Abstract
The aim of this study was to compare the release of bone markers during osseointegration of immediately loaded and nonloaded implants. Forty patients who were indicated for rehabilitation with dental implants randomly received either implant and prosthesis placement within 72 hours (group IM) or implant insertion and no prosthesis placement (group NL). Peri-implant crevicular fluid was collected immediately after implant insertion and 7, 15, 30, 60, 90, and 120 days after surgery and levels of osteoprotegerin, transforming growth factors, osteocalcin, osteopontin, and parathyroid hormone were evaluated using Luminex assay. Bleeding index and peri-implantar sulcus depth were also evaluated. The data were compared using statistical tests (α = 5%). No statistical difference was found regarding demographic and clinical parameters (p > .05). Transforming growth factors, osteoprotegerin, osteopontin, and parathyroid hormone presented an earlier release peak in group IM than in NL group (p < .05). Osteocalcin achieved higher levels in group IM versus group NL between 7 and 30 days of evaluation (p < .05). It may be concluded that earlier loading positively modulates bone mediators release around immediately loaded implants when compared with nonloaded dental implants (ClinicalTrials.gov NCT01909999).
Keywords: osseointegration, osteogenesis, biological markers, implant-supported dental prosthesis, immediate dental implant loading, edentulous jaws
Introduction
Aesthetic and functional rehabilitation using dental implants is an alternative for the treatment of edentulous areas with high success rates. Based on knowledge about the events of osseointegration and in an endeavor to reduce the waiting period before rehabilitation, the immediate loading protocol was developed, reducing healing time and allowing prosthetic placement after implant insertion (Chiapasco et al., 2006; Goiato et al., 2012). This technique has become an attractive option for the rehabilitation of edentulous patients, providing greater psychological and functional patient satisfaction, with success rates ranging from 85% to 100% (Chiapasco et al., 2006; Goiato et al., 2012). However, although studies have evaluated the clinical success of immediately loaded implants, little is known about the molecular events associated with early loaded dental implants in humans.
Animal studies suggested that in the presence of loading, osseointegration can occur early (Branemark et al., 1999) with greater deposition of mineralized tissue around the implant (Ogawa et al., 2011). This phenomenon suggests that functional stimulation could alter the osseointegration process through the release of molecules that act as modulators of osteogenesis and osteoclastogenesis. However, the assessment of bone markers’ concentration and their release kinetics has not yet been evaluated during osseointegration, particularly following immediate implant loading. Thus, the aim of this study was to compare the levels of transforming growth factor α (TGFα), osteoprotegerin (OPG), osteocalcin (OCN), osteopontin (OPN), and parathyroid hormone (PTH) in the peri-implant crevicular fluid of immediately loaded and nonloaded implants during osseointegration.
Materials & Methods
Population and Clinical Strategy
The population of this prospective, parallel, randomized controlled clinical laboratory trial was recruited from the patients referred to Paulista University. The study was conducted within the standards of the ethics committee (10280251000-11), and all participants signed the informed consent form.
Subject recruitment started March 2010. The clinical procedures were carried out between June 2010 and October 2012. Data entry and statistical analyses were performed December 2012. The inclusion criteria were as follows: patients with mandibular and/or maxillary edentulous arch indicated for rehabilitation with implants; extractions occurring at least 4 months before treatment; good oral hygiene (plaque index < 20%) (Ainamo & Bay, 1975); age between 18 and 65 years. The exclusion criteria were as follows: presence of systemic disease (including diabetes, arthritis, hypothyroidism, hyperparathyroidism, osteoporosis, etc.), use of medication that contraindicated placement or altered implant osseointegration (including anti-inflammatories) 6 months before surgery; bone grafts in the area of the implant; pregnant or breastfeeding women; smokers or ex-smokers.
For random allocation, a computer-generated list was created (under responsibility of R.C.V.C.), and immediately after implant installation, the patient and examiner were advised of which type of rehabilitation was to be performed, according to groups:
IM group (n = 20): patients who received placement of implants and prosthesis within 3 days, characterizing the immediately loaded protocol.
NL group (n = 20): patients who received single-stage dental implants with no placement of prostheses within a period of 4 months, characterizing the nonloaded implants.
The surgeries (performed by A.J.P./G.P.P.), as well as all postoperative follow-up, were performed at the dental clinic of Paulista University. Surgical areas were anesthetized (2% mepivacaine/1:100,000 epinephrine), and mucoperiosteal incisions in the alveolar-ridge mucosa were made. The surgical sequence follows the protocol described by the implant company (SIN, São Paulo, Brazil), which required torque within 32 to 60N. Six implants were installed for maxilla rehabilitation and 5 for mandible. Sutures were done with interrupted sutures using absorbable polygalactin-910. Postoperative care was as follows: amoxicillin, 500 mg (every 8 hours/7 days); sodic-dipyrone, 500 mg (every 6 hours/3 days); 0.12% chlorhexidine mouthwash (every 12 hours/10 days).
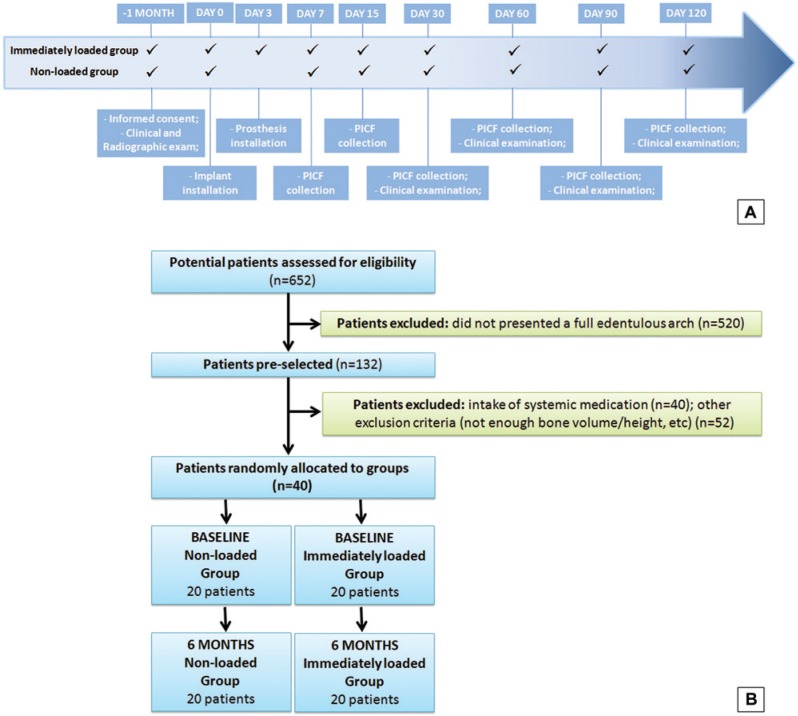
The patients in the IM group received Brånemark full-arch prosthesis within 3 days after the implant (Figure 1A). All prosthesis used straight miniabutments (SIN) and received occlusal adjustment and clinical monitoring.
Figure 1.
Timeline (A) and flowchart (B) of the study.
Clinical Evaluation
Clinical examination of implants was performed using a plastic probe (Colorvue, Hu-Friedy, Chicago, IL) by a single examiner (A.J.P., intraclass correlation = 85%) at the 2 intermediary implants of each side. Four regions per implant (mesial-buccal/lingual, distobuccal/lingual] were assessed regarding:
Peri-implant sulcus depth: distance from the margin of the peri-implant mucosa to the bottom of the peri-implant sulcus.
Modified bleeding on probing index: the presence or absence of bleeding after 10 seconds on probing.
For both groups, the implants were evaluated at 30, 60, 90, and 120 days (Figure 1A). Moreover, the opposite arch was screened regarding the number of remaining teeth and alveolar bone loss (Buhlin et al., 2011).
Evaluation of Bone Markers
The collection of peri-implant crevicular fluid was performed immediately after implant insertion (baseline) and after 7, 15, 30, 60, 90, and 120 days, at the same 2 implants, by the same operator (A.J.P.) (Figure 1A). The site was dried and isolated, and absorbent paper strips (Periopaper, Oralflow, Smithtown, New York) were inserted in peri-implant sulcus, until resistance, for 30 seconds at 4 sites per implant, and conditioned in separate tubes. The volume of fluid was measured (Periotron, Oraflow) and conditioned at PBS/Tween.
The levels of the bone markers (OPG, OCN, OPN, TGFα, and PTH) were determined using the LUMINEX/Magpix system (HBN1A-51K and HCCBP1MAG-58K, Millipore Corporation, Billerica, Massachusetts). The samples were analyzed individually, and the levels were estimated using a 5-parameter polynomial curve (Xponent software, Millipore Corporation). All results were adjusted for peri-implant crevicular fluid volume collected in each implant, and values were expressed in pg/mL. A mean of the 2 implants was considered the value per patient.
Data Analysis
The null hypothesis considered in the study was the absence of difference in the bone markers’ concentration between the IM and NL groups. Initially, the data were analyzed for homogeneity using the Shapiro-Wilk test. After that, the data were compared between groups using Student t test (age), Fisher exact test (sex, arch, modified bleeding on probing index), analysis of variance and Tukey (peri-implant sulcus depth), and Friedman and Mann-Whitney tests (bone markers). All analyses were done using SAS 9.1 (SAS Institute Inc., Cary, North Carolina), considering the patient as experimental unit and α = 5%.
Results
All patients completed the follow-up (Figure 1B). Table 1 shows the data concerning sex, age, and implants’ distribution, and no statistically significant differences were observed (p > .05). Moreover, no significant differences were seen regarding number of remaining teeth and alveolar bone loss (p > .05). The values for the modified bleeding on probing index and peri-implant sulcus depth indicate no statistical difference between groups (Table 2) (p > .05).
Table 1.
Demographic and Clinical Data of Subjects Included in the Study
| Group | |||
|---|---|---|---|
| Immediately Loaded Implants | Nonloaded Implants | p | |
| Age, ya | 58.9 ± 4.7 | 52.0 ± 9.5 | .06 |
| Femaleb | 55 (11) | 73 (15) | .69 |
| Maxillary archb | 25 (5) | 40 (8) | .46 |
| No. of teeth at opposite archa | 5.7 ± 5.7 | 7.4 ± 5.5 | .40 |
| Radiographic bone loss of remaining teethc | 1 (1.38, 1.50) | 1 (1.25, 1.62) | .48 |
| Implant success of installed implantsb | 100 | 100 | 1.00 |
Student t test (p < .05), mean ± standard deviation.
Fisher exact test (p < .05), % (n).
Mann-Whitney test (p < .05), median (95% confidence interval).
Table 2.
Peri-implant Sulcus Depth and Modified Bleeding on Probing Index in Implants of Immediately Loaded and Nonloaded Groups After Implant Placement
| Days After Implant Placement | ||||
|---|---|---|---|---|
| Group | 30 | 60 | 90 | 120 |
| Peri-implant sulcus depth, mm | ||||
| Immediately loaded | 3.3 ± 0.6c | 2.2 ± 0.3d | 2.2 ± 0.4d | 2.1 ± 03d |
| Nonloaded | 3.0 ± 0.6c | 2.6 ± 0.4d | 2.3 ± 0.5d | 2.1 ± 0.3d |
| p a | .32 | .06 | .31 | .96 |
| Modified bleeding on probing index, % | ||||
| Immediately loaded | 53.3c | 6.7d | 13.3d | 6.7d |
| Nonloaded | 66.7c | 26.7d | 20.0d | 6.7d |
| p b | .71 | .33 | 1.00 | 1.00 |
Analysis of variance/Tukey.
Fisher exact test.
Distinct lowercase letters (c,d) indicate significant differences among time intervals within each group (p < .05).
Bone Markers
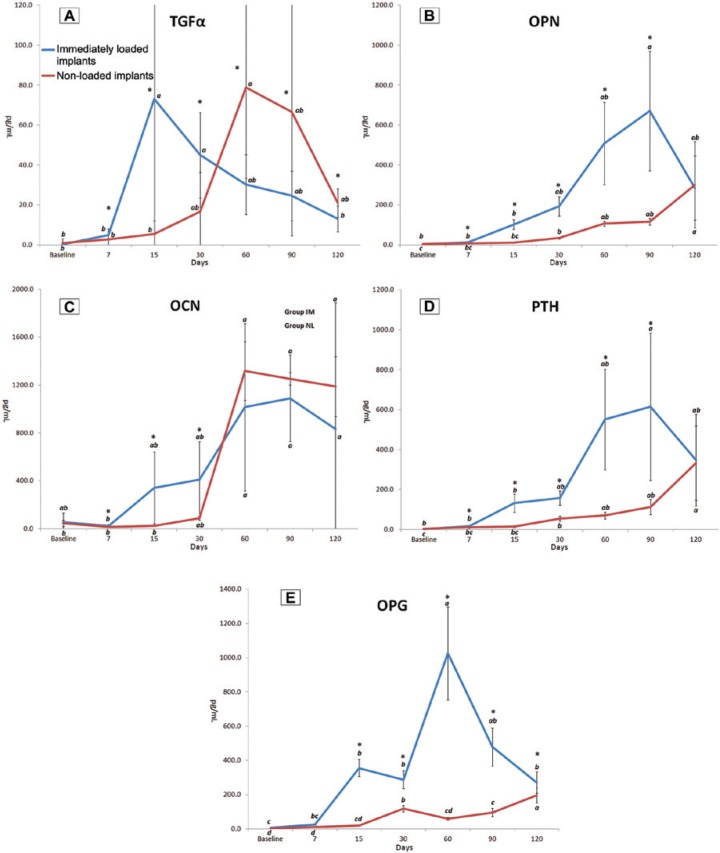
At 7 days, the intergroup analyses showed that TGFα, OPN, OCN, and PTH levels were higher in the IM group versus the NL group (p < .05) (Figure 2A -2D). These levels remained augmented in the IM implants until 30th day for TGFα and OCN and until 90th day for OPN and PTH when compared with the nonloading implants (p < .05). OPG concentration became higher in the IM group at 15 days when compared with the other treatment modality and remained more pronounced until 120 days (p < .05) (Figure 2E). Conversely, intergroup comparisons demonstrated a reversal in this pattern of response—that is, higher levels of TGFα in the NL group, revealing a higher concentration of this mediator at 60, 90, and 120 days in the nonloading implants when compared with IM ones (p < .05).
Figure 2.
Levels of transforming growth factor α (TGFα) (A), osteopontin (OPN) (B), osteocalcin (OCN) (C), parathyroid hormone (PTH) (D), and osteoprotegerin (OPG) (E) in the peri-implant fluid (pg/mL) in immediately loaded and nonloaded implants during the evaluation periods.
Asterisk (*) indicates a statistically significant difference between the groups (Mann-Whitney test, p < .05); lowercase letters indicate a statistically significant difference among the periods of evaluation within each group (Friedman test, p < .05).
Regarding the IM group, intragroup analyses showed that the first marker released in these sites was TGFα, demonstrating a peak release on the 15th day and remaining elevated until the 30th day, as compared with baseline values (p < .05). OPN and PTH levels were augmented at 90 days, whereas OPG presented elevated levels from 15 to 120 days (peak concentration at 60th day), when compared with baseline concentrations (p < .05). Levels of OCN were higher from 60 to 120 days when compared with 7th day (p < .05). Concerning the NL group, intragroup analyses revealed that TGFα and OCN levels were higher at 60 days, whereas OPN and PTH concentrations were higher from 30 to 120 days (peak at 120th day), when compared with the baseline (p < .05). Levels of OPG presented elevated values from baseline at 30, 90, and 120 days (p < .05). Figure 2 shows bone markers outcomes.
Discussion
Immediately loaded implants are an alternative in the rehabilitation of edentulous patients. However, to date, only animal model studies have been conducted in an attempt to understand the impact of immediate loading on osseointegration. So, this study evaluated the release of bone markers during osseointegration, and the results indicate that the presence of a functional load modulates the bone markers’ release.
Several preclinical studies assessing the impact of loading on the implants’ osseointegration showed that the replacement of the old bone occurred more rapidly and with greater bone-implant contact (Vandamme et al., 2007; Esaki et al., 2012; Blanco et al., 2013; Yamamoto et al., 2013). These conclusions could be associated with the altered release of bone markers in IM implants, which are seen in the present study.
The first bone marker presenting a peak of release was TGFα, in the beginning of osseointegration in the IM group (15 days), while the peak release in nonloading implants did not occur until the 60th day. To understand the role of TGFα, it is important to remember that during the first stages of osseointegration, a fibrin-rich vascularized matrix populated by fibroblast-like cells is created (Berghlund et al., 2003), providing a template for future bone (Mackie et al., 2008). TGFα controls the factors involved in the conversion of initial matrix to bone. TGFα directly affects runt-related transcription factor 2 (RUNX2) production in chondrocytes; mediates the release of matrix metalloproteinases 9, 13, and 14; and stimulates vessel formation, as well as patterns of matrix removal (Usmani et al., 2012). Additionally, the TGF family is linked to osteoblastic proliferation, differentiation, activity, and collagen synthesis (Stein et al., 1993). Interestingly, this action during the initial phase of osteogenesis could be noted in our results: after the peak, TGFα levels significantly decrease. This result suggests that loading stimulates the first stage of ossification and, consequently, the next steps occur faster.
This idea is confirmed by the levels of OPN, which is related to the binding of basic elements to the extracellular bone matrix, and OCN, responsible to the calcium-ion binding to the same matrix (Nilsson et al., 1999; Sato et al., 2011). Both markers, important in matrix mineralization, were released early in the presence of loading. Sato et al. (2011) also found higher OCN RNAm levels in the bone tissue around the immediately loaded implants placed in dogs, while Pavlin et al. (2001) identified OCN as a mechanically responsive gene, increasing its concentration during stimulus. Furthermore, OPN showed significantly higher levels throughout the osseointegration, suggesting greater activity during bone mineralization.
An interesting result of the current trial was the increased and earlier release of PTH and OPG in IM implants when compared with NL ones. OPG binds to RANKL and prevents binding to its membrane receptor (RANK) present in the preosteoclasts. This way, OPG could be considered a modulator of bone maturation and resorption (Belibasakis & Bostanci, 2012). The results showed that the presence of loading promoted OPG release, indicating a predominant osteoblastic activity. However, the most interesting point is the coincidence of the OPG and PTH release peaks. PTH—controlling the levels of serum, calcium, and phosphorus—initially and rapidly acts on the bone formation because of its receptor present in the membrane of the osteoblastic cells (Pierroz et al., 2010). However, secondarily and more durably, it is responsible for bone resorption due to the increase in the osteoclast population by increasing RANKL, inducing preosteoclasts turn to osteoclasts (Pierroz et al., 2010). Meanwhile, recent literature has shown that the adjunctive action of OPG-PTH could shift the bone turnover toward an osteogenic environment. Animal studies indicate that OPG-PTH combination is effective in inhibiting PTH-induced osteoclast activity and could augment the effects of PTH on bone mass while preventing PTH-associated hypercalcemia (Kostenuik et al., 2001; Redlich et al., 2004; Padagas et al., 2006; Pierroz et al., 2010). This could be related to the fact that OPG eliminates the PTH receptor active in osteoclasts, a potential therapy in severe osteoporosis (Valenta et al., 2005). In this context, the simultaneous elevation of both markers and the knowledge of an increased bone deposition in loaded implants could lead to new insight on changes during bone formation around IM implants.
Based on all these points, the application of force during osseointegration changes the metabolism and action of cells involved in the process of osteogenesis. At the implant interface, osteoblasts and osteocytes act as force transducers, which could lead to bone apposition and an initiate remodeling phase (Sato et al., 2011). Studies showed that loading on the bone regulates the activation of osteocytes, which has a mechanism for a precise targeting of osteoclasts for bone adaptation (Noble et al., 2003), blood circulation stimulation, and improved bone remodeling (Myata et al., 2000). Berglundh et al. (2003) and Yamamoto et al. (2013) showed that functional loading on newly inserted implants caused high cell activity and, in a few days, newly formed bone could be seen around implants.
This knowledge and the possibility of a noninvasive form to assess the events occurring around implants could lead to future studies for a better understanding of osseointegration and/or the peri-implantar conditions. This idea was the target of studies trying to determine if the evaluation of some proteins could be used. Tatarakis et al. (2013), assessing the levels of OPG in the saliva of diabetic and nondiabetic subjects before and after implant installation, observed a reduction in OPG levels occurring in the former and an increase in the latter. This result, however, was not correlated with clinical signs. At the same time, Arikan et al. (2008, 2011) observed that OPG is a potential marker to assess implant conditions and its local levels may help to distinguish diseased and healthy implants. Another marker that could represent the destruction of tissues—pyridinoline cross-linked carboxyterminal-telopeptide of type I collagen (ICTP)— has been evaluated in previous clinical studies. However, although the increase of ICTP levels in some species was associated with periodontal disease progression (Oringer et al., 1998), although its local levels increased after regenerative treatment (Sarment et al., 2006), and although its levels were higher in peri-implant lesions than in healthy implants (Arikan et al., 2011), future studies should still be done to confirm ICTP and other proteins as potential biomarkers.
Finally, another important discussion regarding the use of markers is their usefulness in clinics and research. Remember that, to date, there is no clinical, radiologic, or biochemical marker that can precisely determine the quality or the impact of different conditions (loading, type of implant, systemic diseases, etc.) during osseointegration. The absence of a definitive marker is probably due to the difficulty in assessing, in humans, the bone’s formation without disturbing the process, notwithstanding the ethical aspects related to this analysis. In this vein, this could be considered a limitation of this study. So, future studies should try to correlate the findings of the present study with the clinical or radiographic assessments of bone repair around dental implants. At any rate, these changes in the profile of bone activity found in IM implants are in agreement with the previously discussed histologic studies (Noble et al., 2003; Berglundh et al., 2003; Yamamoto et al., 2013).
In conclusion, immediate loading promotes a higher and accelerated release of bone mediators around implants when compared with nonloaded implants. This result, however, should be confirmed and deeply explored in future studies.
Footnotes
This study was partially funded by Paulista University.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ainamo J, Bay I. (1975). Problems and proposals for recording gingivitis and plaque. Int Dent J 25:229-235 [PubMed] [Google Scholar]
- Arikan F, Buduneli N, Kütükçüler N. (2008). Osteoprotegerin levels in peri-implant crevicular fluid. Clin Oral Implants Res 19:283-288 [DOI] [PubMed] [Google Scholar]
- Arikan F, Buduneli N, Lappin DF. (2011). C-telopeptide pyridinoline crosslinks of type I collagen, soluble RANKL, and osteoprotegerin levels in crevicular fluid of dental implants with peri-implantitis: a case-control study. Int J Oral Maxillofac Implants 26:282-289 [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. (2012). The RANKL-OPG system in clinical periodontology. J Clin Periodontol 39:239-248 [DOI] [PubMed] [Google Scholar]
- Berglundh T, Abrahamsson I, Lang NP, Lindhe J. (2003). De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res 14:251-262 [DOI] [PubMed] [Google Scholar]
- Blanco J, Mareque S, Liñares A, Pérez J, Muñoz F, Ramos I. (2013). Impact of immediate loading on early bone healing at two-piece implants placed in fresh extraction sockets: an experimental study in the beagle dog. J Clin Periodontol 40:421-429 [DOI] [PubMed] [Google Scholar]
- Brånemark PI, Engstrand P, Ohrnell LO, Gröndahl K, Nilsson P, Hagberg K, et al. (1999). Brånemark Novum: a new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow-up study. Clin Implant Dent Relat Res 1:2-16 [DOI] [PubMed] [Google Scholar]
- Buhlin K, Mäntylä P, Paju S, Peltola JS, Nieminen MS, Sinisalo J, et al. (2011). Periodontitis is associated with angiographically verified coronary artery disease. J Clin Periodontol 38:1007-1014 [DOI] [PubMed] [Google Scholar]
- Chiapasco M, Gatti C. (2003). Implant-retained mandibular overdentures with immediate loading: a 3- to 8-year prospective study on 328 implants. Clin Implant Dent Relat Res 5:29-38 [DOI] [PubMed] [Google Scholar]
- Esaki D, Matsushita Y, Ayukawa Y, Sakai N, Sawae Y, Koyano K. (2012). Relationship between magnitude of immediate loading and peri-implant osteogenesis in dogs. Clin Oral Implants Res 23:1290-1296 [DOI] [PubMed] [Google Scholar]
- Goiato MC, Bannwart LC, Pesqueira AA, Dos Santos DM, Haddad MF, Santos MR, et al. (2013). Immediate loading of overdentures: systematic review [published online July 5, 2013]. Oral Maxillofac Surg. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Capparelli C, Morony S, Adamu S, Shimamoto G, Shen V, et al. (2001). OPG and PTH-(1-34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology 142:4295-4304 [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. (2008). Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40:46-62 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kobayashi Y, Araki H, Ohto T, Shin K. (2000). The influence of controlled occlusal overload on peri-implant tissue: part 3. A histologic study in monkeys. Int J Oral Maxillofac Implants 15:425-431 [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Weier HU, Frenkel B, Lian JB, Stein GS, et al. (1999). Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J Exp Med 15:729-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, et al. (2003). Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 284:C934-C943 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Possemiers T, Zhang X, Naert I, Chaudhari A, Sasaki K, et al. (2011). Influence of whole-body vibration time on peri-implant bone healing: a histomorphometrical animal study. J Clin Periodontol 38:180-185 [DOI] [PubMed] [Google Scholar]
- Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, et al. (1998). C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res 9:365-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padagas J, Colloton M, Shalhoub V, Kostenuik P, Morony S, Munyakazi L, et al. (2006). The receptor activator of nuclear factor-kappaB ligand inhibitor osteoprotegerin is a bone-protective agent in a rat model of chronic renal insufficiency and hyperparathyroidism. Calcif Tissue Int 78:35-44 [DOI] [PubMed] [Google Scholar]
- Pavlin D, Zadro R, Gluhak-Heinrich J. (2001). Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect Tissue Research 42:135-148 [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Bonnet N, Baldock PA, Ominsky MS, Stolina M, Kostenuik PJ, et al. (2010). Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem 285:28164-28173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich K, Görtz B, Hayer S, Zwerina J, Doerr N, Kostenuik P, et al. (2004). Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am J Pathol 164:543-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarment DP, Cooke JW, Miller SE, Jin Q, McGuire MK, Kao RT, et al. (2006). Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol 33:135-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R, Matsuzaka K, Kokubu E, Inoue T. (2011). Immediate loading after implant placement following tooth extraction up-regulates cellular activity in the dog mandible. Clin Oral Implants Res 22:1372-1378 [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB. (1993). Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrin Rev 14:424-442 [DOI] [PubMed] [Google Scholar]
- Tatarakis N, Kinney JS, Inglehart M, Braun TM, Shelburne C, Lang NP, et al. (2013). Clinical, microbiological, and salivary biomarker profiles of dental implant patients with type 2 diabetes [published online February 27, 2013]. Clin Oral Implants Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani SE, Pest MA, Kim G, Ohora SN, Qin L, Beier F. (2012). Transforming growth factor alpha controls the transition from hypertrophic cartilage to bone during endochondral bone growth. Bone 51:131-141 [DOI] [PubMed] [Google Scholar]
- Valenta A, Roschger P, Fratzl-Zelman N, Kostenuik PJ, Dunstan CR, Fratzl P, et al. (2005). Combined treatment with PTH (1-34) and OPG increases bone volume and uniformity of mineralization in aged ovariectomized rats. Bone 37:87-95 [DOI] [PubMed] [Google Scholar]
- Vandamme K, Naert I, Geris L, Vander Sloten J, Puers R, Duyck J. (2007). The effect of micro-motion on the tissue response around immediately loaded roughened titanium implants in the rabbit [erratum in Eur J Oral Sci 115:167, 2007]. Eur J Oral Sci 115:21-29 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ogawa T, Yokoyama M, Koyama S, Sasaki K. (2013). Influence of immediate and early loading on bone metabolic activity around dental implants in rat tibiae [published online June 27, 2013]. Clin Oral Implants Res. [DOI] [PubMed] [Google Scholar]