR TI “regenerat*” OR AB “regenerat*”) [Limits: 1990-2013; peer-reviewed journals]
Furthermore, cross-referencing from included and excluded papers and review articles was used to identify additional publications. Potential articles were examined by 2 reviewers (VK and HLC). Disagreement between the reviewers was resolved with discussion. The level of agreement between the reviewers regarding study inclusion was expressed with the kappa value. In addition, funnel plots were used to assess the presence of publication biases.
Risk of Bias Assessment
The following criteria were used, as modified from the randomized clinical trial checklist of the Cochrane Center (Higgins and Green, 2011) and the CONSORT statement (Schulz et al., 2010): representative of general population, defined inclusions/exclusions, randomization methods, allocation concealment method, masking of the examiner, intervention difference only, and participant dropout and analysis accounts for patient losses. The degrees of bias were categorized as follows: low risk, if all the criteria were met; moderate risk, when only one criterion was missing; and high risk, if 2 or more criteria were missing.
Data Analysis
The primary outcome was the RDF. The pooled weighted mean (WM) and the 95% confidence interval (CI) of each variable were estimated with Comprehensive Meta-analysis (Version 2, Biostat, Englewood, NJ, USA). The random effect model was applied during meta-analysis. Forest plots were produced to graphically represent WM and 95% CI for the primary and secondary outcomes, with the implant as the analysis unit. For studies with more than one reconstructive treatment arm, the results from all arms were combined. Heterogeneity was assessed with the I 2 test, which ranges between 0% and 100%, with lower values representing less heterogeneity. To evaluate the potential influences of different treatment modalities, WM and 95% CI were calculated separately for each type of membrane, bone graft, and type of the flap manipulation (submerge/nonsubmerge healing). The reporting of this meta-analysis adhered to the PRISMA statement (i.e., Preferred Reporting Items for Systematic Review and Meta-analysis) (Liberati et al., 2009).
Results
Because of only 1 comparative study (Wohlfahrt et al., 2012) available in the literature addressing the first focused question, case series implementing reconstructive procedures without controls were also included to evaluate the overall performances of the reconstructive procedures. The screening process is illustrated in Appendix Figure 1. The search initially retrieved 802 total citations, 378 of which were identified as duplicates. An additional 6 studies were retrieved through cross-referencing. After the titles and abstracts were reviewed, 55 articles were identified as full-text articles. Eighteen citations were selected for full evaluation, of which 6 were excluded. The reasons for article exclusion included the following: outcome variables in median values only (1 study) or ranges only (1 study), redundant cohorts (3 studies), and insufficient data (1 study). A total of 12 studies were included in this review (Tables 1 and 2). The kappa value for the interreviewer agreement of the included publications was 0.94. The reference numbers allocated to the included articles in the tables will be used throughout the rest of this review.
Table 1.
Features of the Included Studies
| Patient Features |
Implant Features |
Surgical Intervention |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | Follow-up, mo | n | Age, y | Smokers, % | BL, n | FE, n | Location | Body Surface | Platform Surface | Detoxification Method | Grafting Material | Membrane | Submerge |
| 1: Khoury and Buchmann (2001) | |||||||||||||
| QE | 36 | 7 | 49.4 ± 5.2 | ND | 12 | 12 | ND | R | S | CHX(0.2%) + CA + H2O2 + NaCl | Auto | N | Y |
| 11 | 55.5 ± 14.1 | 20 | 20 | e-PTFE | |||||||||
| 7 | 48.6 ± 8.1 | 9 | 9 | Resorb | |||||||||
| 2: Deppe et al. (2007) a | |||||||||||||
| QE | 63 | 7 | ND | ND | 15 | 11 | ND | ND | ND | AA | Auto + ß-TCP | e-PTFE | Y |
| 9 | 17 | 13 | AA + CO2 laser | ||||||||||
| 3: Roos-Jansåker et al. (2007a) | |||||||||||||
| CS | 12 | 12 | 64.4 ± 6.0 | 66.7 | 16 | 16 | ND | S | S | H2O2 (3%) + NaCl | PCC | Resorb | Y |
| 4: Roos-Jansåker et al. (2007b) | |||||||||||||
| QE | 12 | 17 | 65.6 ± 7.4 | 70.6 | 29 | 29 | ND | R 1, S 28 | ND | H2O2 (3%) + NaCl | PCC | Resorb | N |
| 19 | 66.3 ± 6.8 | 68.4 | 36 | 36 | R 1, S 35 | PCC | N | ||||||
| 5: Schwarz et al. (2009) | |||||||||||||
| CS | 48 | 9 | 54.4 ± 12.5 | 0 | 9 | 9 | ND | ND | ND | PC + NaCl | AP | N | N |
| 11 | 11 | 10 | XG | Resorb | |||||||||
| 6: Schwarz et al. (2010) | |||||||||||||
| CS | 12 | 27 | 48.5 ± 14.6 | ND | 9 | 9 | 7 mand, 2 max | R | S | IP + CC + NaCl | XG | Resorb | N |
| 9 | 9 | 4 mand, 5 max | R 8, S 1 | ||||||||||
| 9 | 9 | 5 mand, 4 max | R | ||||||||||
| 7: Roccuzzo et al. (2011) | |||||||||||||
| CS | 12 | 12 | 60 ± 8.8 | 16.7 | 12 | 12 | 7 mand, 5 max | R | S | PC + EDTA gel + CHX + NaCl | XG | N | N |
| 14 | 59.9 ± 7.0 | 14.3 | 14 | 14 | 8 mand, 6 max | R | |||||||
| 8: Aghazadeh et al. (2012) | |||||||||||||
| RCT | 12 | 22 | 70.1 ± 6.2 | ND | 22 | 22 | ND | R 44, ND 1 | ND | H2O2 (3%) + NaCl | Auto | Resorb | N |
| 23 | 67.0 ± 7.5 | 70.0 | 23 | 23 | XG | ||||||||
| 9: Froum et al. (2012) | |||||||||||||
| CS | 49 | 15 | 58 | ND | 19 | 19 | ND | R | ND | GC + AA + NaCl + TCN + AA + CHX + NaCl + EMD + PDGF | XG or allograft | Resorb or SCTG | N |
| 23 | 32 | 32 | |||||||||||
| 10: Schwarz et al. (2012) | |||||||||||||
| RCT | 24 | 14 | 62.3 ± 10 | ND | 16 | 14 | ND | R 13, S 1 | ND | IP + PC + NaCl | XG | Resorb | N |
| 10 | 16 | 10 | R 5, S 4, ND 1 | IP + Er:YAG | |||||||||
| 11: Wiltfang et al. (2012) | |||||||||||||
| CS | 12 | 22 | 24-83 | ND | 36 | 36 | 26 mand, 10 max | ND | ND | IP + Etching gel | Auto + XG | N | N |
| 12: Wohlfahrt et al. (2012) a | |||||||||||||
| RCT | 12 | 16 | 65 ± 10 | 31.2 | 16 | 16 | ND | R | ND | TC + EDTA | PTG | N | Y |
The control arms were not listed in this table because they are not reconstructive procedures.
AA, air abrasive; auto, autogenous; BL, baseline; CA, citric acid (pH, 1); CC, carbon curette; CHX, chlorhexidine; CS, case series; EMD, enamel matrix derivatives; FE, final examination; GC, graphite curette; IP, implantoplasty; mand, mandible; max, maxilla; ND, not determined or reported; PC, plastic curette; PCC, phytogenic carbonate calcium; PDGF, platelet-derived growth factor; PTG, porous titanium granules; QE, quasi-experimental studies; R, rough; RCT, randomized controlled trial; resorb, resorbable membrane; S, smooth; SCTG, subepithelial connective tissue graft; TC, titanium curette; TCN, tetracycline; XG, xenograft.
Table 2.
Summary of the Peri-implant Reconstructive Outcomes Investigated of Selected Studies
| Other Clinical Outcomes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radiographic Outcome |
PD, mm |
CAL, mm |
BOP, % |
|||||||
| Initial Bone Loss | Bone Fill, mm | PI Red | In | Red | In | Gain | In | Red | Muc Rec, mm | Complications |
| 1: Khoury and Buchmann (2001) | ||||||||||
| 3.5 ± 3.4 | 2.4 ± 2.7 | ND | 8.0 ± 0.5 | 5.1 ± 2.7 | ND | ND | ND | ND | ND | None |
| 5.1 ± 3.1 | 2.8 ± 3.1 | ND | 8.2 ± 1.0 | 5.4 ± 3.0 | ND | ND | ND | ND | ND | 60% implantsa |
| 6.4 ± 3.2 | 1.9 ± 3.2 | ND | 7.7 ± 0.5 | 2.6 ± 1.6 | ND | ND | ND | ND | ND | 56% implantsb |
| 2: Deppe et al. (2007) | ||||||||||
| 6.8 ± 1.2 | ND | ND | 4.8 ± 1.4 | 2.3 ± 0.5 | 5.9 ± 1.1 | 2.1 ± 0.4 | ND | ND | 0.2 ± 0.6 | Severe infection in 1 patient, resulting in loss of 4 implants |
| 6.7 ± 1.5 | ND | ND | 5.0 ± 1.3 | 2.5 ± 0.5 | 6.3 ± 1.3 | 2.7 ± 0.5 | ND | ND | −0.2 ± 0.6 | Grafts and 4 implants were lost after 10 mo in 1 patient |
| 3: Roos-Jansåker et al. (2007a) | ||||||||||
| 3.8 ± 1.0c | 2.3 ± 1.2 | ND | 5.1 ± 1.6 | 4.2 ± 1.5 | ND | 1.4 ± 1.7 | 81.2 | ND | −2.8 ± 1.4 | Membrane exposure,75.1% |
| 4: Roos-Jansåker et al. (2007b) | ||||||||||
| 3.3 ± 1.1 | 1.5 ± 1.1 | ND | 5.4 ± 1.7 | 2.8 ± 2.0 | 6.8 ± 1.9 | 1.5 ± 2.0 | ND | ND | −1.2 ± 1.5 | Uneventful |
| 2.8 ± 0.8 | 1.4 ± 1.2 | ND | 5.6 ± 1.8 | 3.4 ± 1.5 | 7.0 ± 2.1 | 1.8 ± 1.3 | ND | ND | −1.6 ± 1.6 | Membrane exposure, 87.6% |
| 5: Schwarz et al. (2009) | ||||||||||
| ND | ND | −0.5 ± 0.5 | 6.9 ± 0.6 | 1.1 ± 0.3 | 7.3 ± 0.8 | 0.6 ± 0.5 | 80 | 34 | 0.4 ± 0.5 | Uneventful |
| ND | ND | −0.2 ± 0.6 | 7.1 ± 0.7 | 2.5 ± 0.9 | 7.5 ± 0.9 | 2.0 ± 1.0 | 79 | 51 | 0.5 ± 0.4 | |
| 6: Schwarz et al. (2010) | ||||||||||
| ND | ND | 0.1 ± 0.4 | 6.7 ± 0.7 | 1.6 ± 0.9 | 7.1 ± 0.9 | 1.2 ± 1.1 | 81.5 | 38.9 | 0.4 ± 0.7 | Uneventful |
| ND | ND | 0.1 ± 0.3 | 7.1 ± 0.6 | 1.6 ± 0.7 | 7.5 ± 0.9 | 1.1 ± 0.9 | 83.3 | 25.9 | 0.5 ± 0.5 | |
| ND | ND | −0.2 ± 0.3 | 7.0 ± 0.5 | 2.7 ± 0.7 | 7.5 ± 0.8 | 2.4 ± 1.0 | 85.2 | 61.1 | 0.3 ± 0.6 | |
| 7: Roccuzzo et al. (2011) | ||||||||||
| 3.9 ± 1.6 | 1.9 ± 1.3 | 29.10% | 6.8 ± 1.2 | 3.4 ± 1.7 | ND | ND | 75.0 | 60.4 | ND | Uneventful |
| 3.0 ± 0.9 | 1.6 ± 0.7 | 33.90% | 7.2 ± 1.5 | 2.1 ± 1.2 | ND | ND | 91.1 | 33.9 | ND | |
| 8: Aghazadeh et al. (2012) | ||||||||||
| 5.8 ± 1.7 | 0.2 ± 1.8 | 10.9 ± 30.6 % | 6.0 ± 1.3 | 2.0 ± 1.2 | ND | ND | 87.5 | 44.8 | ND | ND |
| 5.2 ± 1.8 | 1.1 ± 1.9 | 5.9 ± 24.6 % | 6.2 ± 1.4 | 3.1 ± 1.2 | ND | ND | 79.4 | 50.4 | ND | |
| 9: Froum et al. (2012) | ||||||||||
| ND | 3.8 ± 1.5 | ND | 8.8 ± 1.9 | 5.4 ± 1.5 | ND | ND | ND | ND | ND | ND |
| ND | 3.0 ± 0.8 | ND | 7.9 ± 1.8 | 5.1 ± 1.9 | ND | ND | ND | ND | ND | |
| 10: Schwarz et al. (2012) | ||||||||||
| ND | ND | 0.0 ± 0.8 | 5.2 ± 1.5 | 1.5 ± 2.0 | 6.5 ± 2.0 | 1.2 ± 2.2 | 100.0 | 54.9 | 0.3 ± 0.6 | Uneventful |
| ND | ND | 0.2 ± 0.6 | 4.9 ± 1.4 | 1.1 ± 2.2 | 6.4 ± 2.0 | 1.0 ± 2.2 | 96.6 | 75.0 | 0.1 ± 0.4 | |
| 11: Wiltfang et al. (2012) | ||||||||||
| 5.1 ± 2.4d | 3.5 ± 2.4 | ND | ND | 4.0 ± 1.8 | ND | ND | 61 | 36 | 1.3 ± 0.2 | Swelling for 5 days |
| 12: Wohlfahrt et al. (2012) | ||||||||||
| 6.8 ± 2.7 | 2.0 ± 1.7 | ND | 6.5 ± 1.9 | 1.7 ± 1.7 | ND | ND | ND | ND | ND | Uneventful |
Four with dehiscences, 5 with membrane exposures, 2 with fistula, 1 with sequester formation.
Two with dehiscences, 1 with membrane exposure, 2 with sequester formation.
Bone loss measured from first thread.
Bone loss measured from the crest.
BOP, bleeding on probing; CAL, clinical attachment level; in, initial; muc rec, mucosal recession; ND, not determined or reported; PD, probing depth; PI, plaque index; red, reduction.
Study Design and Subject Features
Six case series (Nos. 3, 5-7, 9, 11), 3 quasi-experimental studies (1, 2, 4), and 3 randomized controlled trials (Nos. 8, 10, 12) were included. Of the 3 randomized controlled trials, only 1 (No. 12) evaluated the effectiveness of the reconstructive procedure over the nonreconstructive procedure; the other 2 made comparisons among different reconstructive procedures. This controlled study (No. 12) failed to show significantly more PD and BOP reduction with the use of porous titanium granules, although better radiographic peri-implant defect fill was found. In this study, the power analysis was not based on the defect fill or other clinical measurements but implant stability quotient values; thus, the power of this study for evaluating other clinical parameters remains uncertain.
Including case series, 390 dental implants were treated and followed up between 12 and 63 months (mean follow-up time, 25 months). The age of the patients ranged from 24 to 83 years (No. 11). Six studies reported the smoking status of the patients; the proportion of the smokers varied from 0% (No. 5) to approximately 70% (Nos. 4, 8). Except for study No. 2, the included studies provided information about the sex of the patients. Information about the location of the treated implants was retrievable from only 3 studies (Nos. 6, 7, 11).
Oral Implant Features
One study (No. 11) did not report information about the features of the treated implants; 2 other studies (Nos. 8, 10) reported nonidentifiable features for some implants. In one study (No. 3), the treated implants had smooth surfaces, while in 4 other studies (Nos. 1, 7, 9, 12), only rough surface implants were included. In 2 studies (Nos. 2, 5), because of a lack of information about the failed implants that were excluded at the final examination, no information about the implant features was retrievable. The implant platforms were reported to be smooth in 5 studies (Nos. 1, 3, 4, 6, 7), whereas the other studies did not describe the platform features.
Defect Features
Defect depths were radiographically measured in 6 studies (Nos. 1, 2, 7-9, 12) from the implant platform to the base of the defects. The mean initial defect depths ranged from 3.0 mm (No. 7) to 6.8 mm (No. 12). In 2 studies (Nos. 3, 4), the reference point was the first thread of the implants. All included studies reported the mean initial PD, which ranged from 4.8 mm (No. 2) to 8.8 mm (No. 9). Six studies (Nos. 2-6, 10) measured the mean initial CAL, with a range of 5.9 mm (No. 2) to 7.5 mm (No. 6). Eight studies (Nos. 3-8, 10, 11) reported the mean initial BOP, with a range of 61% (No. 11) to 100% (No. 10).
Surgical Features
All studies used bone grafting materials, including autografts (Nos. 1, 2, 8), a combination of autografts and xenografts (No. 11), allografts (No. 10), xenografts (Nos. 5-7, 8-10), and others (Nos. 3, 4, 12). Membranes were commonly applied, nonresorbable (Nos. 1, 2) and resorbable (Nos. 1, 3-6, 8-10), while no membranes were used in some or all patients in 6 studies (Nos. 1, 4, 5, 7, 11, 12). Four studies (Nos. 1-3, 12) submerged the implants during the healing period, and the remaining did not.
Results of the Meta-analysis
The forest plots of the meta-analysis for RDF, PD reduction, CAL gain, and BOP reduction are demonstrated in Figures 1 -4. The WM RDF, calculated from 8 studies (Nos. 1, 3, 4, 7-9, 11, 12), was 2.17 mm (95% CI: 1.46-2.87 mm). All included articles reported the amount of PD reduction, with the WM being 2.97 mm (95% CI: 2.38-3.56 mm). Eleven articles (except for No. 11) provided initial PD; therefore, the percentage of PD reduction was available, equated as the amount of PD reduction divided by the amount of initial PD. The WM percentage of PD reduction was 45.5% (95% CI: 35.9%-55.5%) (Appendix Fig. 2). Six articles (Nos. 2-6, 10) reported the amount of CAL gain, and the WM was 1.65 mm (95% CI: 1.17-2.13 mm). Likewise, the WM percentage of CAL gain was 24.7% (95% CI: 18.4%-32.3%) (Appendix Fig. 3).
Figure 1.
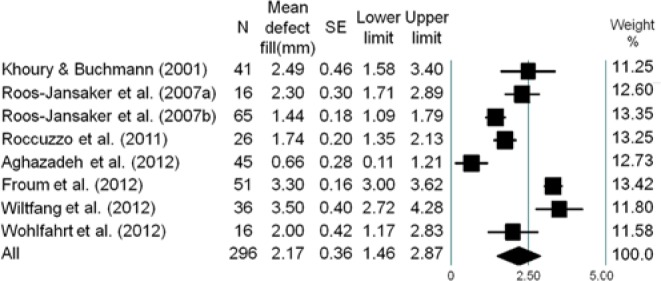
Meta-analysis for the amount of defect fill among selected studies. The weighted mean was 2.17 mm (range, 0.66-3.50 mm), with a 95% confidence interval of 1.46 mm to 2.87 mm.
Figure 2.
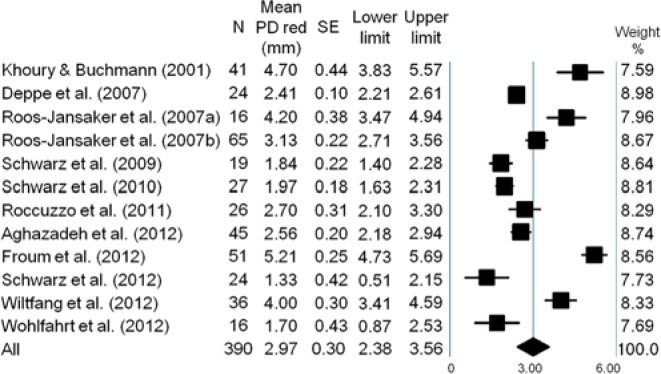
Meta-analysis for probing depth reduction among selected studies. The weighted mean was 2.97 mm (range, 1.33-5.21 mm), with a 95% confidence interval of 2.38 mm to 3.56 mm.
Figure 3.
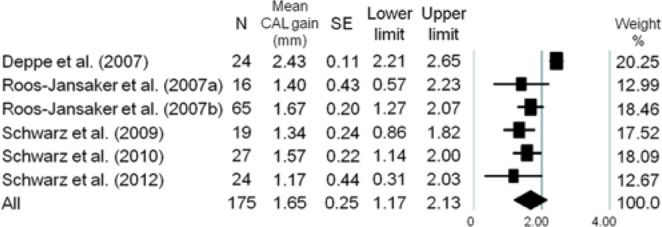
Meta-analysis for clinical attachment level gain among selected studies. The weighted mean was 1.65 mm (range, 1.17-2.43 mm), with a 95% confidence interval of 1.17 mm to 2.13 mm.
Figure 4.
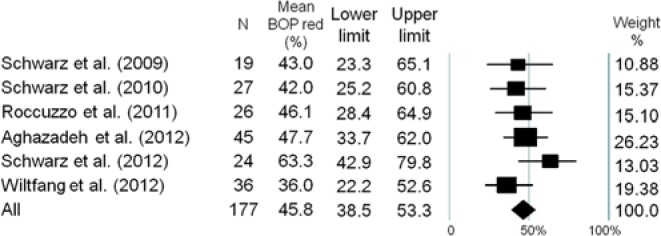
Meta-analysis for the amount of bleeding on probing reduction among selected studies. The weighted mean was 45.8% (range, 36.0%-63.3%), with a 95% confidence interval of 38.5% to 53.3%.
The percentage of BOP reduction was reported in 6 articles (Nos. 5-8, 10, 11), with the WM being 45.8% (95% CI: 38.5%-53.3%). Regarding mucosal recession, 1 study (No. 3) was not included for the meta-analysis due to reporting an outlier value (mean gain, 2.8 mm). The WM mucosal margin, calculated from the other available 6 studies, was 0.17 mm (95% CI: –0.51 to 0.84 mm) (Appendix Fig. 4).
Based on the aforementioned criteria in the Materials & Methods section, the included randomized controlled trial (No. 12) was with moderate risk of bias. Funnel plots evaluating the publication bias of each parameter were prepared (see Appendix Fig. 5). Because of inadequate controlled studies, comparisons among different bone grafting materials, membrane types, and healing protocols were deemed impossible and not intended.
Discussion
The effort to answer the first focused question fell short because only 1 comparative study (Wohlfahrt et al., 2012) was available. As an alternative, the present review evaluated the overall clinical and radiographic performances of the reconstructive procedures. This meta-analysis shows that the mean RDF achieved after reconstructive procedures was 2.17 mm. The lowest bone fill—0.2 mm and 1.1 mm with the use of autografts and xenografts, respectively—was reported by a controlled study (Aghazadeh et al., 2012). This difference might be a result of the radiopaque nature and slow resorption rate of the xenograft. It is noteworthy that the inclusion of a large number of smokers (70%) might have contributed to the observed unfavorable outcomes. The highest values were reported to be approximately 3.5 mm in 2 case series (Froum et al., 2012; Wiltfang et al., 2012). However, this above-average amount of defect fill should be interpreted with caution because identifying the first bone-to-implant contact is difficult when xenografts are used because of their radiopaque nature. The methods to decontaminate implant surfaces, including the use of air abrasives and the use of biological agents (enamel matrix derivatives and platelet-derived growth factors) in combination with bone grafts (Froum et al., 2012), might be associated with higher bone fill. The use of “xenograft derived bone substitutes with autografts” (Wiltfang et al., 2012) yielded an above-average defect fill. Defect features (Schwarz et al., 2010), implant surfaces (Roccuzzo et al., 2011), and grafting materials (Aghazadeh et al., 2012) are also factors that may explain the variability in defect fill among the included studies.
A variety of bone graft materials was used in the included studies. Two studies (Schwarz et al., 2009; Aghazadeh et al., 2012) evaluated the effect of different bone materials on the amount of RDF. The results showed that xenografts provided more RBF than autogenous grafts (Aghazadeh et al., 2012). The better outcome obtained from xenografts might be attributed to slower resorption rate of the material and the radiopaque property. In another (Schwarz et al., 2009), a poorer outcome was obtained with hydroxyapatite particles than xenografts in long term. Because of limited publications and lack of controlled studies, comparisons with an aim to explore the most effective material were not feasible. Therefore, at this moment, the choice of optimal bone grafting materials is unclear and is primarily determined by surgeon preference to provide good space-making and bone stimulatory activities.
Whether the use of membranes might have an adjunctive effect on defect fill is equivocal. Two studies (Khoury and Buchmann, 2001; Roos-Jansaker et al., 2007b) compared RDF with and without membranes. The best results were obtained by the use of nonresorbable membranes (2.8 ± 3.1 mm), followed by no membrane use (2.4 ± 2.7 mm) and the use of absorbable membranes (1.9 ± 3.2 mm) (Khoury and Buchmann, 2001). However, no statistical significant differences were found among the 3 approaches at the end of this 3-year study. No benefit was found by adding absorbable membranes. These results are consistent with preclinical studies (Nociti et al., 2001; Schou et al., 2003). High exposure rate of membranes might have washed out the potential beneficial effects from the use of these occlusive materials. The exposure rate was reported to be as high as 87.6%; therefore, wound closure appeared to be key in the promotion of better reconstructive outcomes.
The use of different reference points for measuring the features of the bony defects might influence the interpretation of the results and make comparisons among citations more challenging. The points that were used included the implant platform (Khoury and Buchmann, 2001; Deppe et al., 2007; Roccuzzo et al., 2011; Aghazadeh et al., 2012; Froum et al., 2012; Wohlfahrt et al., 2012), the first thread (Roos-Jansaker et al., 2007a, 2007b), and the bone crest (Schwarz et al., 2009; Schwarz et al., 2010; Schwarz et al., 2012; Wiltfang et al., 2012). It might be preferable to describe both the supracrestal and the subcrestal components of bony defects so that the changes of the crestal location in relation to the implant platform and the amount of defect fill at the bottom of the defects can be readily evaluated. In addition, whether the amount of defect fill is positively correlated with the initial defect depth is an interesting subject. If proven so, the amount of bone gain might be estimated on the basis of initial defect depth.
RDF is accompanied by improved clinical parameters, including PD reduction, CAL gain, and BOP reduction. A moderate correlation was found between RDF and PD reduction. Based on samples from this systematic review, an estimated 56% of the variations in PD reduction can be explained by RDF in a linear model. Resolution of inflammation and restoration of collagen content also contribute to PD reduction (Lang and Berglundh, 2011). The mucosal margin most likely stays at the same level after reconstructive procedures, as shown in this systematic review. In this regard, in the esthetic zone, reconstructive procedures might be more desirable than resective procedures.
Limitations of this meta-analysis are as follows. First, most of the included manuscripts were case series with small sample sizes and short follow-up periods. Second, there were inconsistencies in methodologies, various treatment modalities, different implant systems, and heavier contributions from the same research group. Therefore, there is a substantial need for randomized controlled studies with proper design and powerful sample size, comparing the reconstructive treatment to open debridement and resective approach, to provide stronger evidence of the possible benefits of the reconstructive procedures for treating peri-implantitis.
Conclusions
No evidence in the literature is currently available to compare the clinical effectiveness of reconstructive and nonreconstructive procedures. Studies without a proper control arm showed that reconstructive procedures for management of peri-implantitis resulted in a mean RDF of 2.17 mm, accompanied by improvement of other clinical parameters. Factors that might influence the reconstructive outcomes include systemic conditions of the patients, defect features, methods to detoxify the implant surfaces, types of bone grafts, and uses of membranes. No superior grafting material or membrane could be identified because of lack of controlled studies. Controlled studies are needed to investigate the effect of biological agents, various bone grafts and detoxification methods, and flap management strategies to enhance the reconstructive outcomes. From a clinical management point of view, the reconstructive procedure is one of several treatment options that may be considered, provided with prudent evaluation of systemic and local factors of the patients affected by peri-implantitis; nonetheless, it should be stressed that there is no available evidence in the literature to show that reconstructive procedures with the use of bone grafts and/or membranes provide better treatment outcomes than nonreconstructive procedures.
Acknowledgments
We would like to acknowledge Professor Kerby A. Shedden, director of the Center for Statistical Consultation and Research, the University of Michigan, for providing statistical consultation.
Footnotes
This article was partially supported by the University of Michigan Periodontal Graduate Student Research Fund.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aghazadeh A, Rutger Persson G, Renvert S. (2012). A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol 39:666-673 [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Buser D, Sennerby L. (2012). Crestal bone loss and oral implants. Clin Implant Dent Relat Res 14:783-791 [DOI] [PubMed] [Google Scholar]
- Aljateeli M, Fu JH, Wang HL. (2012). Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res 14(suppl 1):e109-e118 [DOI] [PubMed] [Google Scholar]
- Deppe H, Horch HH, Neff A. (2007). Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants 22:79-86 [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Worthington HV. (2012). Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane Database Syst Rev 1:CD004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson C, Wennstrom J, Tomasi C, Berglundh T. (2009). Extent of peri-implantitis-associated bone loss. J Clin Periodontol 36:357-363 [DOI] [PubMed] [Google Scholar]
- Fransson C, Tomasi C, Pikner SS, Grondahl K, Wennstrom JL, Leyland AH, et al. (2010). Severity and pattern of peri-implantitis-associated bone loss. J Clin Periodontol 37:442-448 [DOI] [PubMed] [Google Scholar]
- Froum SJ, Froum SH, Rosen PS. (2012). Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent 32:11-20 [PubMed] [Google Scholar]
- Higgins JP, Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration URL accessed on 9/25/2013 at: http://www.cochrane-handbook.org
- Khoury F, Buchmann R. (2001). Surgical therapy of peri-implant disease: a 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J Periodontol 72:1498-1508 [DOI] [PubMed] [Google Scholar]
- Koldsland OC, Scheie AA, Aass AM. (2010). Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol 81:231-238 [DOI] [PubMed] [Google Scholar]
- Lang NP, Berglundh T; Working Group 4 of Seventh European Workshop on Periodontology (2011). Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38(suppl 11):178-181 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-e34 [DOI] [PubMed] [Google Scholar]
- Mir-Mari J, Mir-Orfila P, Figueiredo R, Valmaseda-Castellón E, Gay-Escoda C. (2012). Prevalence of peri-implant diseases: a cross-sectional study based on a private practice environment. J Clin Periodontol 39:490-494 [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Machado MA, Stefani CM, Sallum EA. (2001). Absorbable versus nonabsorbable membranes and bone grafts in the treatment of ligature-induced peri-implantitis defects in dogs: a histometric investigation. Int J Oral Maxillofac Implants 16:646-652 [PubMed] [Google Scholar]
- Roccuzzo M, Bonino F, Bonino L, Dalmasso P. (2011). Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol 38:738-745 [DOI] [PubMed] [Google Scholar]
- Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. (2007a). Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a prospective cohort study. J Clin Periodontol 34:625-632 [DOI] [PubMed] [Google Scholar]
- Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. (2007b). Submerged healing following surgical treatment of peri-implantitis: a case series. J Clin Periodontol 34:723-727 [DOI] [PubMed] [Google Scholar]
- Schou S, Holmstrup P, Jørgensen T, Stoltze K, Hjørting-Hansen E, Wenzel A. (2003). Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis: I. Clinical and radiographic observations in cynomolgus monkeys. Clin Oral Implants Res 14:391-403 [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D; CONSORT Group (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Sahm N, Bieling K, Becker J. (2009). Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol 36:807-814 [DOI] [PubMed] [Google Scholar]
- Schwarz F, Sahm N, Schwarz K, Becker J. (2010). Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol 37:449-455 [DOI] [PubMed] [Google Scholar]
- Schwarz F, John G, Mainusch S, Sahm N, Becker J. (2012). Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination: a two-year clinical follow up report. J Clin Periodontol 39:789-797 [DOI] [PubMed] [Google Scholar]
- Wiltfang J, Zernial O, Behrens E, Schlegel A, Warnke PH, Becker ST. (2012). Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: a series of 36 defects. Clin Implant Dent Relat Res 14:421-427 [DOI] [PubMed] [Google Scholar]
- Wohlfahrt JC, Lyngstadaas SP, Rønold HJ, Saxegaard E, Ellingsen JE, Karlsson S, et al. (2012). Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int J Oral Maxillofac Implants 27:401-410 [PubMed] [Google Scholar]
- Zitzmann NU, Berglundh T. (2008). Definition and prevalence of peri-implant diseases. J Clin Periodontol 35(8):286S-291S [DOI] [PubMed] [Google Scholar]


