Abstract
Dimensional alterations of the facial bone wall following tooth extractions in the esthetic zone have a profound effect on treatment outcomes. This prospective study in 39 patients is the first to investigate three-dimensional (3D) alterations of facial bone in the esthetic zone during the initial 8 wks following flapless tooth extraction. A novel 3D analysis was carried out, based on 2 consecutive cone beam computed tomographies (CBCTs). A risk zone for significant bone resorption was identified in central areas, whereas proximal areas yielded only minor changes. Correlation analysis identified a facial bone wall thickness of ≤ 1 mm as a critical factor associated with the extent of bone resorption. Thin-wall phenotypes displayed pronounced vertical bone resorption, with a median bone loss of 7.5 mm, as compared with thick-wall phenotypes, which decreased by only 1.1 mm. For the first time, 3D analysis has allowed for documentation of dimensional alterations of the facial bone wall in the esthetic zone of humans following extraction. It also characterized a risk zone prone to pronounced bone resorption in thin-wall phenotypes. Vertical bone loss was 3.5 times more severe than findings reported in the existing literature.
Keywords: bone remodeling, bone resorption, three-dimensional imaging, clinical trial, dental implants, maxilla
Introduction
Esthetics poses a challenge in clinical practice and is critical for successful implant-supported prostheses in the anterior maxilla (Belser et al., 2009). A key prerequisite for esthetic outcomes is adequate three-dimensional (3D) osseous volume of the alveolar ridge, including an intact facial bone wall of sufficient thickness and height (Buser et al., 2004; Grunder et al., 2005). Deficiency of facial bone anatomy has a negative impact on esthetics and is a critical causative factor for esthetic implant complications and failures (Chen and Buser, 2009). Experimental studies on canine mandibular premolar sites revealed substantial structural and dimensional alterations to the facial bone wall of the extraction socket (Cardaropoli et al., 2003; Araujo and Lindhe, 2005). These catabolic changes are initiated by resorption of the bundle bone that lines the extraction socket. They are correlated with the disruption of blood supply from the periodontal ligament and significant osteoclastic activity (Cardaropoli et al., 2003; Araujo and Lindhe, 2005). Importantly, experimental findings on facial bone resorption in extraction sites of canine mandibular premolars have never been confirmed for the esthetic zone in humans, since ethical concerns prohibit histological studies being carried out. Alternative, non-invasive techniques must be adopted. Recently, cone beam computed tomography (CBCT) has become a commonly accepted diagnostic tool (Tyndall et al., 2012). Current CBCT technology offers extremely accurate 3D diagnostics allowing for small Fields of View (FOV), good image quality, and low radiation doses (European Commission, 2011).
The aim of the present prospective study was to investigate dimensional alterations of the facial bone wall following tooth extraction in the esthetic zone. A novel 3D method utilizing digital model superimpositions based on 2 consecutive CBCTs was used to characterize the extent of bone loss and to identify risk zones and the respective modulating factors for facial bone resorption. This study should provide a better understanding of underlying tissue biology and facilitate the selection of an appropriate treatment protocol to achieve esthetic outcomes.
Materials & Methods
Study Sample
Thirty-nine patients were consecutively enrolled in this prospective case series study. All patients were referred to the Department of Oral Surgery at the University of Berne (Switzerland) with a need for a single-tooth replacement in the anterior maxilla, subsequent to an inevitable tooth extraction. Exclusion criteria were systemic diseases that could alter bone and soft-tissue healing, pregnancy, and participants < 18 yrs of age (Buser et al., 2000). The study was approved by the standing ethical committee of the state of Berne, Switzerland (Number 079/09) and was conducted as a prospective clinical trial in accordance with the Helsinki Declaration (Version 2008) and the STROBE statement guidelines.
Surgical Procedure
Prior to extraction, the patients rinsed with a 0.1% chlorhexidine solution for 1 min. Following the administration of local anesthesia, tooth extraction was performed without flap elevation by means of a low-trauma technique. The extraction sockets were thoroughly debrided to remove all soft and granulation tissues. A collagen sponge was placed into the socket to stabilize the blood clot (Tissue Cone™, Baxter, Chicago, IL, USA). Medication prescribed to all patients included analgesics and a chlorhexidine mouthwash (0.1%). A removable prosthesis was inserted and adapted to avoid any direct pressure to the underlying soft tissues. Patients were recalled at 2, 4, 6, and 8 wks for monitoring of the healing progress.
Radiographic Examination
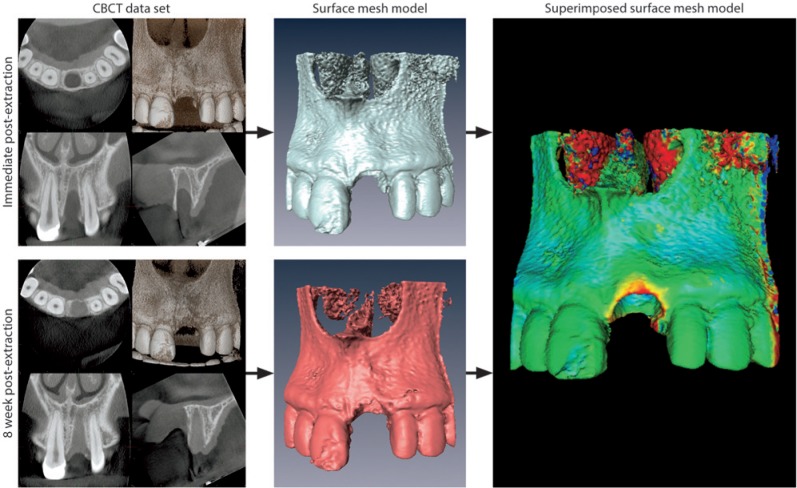
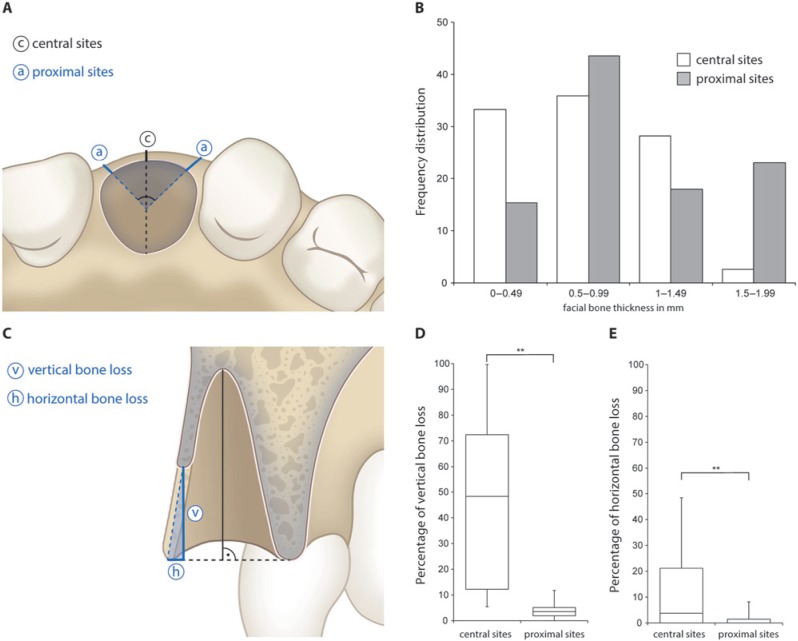
Two consecutive CBCTs were obtained, one immediately post-extraction and the second after 8 wks (3D Accuitomo XYZ Slice View Tomograph, Morita, Kyoto, Japan). The smallest FOV of 4 × 4 cm with a voxel size of 0.125 mm was used with an exposure setting of 5.0 mA/80 kV and a scanning time of 17.5 sec. The DICOM data (Digital Imaging and Communications in Medicine) were segmented by digital imaging software (Amira, FEI Visualization Sciences Group, Hillsboro, OR, USA).Based on the result of segmentation, a surface mesh model was generated according to conventional marching cube algorithms (Lorenson and Cline, 1987), followed by automated surface mesh model generation (Fig. 1). The eight-week mesh model was superimposed on the baseline mesh model and rigidly aligned by anatomical landmarks with the help of Di2Mesh software (Institute for Surgical Technology & Biomechanics, Bern, Switzerland) (Fig. 1). The distance between the 2 surface meshes was presented as color-coded figures to identify zones of facial bone resorption (Kim et al., 2010) (Fig. 1). Baseline facial bone thickness was measured at distances of 1, 3, and 5 mm from the most coronal point of the bone crest (Araujo and Lindhe, 2005). The analysis was performed in central (c) and proximal sites (a) oriented at a 45° degree angle, with the tooth axis as a reference (Fig. 2A). A horizontal reference line was traced connecting the facial and palatal crest for standardized measurements (Fickl et al., 2008). The point-to-point distance between the 2 surface meshes with the respective angle to the reference line was obtained for each sample, and the vertical and horizontal bone losses were calculated accordingly (Fig. 2C).
Figure 1.
Radiographic examination. The DICOM files of the obtained CBCT datasets, immediately post-extraction and following 8 wks of healing, were converted into a surface mesh model with digital imaging software. The 2 surface mesh models were superimposed and rigidly aligned with anatomical landmarks. The distance between the 2 surface meshes was presented as color-coded figures to identify zones of facial bone resorption.
Figure 2.
Baseline measurements and dimensional and vertical bone loss after 8 wks of healing. (A) The analysis was performed in central (c) and proximal sites (a) oriented at a 45° degree angle with the tooth axis as a reference. (B) Frequency distribution of facial bone wall thickness in central and proximal sites. (C) A horizontal reference line was traced connecting the facial and palatal bone wall for standardized measurements. The point-to-point distance between the 2 surface meshes with the respective angle to the reference line was obtained for each sample, and the vertical and horizontal bone losses were calculated accordingly. (D) Percentage of vertical bone loss in central and proximal sites. (E) Percentage of horizontal bone loss in central and proximal sites. **p < .0001.
Statistical Analysis
All data were expressed as median, mean values (± standard error), minimum, and maximum values. To test whether there was a statistical significance between/among the groups, we used a non-parametric Wilcoxon signed-rank test. Multivariate linear regression was applied in the central measurements to identify modulating factors (Oja, 2010). Age, gender, and baseline central wall thickness were used as explanatory variables. Differences were considered statistically significant at *p < .05 and **p < .0001. All statistical analyses were calculated with an open-source R software package (R 2.14.1, http://www.r-project.org).
Results
Study Sample
The study population consisted of 18 women and 21 men aged between 21 and 69 yrs (mean, 45.8 ± 12.6 yrs). No post-operative complications were observed at the extraction sites. Thirty-three patients were non-smokers, three were light smokers (≤ 10 cigarettes/day), and three were heavy smokers (> 10 cigarettes/day). The extraction sites included 29 central incisors, 8 lateral incisors, and 2 canines.
Baseline Measurements
The facial bone wall in central sites revealed a mean thickness of 0.8 ± 0.5 mm (range, 0-1.7), whereas in proximal sites the mean facial thickness was 1.0 ± 0.6 mm (range, 0-1.9) (Fig. 2A). The difference in facial bone thickness between central and proximal sites was statistically significant (p < .0001). The frequency distribution analysis yielded a facial wall thickness of ≤ 1 mm in 69% of central sites and in 59% of the proximal sites (Fig. 2B). The multivariate regression analysis revealed no correlation between ridge alterations and age or gender.
Dimensional Changes and Identification of Risk Zones for Bone Resorption
Central sites yielded progressive bone resorption, with a median vertical bone loss of 5.2 mm or 48.3% of the original bone wall height (range, 0.7-12.2 mm or 5.5-99.6%) and a median horizontal bone loss of 0.3 mm or 3.8% of the original bone wall width (range, 0-3.4 mm or 0-49.5%) (Figs. 2C-2E). In proximal sites, there was significantly less bone resorption, not only vertically but also horizontally (p < .0001). Proximal sites revealed a median vertical bone loss of 0.5 mm or 4.5% (range, 0.1-1.1 mm or 1.0-12.7%) and a median horizontal bone loss of 0 mm or 0% (range, 0-0.4 mm or 0-5.9%) (Figs. 2C-2E). These results indicated that central sites were of special interest from a clinical point of view, and were further analyzed.
Characteristic Bone Resorption Patterns and Correlation Analysis in the Central Risk Zone Area
The superimposed color-coded figures showed characteristic resorption patterns (Fig. 3). A critical facial bone wall thickness of 1 mm was identified (Fig. 4A). A thin-wall phenotype exhibiting a facial bone wall thickness of ≤ 1 mm showed a median thickness of 0.7 mm (range, 0.4-1.0 mm; Appendix Fig. 1). A thick-wall phenotype, with a facial bone wall thickness of > 1 mm, revealed a median thickness of 1.4 mm (range, 1.1-1.7 mm; Appendix Fig. 2). Significant bone resorption was associated with a thin-wall phenotype, showing median vertical bone loss of 7.5 mm or 62.3% (range, 0.8-12.2 mm or 8-99.6%), and median horizontal bone loss of 0.8 mm, or 10.5% (range, 0-3.4 mm or 0-49.5%; Fig. 3A, Fig. 4B, Appendix Fig. 1). A less pronounced resorption pattern was associated with facial bone wall thickness of > 1 mm at baseline. The thick-wall phenotype displayed a median vertical bone loss of 1.1 mm or 9.1% (range, 0.7-3.2 mm or 5.5-38.6%), and a median horizontal bone loss of 0 mm or 0% (0-1.5 mm or 0-21.2%; Fig. 3B, Fig. 4B, Appendix Fig. 2). The difference of vertical bone loss in sites with a thin or thick phenotype was significant (p < .0001; Fig. 4B).
Figure 3.
Characteristic bone resorption patterns. (A) A thin-wall phenotype showed a facial bone wall thickness of ≤ 1 mm and revealed a progressive bone resorption pattern after 8 wks of healing. (B) A thick-wall phenotype, with a facial bone wall thickness of > 1 mm, exhibited a less-pronounced bone resorption pattern after 8 wks of healing.
Figure 4.
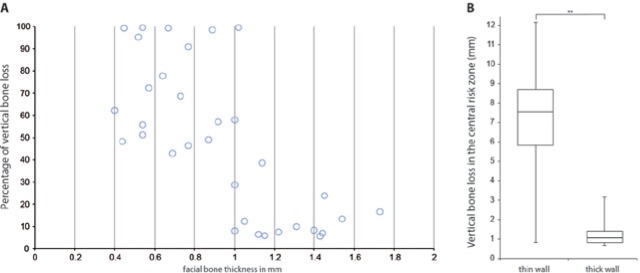
Correlation analysis in central risk zones. (A) A critical facial bone wall thickness of 1 mm was identified. (B) The difference between the thin and thick phenotypes in relation to vertical bone loss was significant [p < .0001 (**)].
Discussion
In the present study, for the first time, a novel 3D analysis allowed for the characterization of dimensional alterations of the facial bone wall in the esthetic zone following extractions in human patients. Although the results confirmed pre-clinical findings established in mandibular premolar sites of dogs (Cardaropoli et al., 2003; Araujo and Lindhe, 2005), dimensional alterations of the facial bone in the esthetic zone were 2 to 3.5 times more severe in humans. In addition, bone resorption patterns were characterized, and revealed 2 novel findings which are of utmost importance for clinical practice. First, central and proximal areas of the facial bone wall displayed a significantly different bone resorption pattern; a risk zone more susceptible to bone loss was identified in central areas. Second, correlation analysis revealed a risk zone prone to pronounced bone resorption in thin-wall phenotypes characterized by a facial bone wall thickness of ≤ 1 mm.
These new findings were based on a novel 3D analysis carried out over 8 wks post-extraction, with the DICOM datasets of 2 consecutive CBCTs. This was followed by surface mesh model generation and digital model superimposition. Alternative clinical methods with periodontal probes (Sanz et al., 2010) or calipers (Huynh-Ba et al., 2010) were not used, since these techniques lack the required precision and are not sequentially reproducible (Roe et al., 2012). Although histological analysis facilitates the observation of resorption patterns, it requires invasive bloc resection, and ethical concerns prohibit its application in clinical studies. The use of 3D radiographic imaging is non-invasive, and it can be applied sequentially. The internal ethical review board required the utilization of CBCT technology with the smallest FOV of 4 × 4 cm. At these settings, a much lower effective radiation dose was measured compared with multislice CTs (20.02 μSv vs. 474-1160 μSv; Hirsch et al., 2008). In addition, CBCT technology offers accurate 3D diagnostics (Loubele et al., 2009). A position paper recently published by the American Academy of Oral and Maxillofacial Radiology (AAOMR) recommended CBCTs as the imaging method of choice for the assessment of dental implant sites (Tyndall et al., 2012).
Successful implant esthetics following tooth extraction requires a better understanding of tissue biology and the associated 3D changes in facial bone architecture. In the mid-2000s, the mechanisms of bone resorption and modeling post-extraction were characterized in canine mandibular premolar sites over an initial eight-week healing period, resulting in a mean facial bone wall resorption of 2.2 mm (Araujo and Lindhe, 2005). Bone loss was histometrically analyzed in the mid-facial areas of the extraction sockets. In the present study, dimensional changes were examined not only in central, but also in proximal areas. The 3D analysis identified the central zone to be at higher risk for bone resorption, with a median bone loss of 5.2 mm vertically and 0.3 mm horizontally. Proximal areas revealed only minor changes of 0.5 mm and 0 mm, respectively (Fig. 2D-E). The minimal bone resorption in proximal areas showing at 8 wks post-extraction provides a favorable two-wall defect morphology at early implant placement. This is considered relevant for predictable regenerative outcomes (Buser, 2009). The regenerative potential of a peri-implant bone defect has been attributed to the ratio between the area of exposed bone marrow and the defect volume to be regenerated (Schenk et al., 1994). Flapless extraction appears important for achieving a favorable 2-wall defect morphology, and avoids additional superficial bone resorption in proximal areas due to open flap elevation (Fickl et al., 2008).
The maintenance of facial bone architecture has been related to a facial bone wall thickness of 2 mm in an experimental dog study (Qahash et al., 2008). In the anterior maxilla, the facial bone wall is usually thinner than 2 mm, as demonstrated in several CBCT studies (Braut et al., 2011; Januario et al., 2011; Vera et al., 2012b). In the present study, none of the central areas revealed a facial wall thickness of ≥ 2 mm. Central facial wall thickness was 1 mm or less in 69% of sites (Fig. 2B). Correlation analysis of the central risk zone revealed a facial bone thickness of ≤ 1 mm to be a critical factor for severe bone resorption. Thin-wall phenotypes resulted in a median vertical bone loss of 7.5 mm in the central risk zone, whereas thick-wall phenotypes showed a median vertical bone loss of only 1.1 mm (Figs. 3, 4). Therefore, thin-wall phenotypes experienced a 3.5-times-greater bone loss than documented in experimental studies (Araujo and Lindhe, 2005). Even thick-wall phenotypes exhibited bone loss of more than 1 mm, which could affect esthetics negatively. Efforts have been made to prevent these dimensional changes following tooth extraction, but neither immediate implant placement nor ridge preservation techniques were able to maintain the facial osseous architecture (Araujo et al., 2005; Ten Heggeler et al., 2011).
The clinician must select an appropriate time point for post-extraction implant placement, to achieve a predictable regenerative and esthetic outcome, and to avoid complications. The concepts of immediate and early implant placement were developed in the 1990s to reduce overall treatment time for post-extraction implant therapy (Hämmerle et al., 2004). The most recent systematic reviews clearly demonstrate that immediate implant placement increases the risk of significant mucosal recessions if this approach is not applied with strict inclusion criteria (Chen and Buser, 2009, 2013). The present study supports these findings, since even thick phenotypes produced a median vertical bone loss of 1.1 mm. Two recent clinical studies performing consecutive CBCTs, the first at implant placement and another 1 yr later, confirmed that significant mid-facial vertical bone resorption occurred in immediate implant cases (Roe et al., 2012; Vera et al., 2012a). Therefore, it is recommended that immediate implants be carried out only in ideal sites with a thick-wall phenotype and thick gingival biotype (Morton et al., 2013).
There are some limitations of the present study. First, since only sites in the anterior maxilla were involved, the results can be applied exclusively to extraction sites in the esthetic zone. Second, the study was limited to an eight-week healing period, since this is standard for early implant placement (Buser et al., 2008). A longer healing period could potentially produce different results.
In conclusion, biological understanding of dimensional alterations of the facial bone wall following extraction aids in the selection of the appropriate surgical protocol needed to achieve esthetic results. The present study clearly showed that facial bone resorption also occurs in post-extraction sites in the anterior maxilla, and that the facial bone wall thickness in central areas determines the extent of resorption. In addition, the observed dimensional alterations in thin-wall biotypes are much more severe than has been documented in pre-clinical studies.
Footnotes
The study was supported by the ITI Foundation (Basel, Switzerland; No. 624_2009).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Araujo MG, Lindhe J. (2005). Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 32:212-218 [DOI] [PubMed] [Google Scholar]
- Araujo MG, Sukekava F, Wennström JL, Lindhe J. (2005). Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol 32:645-652 [DOI] [PubMed] [Google Scholar]
- Belser UC, Grutter L, Vailati F, Bornstein MM, Weber HP, Buser D. (2009). Outcome evaluation of early placed maxillary anterior single-tooth implants using objective esthetic criteria: a cross-sectional, retrospective study in 45 patients with a 2- to 4-year follow-up using pink and white esthetic scores. J Periodontol 80:140-151 [DOI] [PubMed] [Google Scholar]
- Braut V, Bornstein MM, Belser U, Buser D. (2011). Thickness of the anterior maxillary facial bone wall—a retrospective radiographic study using cone beam computed tomography. Int J Periodontics Restorative Dent 31:125-131 [PubMed] [Google Scholar]
- Buser D. (2009). Implant placement with simultaneous guided bone regeneration: selection of biomaterial and surgical principles. In: 20 years of guided bone regeneration in implant dentistry. Chicago, IL: Quintessence Inc., pp. 123-152 [Google Scholar]
- Buser D, von Arx T, ten Bruggenkate C, Weingart D. (2000). Basic surgical principles with ITI implants. Clin Oral Implants Res 11(Suppl 1):59-68 [DOI] [PubMed] [Google Scholar]
- Buser D, Martin W, Belser UC. (2004). Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants 19(Suppl):43-61 [PubMed] [Google Scholar]
- Buser D, Bornstein MM, Weber HP, Grutter L, Schmid B, Belser UC. (2008). Early implant placement with simultaneous guided bone regeneration following single-tooth extraction in the esthetic zone: a cross-sectional, retrospective study in 45 subjects with a 2- to 4-year follow-up. J Periodontol 79:1773-1781 [DOI] [PubMed] [Google Scholar]
- Cardaropoli G, Araujo M, Lindhe J. (2003). Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol 30:809-818 [DOI] [PubMed] [Google Scholar]
- Chen S, Buser D. (2013). Esthetic outcomes following immediate and early implant placement in the anterior maxilla – a systematic review. Int J Oral Maxillofac Implants [Epub ahead of print 8/1/2013] (in press). [DOI] [PubMed] [Google Scholar]
- Chen ST, Buser D. (2009). Clinical and esthetic outcomes of implants placed in postextraction sites. Int J Oral Maxillofac Implants 24(Suppl):186-217 [PubMed] [Google Scholar]
- European Commission (2011). Protection Radiation No. 172. Cone beam CT for dental and maxillofacial radiology. Evidence-based guidelines. In: RP Unit editor, pp. 73-76 URL accessed on 9/5/2013 at: http://ec.europa.eu/energy/nuclear/radiation_protection/doc/publication/172.pdf
- Fickl S, Zuhr O, Wachtel H, Bolz W, Huerzeler M. (2008). Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. J Clin Periodontol 35:356-363 [DOI] [PubMed] [Google Scholar]
- Grunder U, Gracis S, Capelli M. (2005). Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent 25:113-119 [PubMed] [Google Scholar]
- Hämmerle CH, Chen ST, Wilson TG., Jr (2004). Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int J Oral Maxillofac Implants 19(Suppl):26-28 [PubMed] [Google Scholar]
- Hirsch E, Wolf U, Heinicke F, Silva MA. (2008). Dosimetry of the cone beam computed tomography Veraviewepocs 3D compared with the 3D Accuitomo in different fields of view. Dentomaxillofac Radiol 37:268-273 [DOI] [PubMed] [Google Scholar]
- Huynh-Ba G, Pjetursson BE, Sanz M, Cecchinato D, Ferrus J, Lindhe J, et al. (2010). Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res 21:37-42 [DOI] [PubMed] [Google Scholar]
- Januario AL, Duarte WR, Barriviera M, Mesti JC, Araujo MG, Lindhe J. (2011). Dimension of the facial bone wall in the anterior maxilla: a cone-beam computed tomography study. Clin Oral Implants Res 22:1168-1171 [DOI] [PubMed] [Google Scholar]
- Kim H, Jurgens P, Weber S, Nolte LP, Reyes M. (2010). A new soft-tissue simulation strategy for cranio-maxillofacial surgery using facial muscle template model. Prog Biophys Mol Biol 103:284-291 [DOI] [PubMed] [Google Scholar]
- Lorenson W, Cline H. (1987). Marching cubes: a high resolution 3d surface construction algorithm. In: Proceedings of the 14th Annual Conference on computer graphics and interactive techniques Stone MC, editor. New York, NY: Association for Computing Machinery (ACM), pp. 163-169 [Google Scholar]
- Loubele M, Bogaerts R, Van Dijck E, Pauwels R, Vanheusden S, Suetens P, et al. (2009). Comparison between effective radiation dose of CBCT and MSCT scanners for dentomaxillofacial applications. Eur J Radiol 71:461-468 [DOI] [PubMed] [Google Scholar]
- Morton D, Chen ST, Martin WC, Levine R, Buser D. (2013). Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. [E-pub ahead of print 8/13/2013] (in press). [DOI] [PubMed] [Google Scholar]
- Oja H. (2010). Multivariate nonparametric methods with R: an approach based on spatial signs and ranks. New York, NY: Springer [Google Scholar]
- Qahash M, Susin C, Polimeni G, Hall J, Wikesjö UM. (2008). Bone healing dynamics at buccal peri-implant sites. Clin Oral Implants Res 19:166-172 [DOI] [PubMed] [Google Scholar]
- Roe P, Kan JY, Rungcharassaeng K, Caruso JM, Zimmerman G, Mesquida J. (2012). Horizontal and vertical dimensional changes of peri-implant facial bone following immediate placement and provisionalization of maxillary anterior single implants: a 1-year cone beam computed tomography study. Int J Oral Maxillofac Implants 27:393-400 [PubMed] [Google Scholar]
- Sanz M, Cecchinato D, Ferrus J, Pjetursson EB, Lang NP, Lindhe J. (2010). A prospective, randomized-controlled clinical trial to evaluate bone preservation using implants with different geometry placed into extraction sockets in the maxilla. Clin Oral Implants Res 21:13-21 [DOI] [PubMed] [Google Scholar]
- Schenk RK, Buser D, Hardwick WR, Dahlin C. (1994). Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants 9:13-29 [PubMed] [Google Scholar]
- Ten Heggeler JM, Slot DE, Van der Weijden GA. (2011). Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: a systematic review. Clin Oral Implants Res 22:779-788 [DOI] [PubMed] [Google Scholar]
- Tyndall DA, Price JB, Tetradis S, Ganz SD, Hildebolt C, Scarfe WC. (2012). Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol 113:817-826 [DOI] [PubMed] [Google Scholar]
- Vera C, De Kok IJ, Chen W, Reside G, Tyndall D, Cooper LF. (2012a). Evaluation of post-implant buccal bone resorption using cone beam computed tomography: a clinical pilot study. Int J Oral Maxillofac Implants 27:1249-1257 [PubMed] [Google Scholar]
- Vera C, De Kok IJ, Reinhold D, Limpiphipatanakorn P, Yap AK, Tyndall D, et al. (2012b). Evaluation of buccal alveolar bone dimension of maxillary anterior and premolar teeth: a cone beam computed tomography investigation. Int J Oral Maxillofac Implants 27:1514-1519 [PubMed] [Google Scholar]