Abstract
Background. Papillary thyroid carcinoma (PTC), follicular thyroid adenoma (FTA), and thyroid nodular hyperplasia (TNH) are the most frequent diseases of the thyroid gland. Previous studies described the involvement of dipeptidyl-peptidase IV (DPPIV/CD26) in the development of thyroid neoplasia and proposed it as an additional tool in the diagnosis/prognosis of these diseases. However, very little is known about the involvement of other peptidases in neoplastic and hyperplastic processes of this gland. Methods. The catalytic activity of 10 peptidases in a series of 30 PTC, 10 FTA, and 14 TNH was measured fluorimetrically in tumour and nontumour adjacent tissues. Results. The activity of DPPIV/CD26 was markedly higher in PTC than in FTA, TNH, and nontumour tissues. Aspartyl aminopeptidase (AspAP), alanyl aminopeptidase (AlaAP), prolyl endopeptidase, pyroglutamyl peptidase I, and aminopeptidase B activities were significantly increased in thyroid neoplasms when compared to nontumour tissues. AspAP and AlaAP activities were also significantly higher in PTC than in FTA and TNH. Conclusions. These data suggest the involvement of DPPIV/CD26 and some cytosolic peptidases in the neoplastic development of PTC and FTA. Further studies will help to define the possible clinical usefulness of AlaAP and AspAP in the diagnosis/prognosis of thyroid neoplasms.
1. Introduction
Solitary and multiple nodules of the thyroid gland are very common in clinical practice, with most of them being benign lesions, either thyroid nodular hyperplasia (TNH) or follicular thyroid adenoma (FTA). Roughly only 5% of thyroid nodules are malignant [1] and 70% of them are papillary thyroid carcinomas (PTC) [2]. The arrival of ultrasound examination to routine practice has discovered a large amount of nonpalpable and clinically silent thyroid nodules. TNH, FTA, and PTC can be distinguished from each other following well-established histopathologic criteria [3]. Interestingly, these lesions are able to be sampled with recognized diagnostic success by sonographically guided fine needle aspiration cytology [1] or by core biopsy [4].
TNH consists of multiple nodules of variable size composed of odd-shaped thyroid follicles with homogeneous bland cytology and partial encapsulation that may reverse and dissapear when the hormonal stimulus ceases. Thyroid hyperfunction is common.
FTA usually appears as asymptomatic solitary nodules. This lesion is composed of small size follicles lying in a fibrous, sometimes edematous stroma and shows a well defined fibrous capsule. There is no evidence of vascular or capsular invasion in these neoplasms; otherwise, the lesion is considered carcinoma [3].
PTC is the most common type of endocrine cancer and shows a varied clinical presentation. Most patients present with a thyroid nodule when they consult for the first time, but some others do not, and lymph node metastases in the neck are the sole manifestation of a tumor that remains occult in the thyroid. Histologically, PTC may display very different growth patterns [5, 6], and its diagnosis relies on the identification of characteristic nuclear features, such as nuclear enlargement, nuclear membrane irregularities, peripheral chromatin margination, and prominent micronucleoli [3].
Immunohistochemical markers like HBME-1, cytokeratin 19, and galectin-3 may be of help in difficult cases [6].
Bioactive peptides are regulated through specific peptidases that hydrolyze them. These peptide-converting enzymes are distributed in the main human tissues and have been originally considered to be only involved in protein and peptide scavenging [7]. However, many studies have shown that they are involved in several physiological functions and play a key role in growth control, differentiation, and signal transduction of many cell systems by modulating the activity of bioactive peptides, degrading extracellular matrix, acting as adhesion molecules and directly participating in the intracellular signaling [7–10]. So, altered expression and catalytic function patterns of these enzymes may contribute to several disease processes, including neoplastic transformation and tumour progression [8–10].
Increased understanding of the underlying pathophysiology of thyroid tumours has led to implicate several proteases in its genesis, growth, and dissemination [11–16]. Metalloproteases such as MMP-2 and 9 and peptidases such as dipeptidyl-peptidase IV/CD26 (DPPIV/CD26) change their expression and activity in these neoplasms and have been recognized as molecular markers in the diagnosis of thyroid tumours [11–18]. However, very little is known about the activity pattern of other peptidases in thyroid neoplastic and hyperplastic diseases.
This study intends to analyse the activity of different peptidases in PTC, FTA, and TNH and in the adjacent non-tumour thyroid tissue, as we have performed before in other neoplasms, such as renal, colorectal and head and neck tumours [19–23]. For such a purpose, we have analyzed in these lesions the activity of 10 acid, basic, neutral, and omega peptidases covering the whole spectrum of peptide-converting activity. On the one hand, we selected five cell-surface peptidases—DPPIV/CD26, APN/CD13, NEP/CD10, APA and CAP—which have been described as tumour markers in haematologic, kidney, skin, prostate, and gynaecologic cancers, among others [8–10, 24]. On the other, five cytosolic enzymes were selected—PEP, alaAP, AspAP, APB and PGI—for better know if these enzymes may also be involved in neoplastic development.”
2. Materials and Methods
The authors declare that all the experiments carried out in this study comply with current Spanish and European Union legal regulations.
2.1. Tissue Specimens
Representative tissue from 30 PTC, 14 TNH and 10 FTA and their corresponding non tumour areas were adequately sampled in fresh at the Pathology Lab within the first 30 minutes after surgical removal, immediately frozen with isopentane, and stored at −80°C until the enzyme assays were performed. Personal data of the patients were appropriately encrypted to preserve the anonymous use of the selected material accomplishing with international recommendations.
2.2. Sample Preparation
Soluble and membrane-bound fractions were obtained following a modified version of Zambotti-Villela et al. [25]. Explanted tissue samples were homogenized in 10 mM Tris-HCl buffer at pH 7.4, for 30 seconds at 800 rpm using a Heidolph PZR 50 Selecta homogenizer, and ultracentrifuged in a Centrikon T-2070 Kontron Instruments apparatus at 100,000 ×g for 35 min. The resulting supernatants were used to measure soluble enzyme activities: APB, AspAP, PG I, PEP and AlaAP. To avoid contamination with soluble enzymes, the resulting pellets were washed three times by suspension in 10 mM Tris-HCl buffer at pH 7.4 The pellets were then homogenized in 10 mM Tris-HCl buffer at pH 7.4, and centrifuged at low speed (800 ×g) for 3 min to purify the samples. The supernatants thus obtained were used to determine membrane-bound enzyme activities: APA, APN/CD13, CAP, DPPIV/CD26, and NEP/CD10. All steps were carried out at 4°C.
2.3. Enzyme Assays
Peptidase activities were measured by incubating samples with saturating concentrations of fluorogen-derived substrates following the method described by Yoshimoto et al. [26], Mantle et al. [27], and Silveira et al. [28]. Selected peptidases, their substrates, and the reaction conditions for each enzymatic assay are summarized in Table 1.
Table 1.
Substrates and the reaction conditions for each enzymatic assay.
| Substrates | (mM) | pHa | DTT (mM) | EDTA (mM) | CaCl2 (mM) | NaCl (mM) | MnCl2 (mM) | |
|---|---|---|---|---|---|---|---|---|
| PEP (EC 3.4.21.26) | Z-Gly-Pro-β-NA | 0,125 | 7,4 | 2 | — | — | — | — |
| PGI (EC 3.4.19.3) | pGlu-β-NA | 0,125 | 7,4 | 2 | 2 | — | — | — |
| APBb (EC 3.4.11.6) | Arg-β-NA | 0,125 | 6,5 | — | — | — | 150 | — |
| APN/CD13b (EC 3.4.11.2) | Ala-β-NA | 0,125 | 7,4 | 1,2 | — | — | — | — |
| AlaAP (EC 3.4.11.14) | Ala-β-NA | 0,125 | 7,4 | 1,2 | — | — | — | — |
|
| ||||||||
| APA (EC 3.4.11.7) | H-Glu-β-NA | 0,125 | 7,4 | 0,65 | — | 0,5 | — | — |
| AspAP (EC 3.4.11.21) | Asp-β-NA | 0,125 | 7,4 | — | — | — | — | 1 |
| DPPIV/CD26 (EC 3.4.14.5) | H-Gly-Pro-β-NA | 0,2 | 8,3 | — | — | — | — | — |
| CAP (EC 3.4.11.3) | L-Cys-di-β-NA | 0,125 | 5,9 | — | — | — | — | — |
| NEPc (EC 3.4.24.11) | N-Dansyl-D-Ala-Gly-pNO2-Phe-Gly | 0,125 | 7,4 | — | — | — | — | — |
aReaction buffers: Phosphate buffer (50 mM) was used for PEP, PGI, APB, APN/CD13, and AlaAP. Tris-HCl (50 mM) was used for APA, AspAP, DPPIV/CD26, CAP, and NEP. b40 μM of the AlaAP inhibitor puromycin was added; c4.6 nM of the angiotensin-converting enzyme inhibitor captopril was added.
Alanine aminopeptidase activities (AlaAP and APN/CD13) were measured in triplicate using Ala-β-naphthylamide as a substrate. Incubations with the specific AlaAP inhibitor puromycin (40 μM) were performed in parallel to discriminate between the AlaAP and APN/CD13 forms of total alanine aminopeptidase activity. DPPIV/CD26 and PEP activities were assayed using H-Gly-Pro-β-naphthylamide, and Z-Gly-Pro-β-naphthylamide respectively. APB (basic) and acid aminopeptidase activities (AspAP, APA) were quantified with Arg-β-naphthylamide, Asp-β-naphthylamide, and Glu-β-naphthylamide substrates, respectively. Omega peptidase (PGI) activity was measured fluorometrically using pGlu-β-naphthylamide as substrate. CAP activity was analyzed using L-cystine-di-β-naphthylamide. NEP/CD10 assay was performed by incubating samples with a saturating concentration of N-dansyl-D-Ala-Gly-pNO2-Phe-Gly (DAGNPG, a dansyl derivative). These assays are based on the fluorescence of products generated from substrate hydrolysis by the enzyme.
Reactions were started by adding 30–50 μL of sample to 1 mL of the appropriate incubation mixture depending on the enzyme and substrate analyzed as follows: AlaAP, APN/CD13, APB, PEP, and PGI activities (50 mM phosphate buffer at pH 7.4 for AlaAP, APN/CD13, and PGI activities, pH 6.5 for APB, and 0.125 mM aminoacyl-β-naphthylamide); AspAP, APA, DPPIV/CD26, CAP, and NEP/CD10 activities (50 mM Tris-HCl buffer at pH 7.4 for AspAP, NEP and APA, pH 8.3 for DPPIV/CD26, pH 5.9 for CAP, and 0.125 mM aminoacyl-β-naphthylamide or [D]AG (pN) PG).
After 30 min incubation at 37°C, 1 mL of 0.1 M sodium acetate buffer (pH 4.2) was added to the mixture to terminate the reaction. The released product was determined by measuring the fluorescent intensity (at 412 nm with excitation at 345 nm for β-naphthylamine and at 562 nm with excitation at 342 nm for [D]AG) with a Shimadzu RF-540 Spectrofluorophotometer. Blanks consisted of buffer (instead the sample) and the same reactives and were used to determine background fluorescence. Relative fluorescence was converted into picomoles of product using a standard curve constructed with increasing concentrations of β-naphthylamine or [D]AG.
In the case of DPPIV and PEP, to verify that the formation of β-naphthylamine was specifically due to the action of these peptidases and not due to other enzymes which could cleave the same substrates (H-Gly-Pro-β-NA by DPP8 and DPP9 and Z-Gly-Pro-β-NA by seprase), we performed inhibition assays with specific inhibitors (diprotin A for DPPIV and KYP-2047 for PEP). 87% of the DPPIV activity was inhibited in PTC, 94% in TNH, and 100% in FTA and uninvolved thyroid tissue. In the case of PEP, over 95% of the activity measured was sensitive to the inhibitor in both tumour and nontumour tissues. Therefore, we concluded that the releasing of β-naphthylamine in both cases is mainly due to DPPIV and PEP.
2.4. Protein Determination
Protein concentration of soluble and membrane-bound fractions of thyroid tissues was measured in triplicate by the Bradford method [29], using BSA (1 mg/mL) as calibrator. Results were recorded as units of peptidase (UP) per milligram of protein (1 unit of peptidase (UP) is 1 pmol of naphthylamine released per minute). Fluorogenic assays were linear with respect to hydrolysis time and protein content. Activity is expressed as pmol of product/min/mg protein (UP/mg protein) and reported as mean ± SE.
2.5. Statistical Analysis
Shapiro-Wilk test was applied to data obtained from tumor samples to know if the numbers followed a normal distribution or not. Based on this information, nonparametric Mann-Whitney and Kruskal-Wallis tests were used to detect differences between two groups and more than two groups, respectively. SPSS 19.0 software was used for the statistical analysis.
3. Results
Females predominated in the series: the PTC group included 6 males and 24 females (average age: 53,7 years) and the FTA 3 males and 7 females (35.4 years). The fourteen cases of the TNH group were all females (43.2 years).
Histopathological subtyping of the PTC group included conventional PTC (16 cases, 53,3%), follicular variant PTC (10 cases, 33,3%), and papillary microcarcinomas (4 cases, 13,3%). Organ confined disease (pT1/2) was detected in 80% of the cases. Six cases presented minimal extracapsular extension (pT3) and two of them regional lymph node metastasis.
Table 2 shows all the peptidase activities measured in the soluble and membrane-bound fractions of hyperplastic, neoplastic, and nontumour adjacent tissues.
Table 2.
(a) Peptidase activities in papillary thyroid carcinoma (PTC) and in non neoplastic adjacent thyroid tissue. (b) Peptidase activities in follicular thyroid adenoma (FTA) and in non neoplastic adjacent thyroid tissue. (c) Peptidase activities in thyroid nodular hyperplasia (TNH) and in non neoplastic adjacent thyroid tissue. Values are means ± SE of peptidase activity recorded as pmol of units of peptidase (UP) per milligram of protein. A value of P ≤ 0.05 was considered statistically significant (Significant results are in bold).
(a)
| Peptidase | PTC | Non neoplastic | P |
|---|---|---|---|
| Soluble fraction | |||
| APB | 7419 ± 1363 | 4259 ± 867 | 0.094 |
| AspAP | 130 ± 15 | 87 ± 13 | 0.039 |
| PG I | 206 ± 39 | 77 ± 18 | 0.008 |
| PEP | 3836 ± 633 | 1977 ± 449 | 0.026 |
| AlaAP | 8819 ± 1194 | 4122 ± 1111 | 0.007 |
| Membrane-bound fraction | |||
| APA | 1250 ± 280 | 1120 ± 258 | 0.96 |
| APN/CD13 | 2301 ± 538 | 2533 ± 516 | 0.554 |
| CAP | 875 ± 253 | 665 ± 180 | 0.425 |
| DPPIV/CD26 | 4274 ± 1301 | 1347 ± 278 | 0.017 |
| NEP/CD10 | 3.1 ± 0.66 | 1.9 ± 0.45 | 0.12 |
(b)
| Peptidase | FTA | Non neoplastic | P |
|---|---|---|---|
| Soluble fraction | |||
| APB | 3772 ± 318 | 1969 ± 210 | 0.001 |
| AspAP | 84 ± 7.2 | 54 ± 21 | 0.003 |
| PG I | 245 ± 20 | 85 ± 12 | 0.001 |
| PEP | 3056 ± 359 | 1482 ± 184 | 0.002 |
| AlaAP | 2022 ± 206 | 792 ± 76 | 0.001 |
| Membrane-bound fraction | |||
| APA | 1513 ± 191 | 1270 ± 141 | 0.247 |
| APN/CD13 | 1670 ± 198 | 2542 ± 274 | 0.105 |
| CAP | 453 ± 48 | 407 ± 47 | 0.354 |
| DPPIV/CD26 | 589 ± 87 | 1700 ± 546 | 0.037 |
| NEP/CD10 | 3.7 ± 0.63 | 2.8 ± 0.45 | 0.643 |
(c)
| Peptidase | TNH | Nonhyperplastic | P |
|---|---|---|---|
| Soluble fraction | |||
| APB | 3713 ± 688 | 5477 ± 721 | 0.054 |
| AspAP | 67 ± 4.3 | 66 ± 14.4 | 0.519 |
| PG I | 73 ± 7.8 | 81 ± 5.3 | 0.687 |
| PEP | 2934 ± 448 | 3580 ± 478 | 0.295 |
| AlaAP | 1834 ± 258 | 2087 ± 310 | 0.227 |
| Membrane-bound fraction | |||
| APA | 789 ± 140 | 475 ± 87 | 0.116 |
| APN/CD13 | 1775 ± 329 | 1856 ± 243 | 0.601 |
| CAP | 317 ± 73 | 394 ± 107 | 0.687 |
| DPPIV/CD26 | 319 ± 44 | 804 ± 198 | 0.116 |
| NEP/CD10 | 1.34 ± 0.25 | 1.74 ± 0.29 | 0.414 |
The cytosolic fraction activity of AspAP, PG I, PEP, and AlaAP was significantly increased in PTC (Table 2(a)) and FTA (Table 2(b)) when compared with the adjacent nontumour tissues. APB activity was also increased in both neoplasms, although this change did not reach statistical significance in PTC. There were no significant changes in the activity of these peptidases in the TNH tissue when compared with the nonhyperplastic tissue (Table 2(c)).
The membrane-bound fraction activity of DPPIV/CD26 was markedly higher in PTC (Table 2(a)) and lower in FTA (Table 2(c)), when compared with the reference of nontumour tissue samples. By contrast, no significant changes were found in the rest of membrane-bound activities tested. TNH tissue did not yield significant changes.
Finally, when the three thyroid lesions were compared to each other, DPPIV/CD26, AlaAP, and AspAP showed significantly higher activities in PTC than in FTA and TNH (Figure 1). There were no significant results in the remaining analyzed peptidases.
Figure 1.
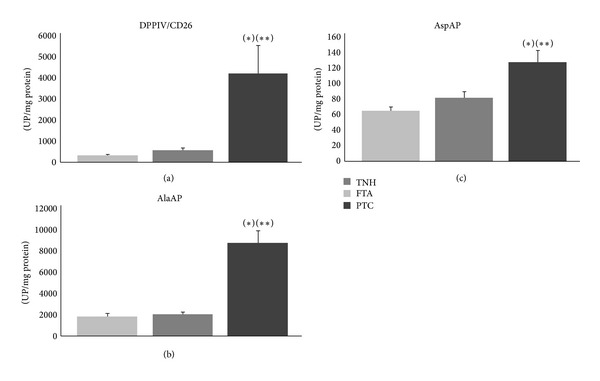
Enzymatic activities: activity in thyroid nodular hyperplasia (TNH), follicular thyroid adenoma (FTA), and papillary thyroid carcinoma (PTC) tissues. Values are means ± SE of peptidase activity recorded as pmol of units of peptidase (UP) per milligram of protein. (a) DPPIV/CD26: a value of P < 0.05 was considered statistically significant. *Kruskal-Wallis test, P ≤ 0.001. **The activity was higher in PTC than in FTA (Mann-Whitney test, P ≤ 0.01) and TNH (Mann-Whitney test, P ≤ 0.01). (b) AlaAP: a value of P < 0.05 was considered statistically significant. *Kruskal-Wallis test, P ≤ 0.001. **The activity was higher in PTC than in FTA (Mann-Whitney test, P ≤ 0.001) and TNH (Mann-Whitney test, P ≤ 0.01). (c) AspAP: a value of P < 0.05 was considered statistically significant. *Kruskal-Wallis test, P ≤ 0.01. **The activity was higher in PTC than in FTA (Mann-Whitney test, P ≤ 0.05) and TNH (Mann-Whitney test, P ≤ 0.05).
4. Discussion
It has been reported that expression and activity patterns of proteases, such as MMPs and peptidases, are altered in malignant tumours, suggesting their implication in tumour cell growth, local invasion, and metastases [8–10]. It has been demonstrated that MMP-2 and 9 are upregulated in the tumour tissue when compared with nontumour tissue in thyroid neoplasms, suggesting that these enzymes could have a role in thyroid neoplastic transformation [14, 15]. These MMPs and peptidases such as DPPIV/CD26 are distinctly expressed in several thyroid neoplasms and have been proposed as useful diagnostic/prognostic thyroid tumour markers [14–18].
In the same way, we observed that several peptidase activities are selectively modified in PTC and FTA when compared with nontumour adjacent tissues. Thus, cytosolic peptidases showed higher activities in both neoplasms, whereas DPPIV/CD26 activity was markedly higher in PTC and lower in FTA. Since TNH did not show any change in peptidase activity, these results indicate a role for these peptidases in the development of thyroid neoplasia.
Interestingly, peptidase activity is distinctly modified in thyroid lesions. Some authors stress that peptidases may either promote or impede tumour development depending on the specific type of tumour or on the phase of development where the tumour is, with this way suggesting that tumour growth regulation by peptides and their converting peptidases occur in a tumour specific manner [8–10]. We have shown in previous studies selective changes in peptidase activities of different neoplasms [19–23]. For example, when those were compared with uninvolved tissues, APN/CD13 and AspAP activities were decreased in renal cell carcinomas [19] and increased in head and neck squamous cell carcinoma [20]. Even more, higher APN/CD13 activity is correlated with high grade renal cell carcinomas [19] and with worse survival of these patients [22]. An exception to the observed tendency could be PEP, whose activity is increased in most of the neoplastic tissues studied to date [23, 30–32].
Recent data show that DPPIV/CD26 is a useful marker of malignancy in thyroid pathology [16]. Previous studies had already demonstrated that this serine ectopeptidase was specifically expressed in follicular-cell derived thyroid carcinomas [17, 18]. Some of these studies have been performed by semiquantitative cytoenzymatic methods and showed the highest activities in PTC [16–18]. Our quantitative fluorimetric assay yields similar results, showing that DPPIV/CD26 activity is markedly higher in PTC than in FTA and TNH. All this accumulated experience advices that this enzyme could be considered a potential marker of practical use in thyroid cancer. Interestingly, AlaAP and AspAP are other enzymes that showed a similar pattern of activity to DPPIV in our analysis. This result opens new possibilities in the study of peptidases as thyroid tumor markers.
DPPIV/CD26 is a glycoprotein which presents well-known pleiotropic effects, and this is probably associated with the varied roles that this enzyme shows in different neoplasms. Two main action mechanisms have been proposed: on one hand, its catalytic activity on bioactive peptides such as substance P, growth hormone-releasing hormone and several chemokines and interleukins which act in cancer growth regulation [33] and, on the other hand, through its direct interaction with certain molecules of the extracellular matrix [34]. These varied actions make it difficult to define the exact role that DPPIV/CD26 plays in cancer, and further investigations are required to elucidate them [24].
Many of the essential alterations occurring in cell physiology during carcinogenesis are mediated via extracellular cell signalling pathways that interact with intracellular circuits. So, both cell surface and intracellular molecules play pivotal roles in cancer pathobiology [35]. Several authors have pointed towards specific roles of peptidases in proliferation and neoplasia, either at the surface or at the intracellular level [36–40].
AlaAP and APB are involved in the regulation of apoptosis and cell cycle regulation, and inhibitors of these enzymes such as bestatin are potential candidates for chemotherapeutic agents [38]. On the other hand, the high activity of the cytosolic PEP in developing tissues, its cellular localization around the nucleus in proliferative cells and tumours, and the inhibition of DNA synthesis by PEP inhibitors seem to involve this enzyme in cell proliferation and differentiation [39–43]. Previous studies have also suggested a role for AspAP, PGI, and other soluble peptidases in different neoplastic processes [19–22, 36]. The higher activity of these peptidases we have detected in thyroid neoplasms with respect to non neoplastic tissue points to a role of cytosolic peptidases in thyroid tumours.
The best known function of peptidases is the conversion of bioactive peptides. Aside from DPPIV/CD26, several peptides converted by cytosolic AlaAP, PEP, AspAP, and PGI are involved in cell growth and proliferative disorders [44–48], for instance, angiotensins that are hydrolyzed by AspAP and PEP, opioid peptides by alaAP, leukotriene A4 by APB, and TRH, by PGI and, indirectly by PEP. However, the role of soluble peptidases in the regulation of bioactive peptides related to proliferative disorders is not so clear as it is in the case of cell surface peptidases. Although the secretion of PEP, APB, and other soluble peptidases into the extracellular space has been suggested as a possible peptide regulatory mechanism [49–51], there is accumulating evidence in favor of intracellular trafficking and action of certain peptides and proteolytic enzymes known as intracrine action [52–54]. Thus, it has been suggested that the aforementioned bioactive peptides may also act as intracrine factors in the intracellular space inducing cell proliferation and angiogenesis in several tissues [52–54]. Therefore, the idea of increasing cytosolic peptidase activities as a result of intracrine peptide dysregulation in thyroid neoplasms should not be ruled out.
In summary, this study analyses the activity of several peptidases in PTC, FTA, and TNH and suggests a potential role of a cell surface peptidase (DPPIV/CD26) and several cytosolic peptidases (PEP, APB, AspAP, AlaAP, and PGI) in thyroid neoplasms. However, further studies are needed to define the exact involvement of peptidases in the pathobiology of these tumours and their possible clinical usefulness.
Conflict of Interests
The authors declare that there is no conflict of interests.
Ethical Approval
The ethic committee of the Basurto University hospital approved this study (CEIC 11/51).
Acknowledgments
The authors wish to thank Arantza Pérez (University of the Basque Country) for her technical contribution to this study. This work was supported by a Grant from the Basque Government (Saiotek SA-2010/00) and UPV/EHU (UFI 11/44).
Abbreviations
- APA:
Aminopeptidase A
- APN/CD13:
Aminopeptidase N/CD13
- AspAP:
Aspartyl aminopeptidase
- APB:
Aminopeptidase B
- DPPIV/CD26:
Dipeptidyl peptidase IV/CD26
- NEP/CD10:
Neutral endopeptidase/CD10
- PEP:
Prolyl endopeptidase
- PGI:
Pyroglutamyl peptidase I
- AlaAP:
Puromycin-sensitive alanyl aminopeptidase
- CAP:
Cystinyl aminopeptidase
- MMP:
Matrix metalloproteinase
- PTC:
Papillary thyroid carcinoma
- FTA:
Follicular thyroid adenoma
- TNH:
Thyroid nodular hyperplasia.
References
- 1.Bahn RS, Castro MR. Approach to the patient with nontoxic multinodular goiter. Journal of Clinical Endocrinology and Metabolism. 2011;96(5):1202–1212. doi: 10.1210/jc.2010-2583. [DOI] [PubMed] [Google Scholar]
- 2.Rosai J, Tallini G. Rosai and Ackerman's Surgical Pathology. 10th edition. chapter 9. Vol. 1. Edinburgh, UK: Elsevier-Mosby; 2011. Thyroid gland; pp. 487–564. [Google Scholar]
- 3.Mete O, Asa SL. Pitfalls in the diagnosis of follicular epithelial proliferations of the thyroid. Advances in Anatomic Pathology. 2012;19(6):363–373. doi: 10.1097/PAP.0b013e318271a5ac. [DOI] [PubMed] [Google Scholar]
- 4.López JI, Zabala R, del Cura JL. Histological diagnosis of the thyroid disease using ultrasound-guided core biopsies. European Thyroid Journal. 2013;2:29–36. doi: 10.1159/000343825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head and Neck Pathology. 2011;5(1):51–56. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LiVolsi VA. Papillary thyroid carcinoma: an update. Modern Pathology. 2011;24(supplement 2):S1–S9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg M, Le Grevès P, Nyberg F. Neuropeptide processing. In: Lendeckel U, Hooper NM, editors. Proteases in the Brain. New York, NY, USA: Springer Science+Business Media; 2004. pp. 203–234. [Google Scholar]
- 8.Ino K, Shibata K, Kajiyama H, Kikkawa F, Mizutani S. Regulatory role of membrane-bound peptidases in the progression of gynecologic malignancies. Biological Chemistry. 2004;385(8):683–690. doi: 10.1515/BC.2004.084. [DOI] [PubMed] [Google Scholar]
- 9.Carl-McGrath S, Lendeckel U, Ebert M, Röcken C. Ectopeptidases in tumour biology: a review. Histology and Histopathology. 2006;21(12):1339–1353. doi: 10.14670/HH-21.1339. [DOI] [PubMed] [Google Scholar]
- 10.Antczak C, De Meester I, Bauvois B. Ectopeptidases in pathophysiology. Bioessays. 2001;23(3):251–260. doi: 10.1002/1521-1878(200103)23:3<251::AID-BIES1035>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Yoshida H, Kakudo K, Nakamura Y, Kuma K, Miyauchi A. Inverse relationships between the expression of MMP-7 and MMP-11 and predictors of poor prognosis of papillary thyroid carcinoma. Pathology. 2006;38(5):421–425. doi: 10.1080/00313020600922496. [DOI] [PubMed] [Google Scholar]
- 12.Kraiem Z, Korem S. Matrix metalloproteinases and the thyroid. Thyroid. 2000;10(12):1061–1069. doi: 10.1089/thy.2000.10.1061. [DOI] [PubMed] [Google Scholar]
- 13.Liang H, Zhong Y, Luo Z, et al. Assessment of biomarkers for clinical diagnosis of papillary thyroid carcinoma with distant metastasis. International Journal of Biological Markers. 2010;25(1):38–45. doi: 10.1177/172460081002500106. [DOI] [PubMed] [Google Scholar]
- 14.Maeta H, Ohgi S, Terada T. Protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in papillary thyroid carcinomas. Virchows Archiv. 2001;438(2):121–128. doi: 10.1007/s004280000286. [DOI] [PubMed] [Google Scholar]
- 15.Marecko I, Cvejic D, Tatic S, Dragutinovic V, Paunovic I, Savin S. Expression of matrix metalloproteinase-2 and its tissue inhibitor-2 in fetal and neoplastic thyroid tissue and their significance as diagnostic and prognostic markers in papillary carcinoma. Cancer Biomark. 2011-2012;11(1):49–58. doi: 10.3233/CBM-2012-0258. [DOI] [PubMed] [Google Scholar]
- 16.De Micco C, Savchenko V, Giorgi R, Sebag F, Henry J-F. Utility of malignancy markers in fine-needle aspiration cytology of thyroid nodules: comparison of Hector Battifora mesothelial antigen-1, thyroid peroxidase and dipeptidyl aminopeptidase IV. British Journal of Cancer. 2008;98(4):818–823. doi: 10.1038/sj.bjc.6604194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotani T, Kawano J, Suganuma T, et al. Immunohistochemical localization of dipeptidyl aminopeptidase IV in thyroid papillary carcinoma. International Journal of Experimental Pathology. 1992;73(2):215–222. [PMC free article] [PubMed] [Google Scholar]
- 18.Umeki K, Tanaka T, Yamamoto I, et al. Differential expression of dipeptidyl peptidase IV (CD26) and thyroid peroxidase in neoplastic thyroid tissues. Endocrine Journal. 1996;43(1):53–60. doi: 10.1507/endocrj.43.53. [DOI] [PubMed] [Google Scholar]
- 19.Blanco L, Larrinaga G, Pérez I, et al. Acid, basic, and neutral peptidases present different profiles in chromophobe renal cell carcinoma and in oncocytoma. American Journal of Physiology. 2008;294(4):F850–F858. doi: 10.1152/ajprenal.00469.2007. [DOI] [PubMed] [Google Scholar]
- 20.Pérez I, Varona A, Blanco L, et al. Increased APN/CD13 and acid aminopeptidase activities in head and neck squamous cell carcinoma. Head and Neck. 2009;31(10):1335–1340. doi: 10.1002/hed.21099. [DOI] [PubMed] [Google Scholar]
- 21.Varona A, Blanco L, López JI, et al. Altered levels of acid, basic, and neutral peptidase activity and expression in human clear cell renal cell carcinoma. American Journal of Physiology. 2007;292(2):F780–F788. doi: 10.1152/ajprenal.00148.2006. [DOI] [PubMed] [Google Scholar]
- 22.Larrinaga G, Blanco L, Sanz B, et al. The impact of peptidase activity on clear cell renal cell carcinoma survival. American Journal of Physiology. 2012;303(12):F1584–F1559. doi: 10.1152/ajprenal.00477.2012. [DOI] [PubMed] [Google Scholar]
- 23.Larrinaga G, Perez I, Blanco L, et al. Increased prolyl endopeptidase activity in human neoplasia. Regulatory Peptides. 2010;163(1–3):102–106. doi: 10.1016/j.regpep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Kikkawa F, Kajiyama H, Shibata K, Ino K, Nomura S, Mizutani S. Dipeptidyl peptidase IV in tumor progression. Biochimica et Biophysica Acta. 2005;1751(1):45–51. doi: 10.1016/j.bbapap.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Zambotti-Villela L, Yamasaki SC, Villarroel JS, Alponti RF, Silveira PF. Aspartyl, arginyl and alanyl aminopeptidase activities in the hippocampus and hypothalamus of streptozotocin-induced diabetic rats. Brain Research. 2007;1170:112–118. doi: 10.1016/j.brainres.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Ogita K, Walter R. Post-proline cleaving enzyme. Synthesis of a new fluorogenic substrate and distribution of the endopeptidase in rat tissues and body fluids of man. Biochimica et Biophysica Acta. 1979;569(2):184–192. doi: 10.1016/0005-2744(79)90053-6. [DOI] [PubMed] [Google Scholar]
- 27.Mantle D, Lauffat B, McDermott J, Gibson A. Characterization of aminopeptidases in human kidney soluble fraction. Clinica Chimica Acta. 1990;187(2):105–113. doi: 10.1016/0009-8981(90)90336-q. [DOI] [PubMed] [Google Scholar]
- 28.Silveira PF, Irazusta J, Gil J, Agirregoitia N, Casis L. Interactions among challenges of hydromineral balance, angiotensin-converting enzyme, and cystine aminopeptidase. Peptides. 2001;22(12):2137–2144. doi: 10.1016/s0196-9781(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Goossens F, De Meester I, Vanhoof G, Scharpé S. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. European Journal of Clinical Chemistry and Clinical Biochemistry. 1996;34(1):17–22. doi: 10.1515/cclm.1996.34.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Liu JL, Kusinski M, Ilic V, et al. Overexpression of the angiogenic tetrapeptide AcSDKP in human malignant tumors. Anticancer Research. 2008;28(5):2813–2817. [PubMed] [Google Scholar]
- 32.Sedo A, Krepela E, Kasafirek E. Dipeptidyl peptidase IV, prolyl endopeptidase and cathepsin B activities in primary human lung tumours and lung parenchyma. Journal of Cancer Research and Clinical Oncology. 1991;117(3):249–253. doi: 10.1007/BF01625433. [DOI] [PubMed] [Google Scholar]
- 33.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clinical Science. 2005;108(4):277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 34.Bušek P, Malík R, Šedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. International Journal of Biochemistry and Cell Biology. 2004;36(3):408–421. doi: 10.1016/s1357-2725(03)00262-0. [DOI] [PubMed] [Google Scholar]
- 35.Grujić M, Renko M. Aminopeptidase inhibitors bestatin and actinonin inhibit cell proliferation of myeloma cells predominantly by intracellular interactions. Cancer Letters. 2002;182(2):113–119. doi: 10.1016/S0304-3835(02)00086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez JM, Prieto I, Ramírez MJ, Cueva C, Alba F, Martínez M. Aminopeptidase activities in breast cancer tissue. Clinical Chemistry. 1999;45(10):1797–1802. [PubMed] [Google Scholar]
- 37.Nanus DM. Of peptides and peptidases: the role of cell surface peptidases in cancer. Clinical Cancer Research. 2003;9(17):6307–6309. [PubMed] [Google Scholar]
- 38.Thompson MW, Hersh LB. The puromycin-sensitive aminopeptidase. Role in neurological, reproductive, inmunological and proliferative disorders. In: Hooper NM, Lendeckel U, editors. Aminopeptidases in Biology and Disease. New York, NY, USA: Kluwer Academic/Plenum Press; 2004. pp. 1–15. [Google Scholar]
- 39.Ishino T, Ohtsuki S, Homma K, Natori S. cDNA cloning of mouse prolyl endopeptidase and its involvement in DNA synthesis by Swiss 3T3 cells. Journal of Biochemistry. 1998;123(3):540–545. doi: 10.1093/oxfordjournals.jbchem.a021970. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Baylach MJ, Felipo V, Männistö PT, García-Horsman JA. Expression and traffic of cellular prolyl oligopeptidase are regulated during cerebellar granule cell differentiation, maturation, and aging. Neuroscience. 2008;156(3):580–585. doi: 10.1016/j.neuroscience.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 41.Myöhänen TT, Venäläinen JI, Tupala E, Garcia-Horsman JA, Miettinen R, Männistö PT. Distribution of immunoreactive prolyl oligopeptidase in human and rat brain. Neurochemical Research. 2007;32(8):1365–1374. doi: 10.1007/s11064-007-9316-y. [DOI] [PubMed] [Google Scholar]
- 42.Hannula MJ, Männistö PT, Myöhänen TT. Sequential expression, activity and nuclear localization of prolyl oligopeptidase protein in the developing rat brain. British Journal of Pharmacology. 2012;166(3):1097–1113. doi: 10.1159/000322082. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Baylach MJ, Puttonen KA, Tenorio-Laranga J, et al. Prolyl endopeptidase is involved in cellular signalling in human neuroblastoma SH-SY5Y cells. NeuroSignals. 2011;19(2):97–109. doi: 10.1159/000326342. [DOI] [PubMed] [Google Scholar]
- 44.DuBois RN. Leukotriene A4 signaling, inflammation, and cancer. Journal of the National Cancer Institute. 2003;95(14):1028–1029. doi: 10.1093/jnci/95.14.1028. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Li N, Wang S, et al. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. Journal of the National Cancer Institute. 2003;95(14):1053–1061. doi: 10.1093/jnci/95.14.1053. [DOI] [PubMed] [Google Scholar]
- 46.Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29(9):1675–1684. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 47.Malaguti A, Della Casa C, Castorina S, et al. Molecular mechanisms for pituitary thyrotroph cell growth. Journal of Endocrinological Investigation. 2004;27(6):151–167. [PubMed] [Google Scholar]
- 48.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr) Brain Research Reviews. 2002;38(3):351–376. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 49.Balogh A, Cadel S, Foulon T, et al. Aminopeptidase B: a processing enzyme secreted and associated with the plasma membrane of rat pheochromocytoma (PC12) cells. Journal of Cell Science. 1998;111(2):161–169. doi: 10.1242/jcs.111.2.161. [DOI] [PubMed] [Google Scholar]
- 50.García-Hornsman JA, Männistö PT, Venäläinen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 2007;41(1):1–24. doi: 10.1016/j.npep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Shrimpton CN, Smith AI, Lew RA. Soluble metalloendopeptidases and neuroendocrine signaling. Endocrine Reviews. 2002;23(5):647–664. doi: 10.1210/er.2001-0032. [DOI] [PubMed] [Google Scholar]
- 52.Re RN, Cook JL. An intracrine view of angiogenesis. BioEssays. 2006;28(9):943–953. doi: 10.1002/bies.20459. [DOI] [PubMed] [Google Scholar]
- 53.Re RN, Cook JL. The intracrine hypothesis: an update. Regulatory Peptides. 2006;133(1–3):1–9. doi: 10.1016/j.regpep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Re RN, Cook JL. Noncanonical intracrine action. Journal of the American Society of Hypertension. 2011;5(6):435–448. doi: 10.1016/j.jash.2011.07.001. [DOI] [PubMed] [Google Scholar]


