Abstract
Oxidative stress involving pre-myelinating oligodendrocytes (OLs) is a major factor in the pathogenesis of preterm white matter injury. In animal and cell culture studies, activation of the lipid-oxidizing enzyme 12/15-lipoxygenase (12/15-LOX) plays a central role as an inflammatory mediator in the pathology of oxidative stress and OL cell death, as well as ischemia and neuronal death. The role of 12/15-LOX, however, is unclear in the developing human brain. The mechanism of 12/15-LOX involves the production of reactive oxygen species through the metabolism of arachidonic acid, as well as direct detrimental effects on organelle membranes. Here we tested the hypothesis that the density of 12/15-LOX-expressing cells is increased in periventricular leukomalacia (PVL). Using immunocytochemistry in human paraffin-embedded tissue, 12/15-LOX expression was seen in macrophages of the focally necrotic lesions in the periventricular white matter, as well as in glial cells throughout the surrounding white matter with reactive gliosis. Interestingly, no significant 12/15-LOX expression was detected in neurons in the cerebral cortex overlying the damaged white matter. Using a scoring system from 0 to 3, we assessed the density of 12/15-LOX-expressing cells in diffusely gliotic white matter from 20–43 postconceptional (PC) weeks in 19 PVL cases [median = 36 PC weeks] and 10 control (non-PVL) cases [median = 34 PC weeks]. The density of 12/15-LOX-positive cells was significantly increased in the diffuse component of PVL [score = 1.17 +/− 0.15] compared to controls [score = 0.48 +/− 0.21] (p = 0.014). Using double-label immunocytochemistry, 12/15-LOX was observed in PVL in OLs of the O4 and O1 premyelinating stages, as well as in mature OLs as determined with the mature OL marker adenomatous polyposis coli (APC). In addition, 12/15-LOX expression was present in a population of CD68-positive activated microglia. There was no 12/15-LOX expression in reactive astrocytes. Finally we observed terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL)–positive cells within the white matter of PVL that expressed 12/15-LOX and/or within close proximity of 12/15-LOX positive cells. Our data support a role for 12/15-LOX activation as an inflammatory mediator of injury in PVL, with a contribution of 12/15-LOX to PVL-induced damage to or cell death of OLs, including at the O1 and O4 stages.
Keywords: hypoxia-ischemia, inflammation, oxidative stress, prematurity, reactive oxygen species
INTRODUCTION
The occurrence of brain injury in the human neonate, particularly in the setting of prematurity, is associated with devastating outcomes, including mortality and life-long neurological deficits. Periventricular leukomalacia (PVL), a lesion of the immature cerebral white matter, is the predominant pathological substrate of the motor and cognitive disorders in long-term survivors of prematurity [1–3]. Pathologically, PVL is composed of a focally necrotic component in the deep periventricular white matter characterized by loss of all cellular elements, and a diffuse component in the surrounding white matter characterized by reactive astrogliosis and microgliosis [4]. While necrotic lesions are still seen at autopsy, with advancements in neonatal care, their pathologic features have changed from grossly visible, large cystic cavities, to now smaller, non-cavitating focal lesions visible only at the microscopic level and potentially below the level of detection in conventional neuroimaging [5–8]. Of particular interest in PVL is the effect of white matter damage on the developing, premyelinating oligodendrocytes (pre-OLs). In the diffuse component of PVL, damage to pre-OLs is thought to underlie the myelin deficits seen in long term survivors of prematurity [3]. This is supported by the finding in the human of acute pre-OL loss in PVL [9–11]. While promising therapies are being tested in the human and in animals [12–16], there are currently no drug interventions that completely prevent or ameliorate white matter damage in the premature infant.
The causes of PVL are complex and likely include cerebral ischemia and reperfusion with the contribution of maternal infection with fetal/neonatal systemic inflammation in some cases [2, 17–20]. Given its involvement in both hypoxia-ischemia (H-I) and inflammation/infection, a major area of interest in PVL is the role of oxidative stress in the development of white matter injury and OL damage. While evidence of oxidative stress has been shown in PVL [9, 10], the sources of ROS in PVL are currently unknown. One potential source of ROS is 12/15-lipoxygenase (12/15-LOX), which catalyzes the addition of oxygen to arachidonic acid (AA) yielding oxidized derivatives, including hydroperoxyeicosatetraenoic acids (HPETEs). In addition, 12/15-LOX can directly oxidize and damage organelle membranes, causing cell death through mitochondrial injury [21, 22]. Under conditions of hypoxia-ischemia (H-I), AA levels in the brain increase due to an increased activation of phospholipase PLA2 which releases AA from cellular membranes [23–26]. In the neonatal rat brain, the RNA encoding leukocyte-type 12-LOX (the rodent isoform of human 12/15-LOX) is also increased under hypoxic conditions [27], which in the setting of increased AA can result in excessive AA metabolism. Upregulation of 12/15-LOX is an inflammatory response to H-I injury and the metabolism of free AA by 12/15-LOX can serve as a source of oxidative stress through the production of unstable byproducts which can function directly as ROS [28, 29]. In addition to these pro-inflammatory mediators, 12/15-LOX can, in conjunction with other enzymes, also generate inflammation-limiting lipoxins, resolvins and protectins, which have been proposed to cause inflammation resolution [30–32]. Besides tissue damaging effects, protective effects of 12/15-LOX up-regulation can thus not a priori be excluded. Interestingly, 12/15-LOX can also directly oxidize AA and other fatty acid substrates esterified as phospholipids resulting in lipid peroxidation of cellular membranes [33, 34]. Important to the role of 12/15-LOX in PVL, under conditions of glutathione depletion, a known consequence of H-I, culture studies have shown that 12/15-LOX activity increases in pre-OLs [35] and that inhibition of 12/15-LOX prevents ROS accumulation and pre-OL cell death [35–37]. These animal observations strongly suggest a role for 12/15-LOX in pre-OL damage in PVL, underscoring that inhibition of this enzyme is a therapeutic candidate aimed at the prevention of oxidative stress in PVL in human infants. Prior to the consideration of 12/15-LOX in translational research, however, evidence of the involvement of 12/15-LOX in human PVL must be shown.
In the following study, we examined the expression of 12/15-LOX in PVL in human infants during the second half of gestation. While 12/15-LOX expression has been shown in the human adult brain [38, 39], little is known in regard to 12/15-LOX expression during human brain development or in the setting of human neonatal brain damage. We hypothesized that the density of 12/15-LOX-immunopositive cells is significantly increased in the diffuse component of PVL compared to controls adjusted for age. While we report on findings in both the focal and diffuse lesion, we focused predominantly on the diffuse component, because damage to the diffuse component may result in the global neurological deficits seen in long-term survivors of prematurity [1]. We examined the expression of 12/15-LOX in inflammatory cells of the white matter, i.e., microglia and reactive astrocytes. In addition, we examined 12/15-LOX expression in OLs of the O4 and O1 pre-myelinating stages in order to determine if this enzyme is up-regulated in this vulnerable cell population in association with the inflammatory changes of PVL.
MATERIALS AND METHODS
Clinicopathologic database information
Cases were collected from the autopsy services of the Departments of Pathology, Boston Children’s Hospital and Brigham and Women’s Hospital with parental permission according to Institutional Review Board protocol. Archival, paraformaldehyde-fixed, paraffin-embedded, brain tissue from either the parietal, occipital, or frontal lobes of 19 PVL cases and 10 controls (i.e., lacking PVL) were used for all single-label ICC and for all double-label ICC with the exception of O4 and O1 OL immunostaining and TUNEL methodology. In the immunostaining with O4 and O1 markers, paraformaldehyde-fixed, non-paraffin-embedded tissue from the parietal-occipital lobes of 5 PVL cases and 4 controls were used In TUNEL combined with 12/15-LOX immunostaining, frozen tissue from the parietal-occipital lobes of 6 PVL and 3 control cases were used. All cases were classified as PVL or control (non-PVL) by the systematic examination of standardized microscopic sections, stained with conventional hematoxylin-and-eosin/Luxol-fast-blue, from each brain and spinal cord. As in other studies from our laboratory [9, 40, 41], PVL was defined as a lesion of the immature cerebral white matter with focal and diffuse components in combination (see above). The control cases were defined as perinatal deaths in which neuropathologic examination did not reveal PVL; in these cases, there were no or minimal neuropathologic changes in the white and gray matter.
Single-label ICC
An affinity purified polyclonal anti-12/15-LOX antibody raised in rabbit was used [42]. Standard methods in deparaffinized tissue sections (5 microns) were performed. Briefly, antigen retrieval involved 10 minutes of microwave at 195°F in citrate buffer, pH 6.0. After retrieval, sections were treated with a dual endogenous enzyme block (DAKO, Carpinteria, Ca) to inhibit endogenous peroxidase activity followed by a 1 hour protein block in phosphate buffered saline (PBS) with 4% normal goat serum. Sections were incubated overnight at 4°C in primary antibody diluted 1:200 in protein block solution. After sections were washed in PBS + 0.1% Tween 20, biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) was applied at 1:200 dilution for 30 minutes at room temperature. Sections were washed and incubated for 30 minutes in ABC reagent (Dako, Carpinteria, Ca) followed by DAB detection. Negative controls omitted the primary antibodies.
Grading method for single-label ICC
Grading of the cell density (positive cells/high power field) of single-labeled tissue sections was performed in both PVL and control tissue, as previously reported by us [9, 40, 41]. After a survey of the entire section, immunopositive cells per high power field (hpf) (x40 magnification) were counted in 3–5 fields within the most intensely stained area of tissue within the periventricular white matter. The three fields were averaged and given an overall standardization score as follows: 0, no cell staining; 0.5, 0–1 immunopositive cell/hpf; 1, 2–10 cells/hpf; 2, 11–20 immunopositive cells/hpf; and 3,>20 cells/hpf. Only cells with a well-defined nucleus and 12/15-LOX-stained cytoplasm were counted as positive. Cells with nuclear-only staining were not considered in this quantitative analysis. In PVL cases, the quantitated fields were in the diffuse component only; the focal necrotic lesion was not included in any scoring. Given the obvious nature of white matter pathology in PVL, it was not possible to score 12/15-LOX-immunopositive cells blinded to diagnosis.
Double-label ICC in paraffin-embedded tissue
Antigen retrieval was performed as described above in 4 to 12 PVL cases. Tissue was blocked in PBS containing 5% goat serum and 0.05% Triton X-100 for 1 h at room temperature, and incubated overnight at 4°C in blocking solution containing antibodies to 12/15-LOX and one of the following monoclonal antibodies: astrocytic marker GFAP (1:5000, Covance [Princeton, NJ]), microglial marker tomato lectin (1:200, Vector Laboratories, [Burlingame, CA]), activated microglial marker CD68 (1:25, Cell Marque [Rocklin, CA]), or mature OL marker APC (1:25, Millipore [Billerica, MA]). After a series of washes, the tissue was incubated in blocking solution containing Alexa-Fluor goat anti-rabbit 488 and Alexa-Fluor goat anti-mouse 590, both at a concentration of 1:1,000 (Molecular Probes, Eugene, OR, USA). Immunofluorescence was visualized with the Olympus BX51 microscope (Olympus America, Inc., Melville, NY). Images were captured with a Cool SNAP fx camera (Photometrics, Tuscan, AZ) and MCID Elite 6.0 software (Life Sciences, Piscataway, NJ).
Double-label ICC for O4 and O1
Double label with monoclonal antibodies O4 and O1 for detection of OLs [43] was performed using paraformaldehyde-fixed, free floating sections. Paraformaldehyde-fixed tissue was cryoprotected in PBS containing 30% sucrose and frozen. Frozen tissue was cut on a cryostat at 40 microns and collected in cold PBS. Tissue was incubated with either O4 or O1 antibody [43] in PBS containing 5% goat serum overnight at 4°C, followed by a 1 hour incubation in Alexa – Fluor goat anti-mouse 594 IgM antibody (Molecular Probes, Eugene, OR, USA). Tissue was then incubated with anti-12/15-LOX in PBS containing 5% goat serum/0.1% Triton X-100 overnight at 4°C. Sections were mounted onto slides and immunofluorescence was visualized as above
Quantitation of O4 and O1 double-label ICC
Sections double-labeled with 12/15-LOX and O4 or O1 OL markers were examined to determine the percent of O4 and O1 OLs that expressed 12/15-LOX. Sections were stained as described above. After a survey of the entire section, 5–10 fields (x40 magnification) within the most intensely stained area of tissue within the periventricular white matter were examined. The percent of O4/O1 + cells that expressed 12/15-LOX was determined by counting all O4/O1 + cells that expressed 12/15-LOX and dividing that by all cells that expressed O4/O1.
TUNEL combined with 12/15-LOX immunocytochemistry
Frozen tissue was sectioned in the cryostat at 30 microns. Tissue was fixed for 10 minutes in 4% paraformaldehyde in PBS followed by three 5 minute washes in PBS. TUNEL was performed using the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Millipore, Billerica, MA) according to the manufacture’s instruction. Following TUNEL, tissue was blocked in PBS containing 5% goat serum and 0.05% Triton X-100 for 1 h at room temperature, and incubated overnight at 4°C in blocking solution containing the antibody to 12/15-LOX at 1:200. After a series of washes, the tissue was incubated in blocking solution containing Alexa-Fluor goat anti-rabbit 590 at a concentration of 1:1,000 (Molecular Probes, Eugene, OR, USA).
Statistics
Age-adjusted differences in PVL relative to controls were accessed using analysis of covariance (ANCOVA) adjusting for postconceptional (PC), postnatal (PN), and gestational age (GA).
RESULTS
Clinical Database
For all immunochemical analyses in frozen or paraformaldehyde-fixed, paraffin-embedded and non-paraffin-embedded tissue, we analyzed 12/15-LOX expression in the cerebral white matter of 28 cases of PVL and 15 control cases without PVL (Table 1). The PVL cases ranged in age from 29 to 43 PC weeks (median of 35.5 PC weeks) and 0–8 PN weeks (median of 1.5 PN weeks); the controls ranged from 20 to 43 PC weeks (median of 33.5 PC weeks) and 0–2.5 PN weeks (median of 1 PN week). The ages and primary causes of death of each case used are listed in Table 1. Also listed is the presence of germinal matrix hemorrhage and infection. The postmortem interval (PMI) ranged from 6 to 25 hours for the PVL cases with outliers at 44 and 89 hours, and 4–25 hours for the control cases.
Table 1.
Clinical summary of cases
| Case | DX | GA | PNA | PCA | Clinical Diagnosis, Cause of Death | Mac PVL | Mic PVL | GMH | Inf | Lox (DAB) | Lox O4/O1 | TUNEL Lox |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | 20 | 0 SB |
20 | CHD | - | - | Y | N | Y | N | N |
| 2 | C | 23 | 0 ET |
23 | Termination for congenital diaphragmatic hernia | - | - | N | N | Y | N | N |
| 3 | C | 23 | 0 SB |
23 | Placental abruption, chorioamnionitis | - | - | Y | Y | N | Y | N |
| 4 | C | 24 | <1 | 24 | Extreme prematurity, Sepsis | - | - | N | Y | Y | N | N |
| 5 | C | 24 | 2.5 | 26.5 | Extreme prematurity, chronic lung disease, patent ductus | - | - | N | N | Y | N | N |
| 6 | C | 28 | <1 | 28 | Prematurity, chorioamnionitis | - | - | N | Y | N | N | Y |
| 7 | C | 30 | <1 | 30 | Prematurity, pulmonary hypoplasia | - | - | N | N | N | Y | N |
| 8 | C | 31 | 2.5 | 33.5 | Prematurity, necrotizing enterocolitis | - | - | N | N | Y | N | N |
| 9 | C | 34 | <1 | 34 | Prematurity, hydrops fetalis | - | - | N | Y | Y | N | N |
| 10 | C | 34 | 0 SB |
34 | Unexplained stillbirth | - | - | N | N | Y | N | N |
| 11 | C | 35 | <1 | 35 | Prematurity, Potter’s sequence, Pulmonary hypertension | - | - | N | N | N | N | Y |
| 12 | C | 38 | <1 | 38 | Multicystic dysplastic kidney Potter’s Sequence | - | - | N | N | Y | Y | N |
| 13 | C | 38 | <1 | 38 | Hypoplastic left heart syndrome | - | - | N | N | N | N | Y |
| 14 | C | 40 | <1 | 40 | Pulmonary Hypoplasia | - | - | N | N | Y | N | N |
| 15 | C | 43 | <1 | 43 | Pulmonary Hypoplasia | - | - | N | N | Y | N | N |
| 16 | P | 25 | 4 | 29 | Extreme prematurity, chronic lung disease | N | Y | Y | Y | N | N | Y |
| 17 | P | 29 | <1 | 29 | Prematurity, skeletal dysplasia | N | Y | Y | N | Y | N | N |
| 18 | P | 27 | 2 | 29 | Prematurity, Sepsis | Y | Y | Y | Y | N | Y | N |
| 19 | P | 28 | 1 | 29 | Prematurity, hyaline membrane disease, pulmonary hemorrhage, CHD | N | Y | Y | N | N | Y | N |
| 20 | P | 25 | 4 | 29 | Extreme prematurity, chronic lung disease | Y | Y | Y | Y | Y | N | N |
| 21 | P | 31.5 | 0 SB |
31.5 | Prematurity, duodenal atresia, chorioamnionitis | Y | Y | N | Y | Y | N | N |
| 22 | P | 30 | 3 | 33 | Prematurity, severe intraventricular hemorrhage | N | Y | Y | Y | Y | N | N |
| 23 | P | 32 | 1 | 33 | Prematurity, sepsis, intraventricular hemorrhage | N | Y | Y | Y | Y | N | N |
| 24 | P | 32 | 2 | 34 | Prematurity, necrotizing enterocolitis | Y | Y | Y | Y | Y | N | N |
| 25 | P | 34 | <1 | 34 | Prematurity, small bowel atresia | Y | Y | Y | N | N | N | Y |
| 26 | P | 34 | 1 | 35 | Prematurity, hyaline membrane disease, metabolic disease, patent ductus arteriosus | N | Y | N | N | Y | N | N |
| 27 | P | 34 | 1 | 35 | Prematurity, respiratory distress syndrome | Y | Y | N | Y | Y | N | N |
| 28 | P | 34 | 1 | 35 | Prematurity, coagulopathy, organ damage due to HSV | N | Y | N | Y | Y | N | N |
| 29 | P | 35 | <1 | 35 | Prematurity, fetal vascular thrombosis in placenta, multiorgan ischemic injury, acute myocardial infarction | N | Y | N | N | N | Y | N |
| 30 | P | 36 | 0 SB |
36 | Prematurity, CMV, hydrops fetalis, skeletal dysplasia | Y | Y | Y | Y | Y | N | N |
| 31 | P | 36 | <1 | 36 | Prematurity, chromosomal translocation (t 12/14) and trisomy 12P; multiorgan failure | N | Y | N | N | Y | N | N |
| 32 | P | 36 | <1 | 37 | Prematurity, CHD | N | Y | N | N | Y | N | N |
| 33 | P | 34 | 4 | 38 | Prematurity, congenital diaphragmatic hernia; pulmonary hypoplasia | N | Y | Y | Y | Y | N | Y |
| 34 | P | 35 | 4 | 39 | Prematurity, CHD | Y | Y | N | Y | Y | N | N |
| 35 | P | 37 | 2 | 39 | Congenital pulmonary dysplasia | Y | Y | N | N | N | Y | N |
| 36 | P | 37 | 2 | 39 | Hypoplastic left heart syndrome | Y | Y | N | N | N | N | Y |
| 37 | P | 35 | 5 | 40 | Prematurity, diffuse mesangial sclerosis, congenital nephrotic syndrome | Y | Y | N | N | Y | N | N |
| 38 | P | 39 | 1 | 40 | Urea cycle defect | Y | Y | N | N | Y | N | N |
| 39 | P | 38 | 2 | 40 | Primary pulmonary hypertension | Y | Y | N | Y | Y | N | N |
| 40 | P | 40 | 1 | 41 | Hypoplastic left heart syndrome | N | Y | N | Y | N | N | Y |
| 41 | P | 36 | 5 | 41 | Prematurity, hemophagocytic lymphohistiocytosis, sepsis, multiorgan failure | Y | Y | Y | Y | N | Y | Y |
| 42 | P | 34 | 8 | 42 | Prematurity, congenital hypotonia, progressive cystic encephalomalacia | Y | Y | N | Y | Y | N | N |
| 43 | P | 40 | 3 | 43 | Haddad syndrome/ CCHS | N | Y | N | Y | Y | N | N |
DX, diagnosis; C, control; P, PVL; PGA, gestational age (weeks); PNA, postnatal age (weeks); PCA, postconceptional age (weeks); Mac PVL, macroscopic PVL; Mic PVL, microscopic PVL; GMH, germinal matrix hemorrhage; Inf, infection; SB, stillbirth; ET, elective termination; DAB, diaminobenzidine; CHD, congenital heart disease; HSV, herpes simplex virus; CMV, cytomegalovirus; CCHS, congenital central hypoventilation syndrome.
Neuropathology of PVL
Each of our PVL cases were designated as such based on the presence of a focal necrotic lesion within the white matter. The stage and degree of severity of the focal necrosis varied, however, reflecting the typical spectrum of this pathology in our autopsy service. 54% (15 out of 28) of the PVL cases had recognizable focal periventricular necrosis at the time of brain cutting (Table 1). These were described grossly as either cystic lesions or chalky-white foci of necrosis, the classic so-called “white spots” of PVL. In the remaining 46% of the total PVL cases in this series (13 out of 28), the necrotic foci were detected only microscopically and varied in size up to approximately 2 mm (Table 1).
Normative expression of 12/15-LOX
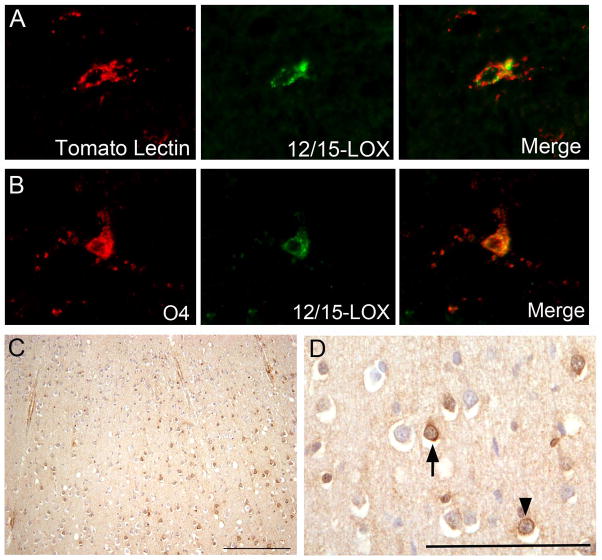
In the time frame examined, the second half of gestation, there was little 12/15-LOX expression in the white matter of controls as seen in paraformaldehyde fixed, paraffin-embedded cases (n=10). Scattered positive cells were detectable in 4 out of 10 control cases and were given density scores of either 0.5 or 1 as indicated: Case 9 at 34 PC weeks (density score of 0.5); Case 12 at 38 PC weeks (density score of 1.0); Case 14 at 40 PC weeks (density score of 0.5); and Case 15 at 43 PC weeks (density score of 1.0). There were 6 control cases from 20 – 34 PC weeks which showed no staining within the white matter (density score of 0) as scored in the DAB stained sections. In the cases with 12/15-LOX positive cells, we examined 12/15-LOX expression in microglia using an antibody to tomato lectin, a general microglial marker that labels resting and activated microglia. Of the scattered 12/15-LOX expressing cells, approximately 80% showed co-localization with tomato lectin (Fig 1A). In these same cases, we looked for co-localization with CD68, an antibody which marks phagocytic macrophages and reactive microglia, and found little expression of CD68 (data not shown). In control cases we also examined the expression of 12/15-LOX in preOLs of the O4 and O1 stage. We did identify scattered O4- and O1-positive OLs with 12/15-LOX expression (Fig 1B). In the cerebral cortex, positive cell bodies of neurons were detected in 3 out of 10 DAB-stained controls (30%) at 34 (Case 10), 40 (Case 14), and 43 (Case 15) gestational weeks (Fig 1C). In these cases, there were neuronal cells of granular and pyramidal morphology expressing 12/15-LOX in all layers of the cortex. The presence of these 12/15-LOX positive cells was seemingly random throughout the cortex examined.
Figure 1.
12/15-LOX expression in controls. A) 12/15-LOX expression shows co-localization with microglial marker tomato lectin in the white matter of a control case at 34 PC weeks (40X magnification). B) A rare 12/15-LOX expressing-O4 cell is detected in the white matter of a control case at 37 PC weeks (40X magnification). C) and (D) Scattered neuronal cells throughout all layers express 12/15-LOX in a control case at 40 PC weeks. Neurons are shown at (C) low magnification (scale bar = 200 μm) and at (D) high magnification (scale bar = 100 μm). 12/15-LOX expression is shown in pyramidal neurons (arrow) and granular neurons (arrowhead) (C).
Cell-specific expression of 12/15-LOX in the focal and diffuse components of PVL
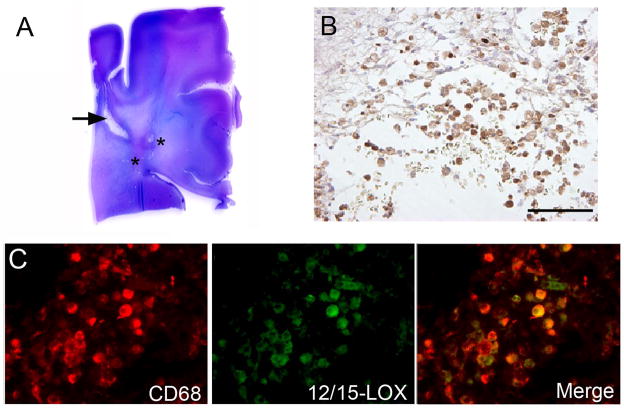
In the paraffin-embedded PVL cases, we identified 12/15-LOX expression in large round cells morphologically consistent with macrophages and/or amoeboid microglia within and around the focally necrotic lesions. These cells were confirmed as macrophages/amoeboid microglia by co-localization with CD68 (Fig 2).
Figure 2.
12/15-LOX expression in macrophages of the focal necrotic lesion of PVL. A) Shown is a representative hemotoxylin and eosin stained section of a posterior frontal section of PVL with a macrocyst (arrow) and microcystic lesions (marked by asterisks) in the periventricular white matter. B) 12/15-LOX is expressed in the macrophages and/or amoeboid microglia of the microcystic lesion as seen by DAB staining at 20X magnification (scale bar = 200 μm). C) Macrophages/amoeboid microglia within the focal lesion show co-localization with macrophage/microglia marker CD68 and 12/15-LOX (40X magnification).
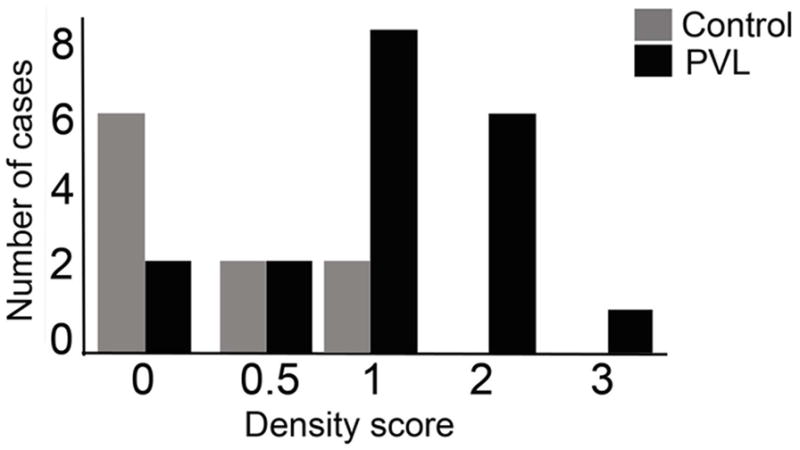
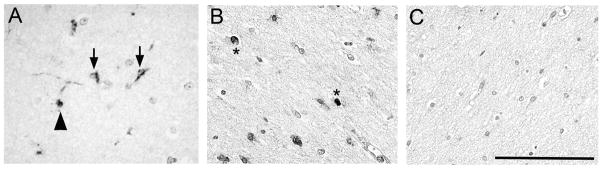
In the diffusely gliotic component of PVL surrounding and distant from the focal necrosis, we found 12/15-LOX positive cells in the white matter. These cells had the morphological appearance of microglia characterized by bipolar thick processes [44](Fig 3A), or of OLs characterized by a central, dark, round, and small nucleus and often with a 12/15-LOX-stained process extending towards an axon (Fig 3A). We also observed scattered cells of unknown type with 12/15-LOX positive nuclei in the white matter of PVL cases (Fig 3B). The 12/15-LOX expression appeared qualitatively increased in the white matter in PVL (Figs 3A and B) compared to controls (Fig 3C). To confirm this visual difference, we used a semi-quantitative scoring system of 0–3 and found a significant increase (p=0.014) in the density of 12/15-LOX expressing cells in PVL (score of 1.17 +/− 0.15 cells/high power field) compared to controls (score of 0.48 +/− 0.21 cells per high power field) during the second half of gestation (Fig 4). Examination of the number of cases scored 0–3 revealed a larger number of control cases with scores of 0 and 0.5 (n=8 out of 10; 80%) relative to the PVL cases which showed an increased number of cases with scores of 1 and 2 (n=15 out of 19, 79%). ANCOVA showed no effect of postmortem interval (p = 0.15) in a model that controlled for diagnosis. Of note, there was no significant expression of 12/15-LOX in the cerebral cortex of PVL cases (data not shown).
Figure 3.
12/15-LOX expression in the diffusely gliotic lesion of PVL. A) 12/15-LOX is expressed in glial cells of the diffusely gliotic lesion in a 40 PC week case with PVL. Arrows indicate cells with the morphological appearance of microglia. The arrowhead indicates a cell with the appearance of an oligodendrocyte. B) 12/15-LOX expression is detected in scattered nuclei (*) of cells in the gliotic white matter of a PVL case at 39 PC weeks. C) There is no detectable expression of 12/15-LOX in the white matter of a control case at 40 PC weeks. All images are 40X magnification. The representative scale bar is 100 μm.
Figure 4.
12/15-LOX cell density in PVL and controls. Semi-quantitative analysis using a standardization density score for 12/15-LOX expressing cells indicates an increase in 12/15-LOX density in PVL cases compared to controls (p = 0.014). The graph shown indicates the number of cases and controls given a specific density score of 0–3.
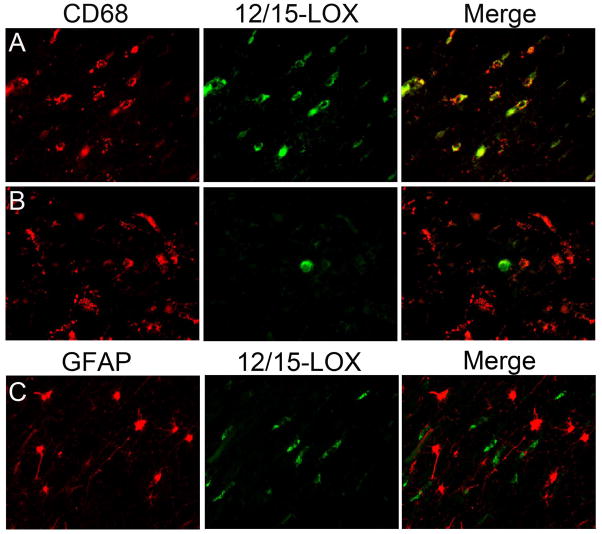
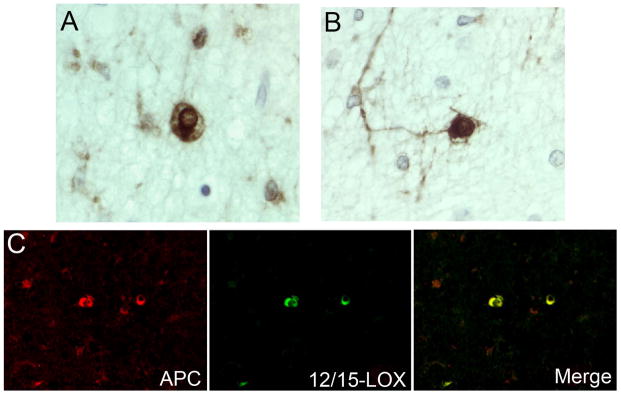
In the diffuse component of PVL, we used double-label ICC to examine the cell-specific expression of 12/15-LOX. Using CD68, in the PVL cases we found that while a population of CD68-positive reactive microglia co-localized with 12/15-LOX (Fig 5A), there was a population of reactive microglia that did not express 12/15-LOX (Fig 5B). In a quantitative analysis of 5 PVL cases, we found that an average of approximately 75% (range of 43–96%) of CD68-positive reactive microglia co-localized with 12/15-LOX. The percentages of CD68 cells that were positive for 12/15-LOX are listed for each case quantitatively examined (Table 2). Using GFAP as a marker of reactive astrocytes, we found no co-localization with 12/15-LOX in GFAP positive cells throughout the white matter of PVL (Fig 5C).
Figure 5.
12/15-LOX expression in reactive microglia and reactive astrocytes of the diffuse component. A) 12/15-LOX is expressed in a population of CD68 positive activated microglia in the diffuse component of PVL. B) There is a population of CD68 positive activated microglia in the diffuse component of PVL that does not show co-localization with 12/15-LOX. Based on morphology the 12/15-LOX positive/CD68 negative cell is likely to be a mature OL. Both A and B are from a PVL case at 40 PC weeks. C) There is no co-localization of 12/15-LOX with the reactive astrocyte marker GFAP in a PVL case at 38 PC weeks. All images are 40X magnification
Table 2.
12/15-LOX double-label data for CD68-positive cells in PVL
| Case | PC Age (wks) | CD68 cells + for 12/15-LOX | Total CD68 cells | % of CD68 cells + for 12/15 LOX |
|---|---|---|---|---|
| 17 | 29 | 2.8 | 3.0 | 93.3 |
| 23 | 33 | 2.2 | 4.2 | 52.4 |
| 28 | 35 | 4.8 | 5.0 | 96.0 |
| 33 | 38 | 2.0 | 4.7 | 42.6 |
| 34 | 39 | 5.6 | 6.3 | 88.9 |
| 39 | 40 | 4.5 | 5.9 | 76.3 |
Case numbers are from Table 1.
Cell numbers are an average of 5–10 representative high power fields (X40)
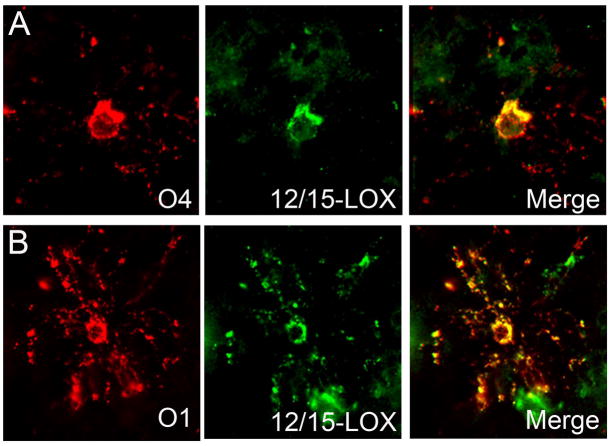
We examined 12/15-LOX expression in O4 positive pre-OLs and O1 positive immature OLs in 5 PVL cases ranging from 29 to 41 postconceptional weeks. We found co-localization of 12/15-LOX with both O4 and O1 OLs (Fig 6). In the population of O4-positive pre-OLs, approximately 10% (range of 6–15%) of OLs expressed 12/15-LOX (Table 3). In our analyses of O1 cells, we found the number of O1 cells (average of 1 to 3 cells per high-power field) to be low compared to O4 cells (average of 4 to 8 cells per high-power field). Within this very small population of O1 cells, however, we did see occasional cells expressing 12/15-LOX (Table 3). In PVL cases, we also examined the expression of 12/15-LOX in mature OLs. 12/15-LOX expression was seen in cells with the morphological appearance of mature OLs characterized by a central, dark, round, and small nucleus and perinuclear halo, the so-called “fried egg” appearance of mature OLs [45] (Fig 7). Expression was also found in these cells with 12/15-LOX positive processes extending towards axons (Fig 7). We found co-localization of 12/15-LOX with the mature OL marker APC (Fig 7). Because APC also labels reactive astrocytes (which are negative for 12/15-LOX), we were unable to get an estimate of the number of mature OLs that express 12/15-LOX.
Figure 6.
12/15-LOX expression in OL of the premyelinating (O4) and immature (O1) stages. A) There is co-localization of 12/15-LOX with the O4 marker of premyelinating OLs in a PVL case at 30 PC weeks. B) There is co-localization of 12/15-LOX with the O1 marker of immature OLs in a PVL case at 30 PC weeks.
Table 3.
12/15-LOX double-label data for O4- and O1-positive cells in PVL
| Case | PC Age (wks) | O4 cells + for 12/15-LOX | Total O4 cells | % O4 cells + for 12/15-LOX |
|---|---|---|---|---|
| 18 | 29 | 0.6 | 3.9 | 15.4 |
| 19 | 29 | 0.4 | 6.4 | 6.2 |
| 29 | 35 | 0.5 | 5.5 | 9.1 |
| 35 | 39 | 0.8 | 7.5 | 10.7 |
| 41 | 41 | 0.6 | 4.8 | 12.5 |
| Case | PC Age (wks) | O1 cells + for 12/15-LOX | Total O1 cells | % O1 cells + for 12/15-LOX |
|---|---|---|---|---|
| 18 | 29 | 1.0 | 1.6 | 62.5 |
| 19 | 29 | 2.6 | 3.2 | 81.2 |
| 29 | 35 | 0.6 | 1.8 | 33.3 |
| 35 | 39 | 1.0 | 2.6 | 38.5 |
| 41 | 41 | 0.3 | 1.0 | 30.0 |
Case numbers are from Table 1.
Cell numbers are an average of 5–10 representative high power fields (X40)
Figure 7.
12/15-LOX expression in mature OLs. A) and B) 12/15-LOX is expressed in cells with the morphological appearance of mature OLs as determined by DAB staining in a PVL case at 38 PC weeks. C. 12/15-LOX is co-localized with the mature OL marker APC in a PVL case at 33 PC weeks (40X magnification).
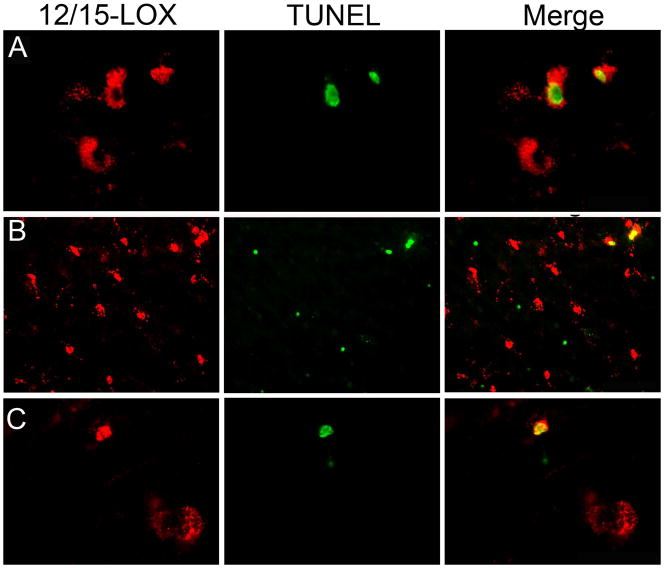
Lastly we examined the relationship between 12/15-LOX expression and cells undergoing cell death using TUNEL methodology combined with 12/15-LOX immunocytochemistry. In the PVL cases examined (n = 6), we found scattered TUNEL-positive cells that expressed 12/15-LOX throughout the damaged white matter (Fig 8A). We also found cells positive only for 12/15-LOX in the vicinity of cells that were positive only for TUNEL (Fig 8B). While this latter pattern was seen in all of the PVL cases examined, each of those PVL cases also had clusters of cells positive only for 12/15-LOX and clusters of cells positive only for TUNEL (data not shown). Also identified in the PVL cases were scattered, albeit rare, cells with nuclear colocalization of TUNEL and 12/15-LOX (Fig 8C). In the control cases examined (n=3), we did identify scattered, rare TUNEL positive cells in the white matter. Very little 12/15-LOX expression was seen in the controls.
Figure 8.
Relationship between TUNEL positive cells and 12/15-LOX expression. A) In a case at 39 PC weeks, 2 TUNEL-positive cells show colocalization with cytoplasmic 12/15-LOX expression. Also shown is a 12/15-LOX positive cell negative for TUNEL. (40X magnification) B) In a case at 41 PC weeks, a low power (20X) magnification shows cells positive for TUNEL-only proximal to cells positive for 12/15-LOX only. Also shown are 2 TUNEL positive cells with colocalization of cytoplasmic 12/15-LOX expression. C) In a case at 39 PC weeks, a TUNEL positive cell is shown with nuclear colocalization of 12/15-LOX. Also shown is a 12/15-LOX positive cell negative for TUNEL (40X magnification).
DISCUSSION
In this study, we present novel evidence for a potential role of 12/15-LOX in the pathogenesis of PVL directly in the developing human brain, with important implications for translational research. In this study, we demonstrate expression of 12/15-LOX in the damaged white matter of cases with PVL during the second half of gestation. Constitutive expression of 12/15-LOX is low as seen in adult human cortical neurons [38, 39] and in mature neurons [46, 47], OL [48], and astrocytes of animal models [47, 48]. This low constitutive level, however, increases significantly in rodent models of transient focal ischemia [42, 46, 49, 50] and in human Alzheimer’s disease [38] and stroke [39], thus implicating 12/15-LOX in the pathology of these conditions. Lipoxygenases are involved in the metabolism of AA to form oxidized derivatives of AA and leukotrienes. The most well characterized role for LOX activity in the brain is the generation of metabolites in inflammatory responses. Below we discuss the potential significance of our finding of 12/15-LOX expression in inflammatory cells within the damaged white matter in PVL, specifically macrophages and activated microglia. We also discuss the implications of our finding of expression of 12/15-LOX in OLs of different stages in PVL in terms of OL loss or damage and potential therapeutic targets for myelin deficits in long-term survivors of PVL.
12/15-LOX in development
In our control tissue, we saw 12/15-LOX expression in scattered resting (tomato lectin-positive/CD68 negative) microglia, scattered and rare O4- and O1-positive OLs, and cortical neurons beginning at 34 gestational weeks. The lack of expression prior to 34 gestational weeks suggests a temporal development of constitutive 12/15-LOX expression within these cells with onset in the third trimester. In studies of mature neurons, evidence suggests a role of 12/15-LOX in synaptic plasticity and neurotransmission [51–53]. Given this evidence, the expression of 12/15-LOX in a population of neurons in our older (> 34 gestational weeks) controls may represent the onset of a mature constitutive, yet low expression of the enzyme. The expression of 12/15-LOX in the small subset of microglia may represent a developmental expression consistent with the developmental changes occurring in the microglia population during this period of time [44]. The function of 12/15-LOX expression in developing OLs is currently unknown. Alternatively, the scattered expression in each of these cell types may represent mild pathologic changes in our controls associated with terminal complications, given that the controls are comprised of infants with systemic illnesses likewise complicated by hypoxia but without PVL, as in virtually all autopsy studies of the neuropathology of prematurity [54].
12/15-LOX in OLs in PVL
In this study, we observed 12/15-LOX expression in PVL in OLs of all stages of cell lineage. In rat cultures of O4 positive preOLs, 12/15-LOX expression plays a role in OL cell death under conditions of oxidative stress [35, 37, 55]. Glutathione depletion results in an increase in 12/15-LOX activity, ROS accumulation, and OL cell death [35]. Inhibition of the 12/15-LOX activity, on the other hand, effectively blocks both the production of ROS and pre-OL cell death [35]. Our finding of 12/15-LOX expression at all stages of OLs suggests that each stage is potentially vulnerable to 12/15-LOX mediated cell damage. An increase in resistance of mature OLs in cultures to oxidative stress-induced cell death [56–60], however, suggests that mature OLs in PVL are likely to be more resistant despite their expression of 12/15-LOX. This is likely due to increased cellular defenses at more developed stages of OL lineage [61, 62].
In PVL, we and others have shown an acute loss of pre-OLs in the diffuse component [9–11] with further evidence of apoptotic pre-OL cell death as determined by TUNEL staining [9, 10]. In addition, we have shown evidence of oxidative injury directly in OLs of the diffuse component of PVL, as determined by the presence of lipid peroxidation marker 4-hydroxynonenal [9]. Given our finding of 12/15-LOX expression in OLs, we hypothesize two different models for 12/15-LOX mediated OL cell death in PVL. The first model involves the direct release of ROS through the activation of 12/15-LOX and subsequent metabolism of AA. These ROS can originate from 2 sources: (1) exogenous expression of 12/15-LOX in microglia within the diffuse component of PVL (see below); and/or (2) endogenous expression of 12/15-LOX within OLs themselves. Either source of ROS can oxidatively damage the OLs and result in OL cell death, particularly in pre-OLs which are known to be more vulnerable to oxidative damage [56–60]. In the damaged white matter of PVL we have shown areas with 12/15-LOX positive cells in close proximity to TUNEL positive cells. We have also identified TUNEL positive cells that express 12/15-LOX. While not direct evidence, these data support our hypothesis of the involvement of 12/15-LOX in cell death in PVL through exogenous (via microglial 12/15-LOX expression) or endogenous AA metabolism and release of ROS. The second model of 12/15-LOX mediated OL cell death involves a potential role for direct mitochondrial damage in OLs and the subsequent release of mitochondrial apoptosis-inducing factor (AIF), which is implicated in caspase-independent forms of cell death [21, 39, 42, 63, 64]. This model of direct contributions of 12/15-LOX to cell death is supported by the finding of cells staining for both TUNEL and 12/15-LOX (Fig 8). Of note, we detected scattered 12/15-LOX positive nuclei in the gliotic white matter of PVL. We also detected scattered TUNEL positive cells with nuclear localization of 12/15-LOX, suggesting that at least some of the cells with nuclear colocalization are in the process of dying. Further analyses need to be done in PVL to examine this population of cells in regard to cell-specific expression and possible AIF co-localization. Of note, while acute preOL cell loss has been shown in PVL [9–11], evidence in human [65, 66] and in perinatal rodent models [67] suggests that this loss is compensated for, likely though proliferation of surviving preOLs [67].
12/15-LOX in inflammatory cells of PVL
In this study, we show that 12/15-LOX is expressed in macrophages/activated microglia of the focal necrosis and in a subpopulation of reactive microglia of the diffuse component of PVL. It is unclear as to what accounts for the differences in the 12/15-LOX expressing and non-expressing microglia. One possibility is that the different expression patterns of 12/15 LOX reflect a distinct timing of expression in the process of microglial activation. Alternatively, the two distinct populations are of different inflammatory related phenotypes, specifically M1 microglia classically activated by microbial compounds or pro-inflammatory cytokines to produce more pro-inflammatory cytokines, or M2 alternatively activated microglia with an anti-inflammatory phenotype promoting tissue repair [68–70]. Characterization of these different phenotypes relative to 12/15-LOX expression is currently underway.
Whether M1 or M2 microglia, the expression of 12/15-LOX by microglia may play a critical role in their downstream inflammatory responses which are activated initially by H-I and/or systemic infection [2, 17–20]. Activation of 12/15-LOX within the microglia may serve as a potent source of ROS in PVL, and widespread release of ROS by activated microglia within the diffuse component of PVL may play an important role in OL damage. We and others have shown evidence of oxidative stress in the white matter of PVL [9, 10]; the current findings suggest that 12/15 LOX upregulation in microglia constitutes at least one important source of this stress. Alternatively, we cannot rule out the possibility that a population of 12/15-LOX expressing microglia may have an anti-inflammatory role given that 12/15-LOX can, in conjunction with other enzymes, generate inflammation-limiting lipoxins, resolvins and protectins [30–32]. Of note, 12/15-LOX, in response to interleukin 4, induces the expression of peroxisome proliferator-activated receptor γ and the subsequent transcription of macrophage-expressed CD36, an antigen associated with the M2, anti-inflammatory phenotype [71].
In PVL, we found no expression of 12/15-LOX in reactive astrocytes in the diffuse component of PVL. This is consistent with animal models of stroke [50], but differs from the finding of 12/15-LOX expression of reactive astrocytes in Alzheimer’s disease [38]. Interestingly, 12-LOX mRNA is significantly increased in cultured rat neonatal astrocytes following hypoxia and reoxygenation [27]. In our study, we did not examine mRNA levels of 12/15-LOX and, therefore, cannot rule out the possibility of increased transcription of 12/15-LOX in astrocytes of PVL. However, the lack of 12/15-LOX protein expression in astrocytes suggests that 12/15-LOX activity does not play a role in the astrocytic inflammatory response in PVL [50].
Lack of 12/15-LOX expression in the cerebral cortex in PVL
In our studies, we surprisingly did not see 12/15-LOX expression in neurons of the cerebral cortex in PVL. This differs from the finding of 12/15-LOX expression in entorhinal cortical neurons in Alzheimer’s disease [38] and in mouse models of transient focal ischemia [42]. It also differs from findings in neuronal cultures showing an involvement in 12/15-LOX activity in models of injury induced by glutathione depletion [22, 42, 72] and peroxynitrite exposure [73]. In PVL, involvement of the cerebral cortex overlying injured white matter is now evident with volumetric MRI studies showing a reduction in volume [2, 74–79]. There is also pathologic data in non-cystic PVL showing evidence of neuronal loss and gliosis in the cerebral cortex [6] and a decrease in layer 5 pyramidal neurons in PVL compared to controls [7]. In each of these findings, it is uncertain whether the neuronal damage is primary, or rather, regressive changes secondary to the underlying white matter (axonal) damage. A third finding, however, of subtle but significant oxidative stress in cortical neurons overlying PVL [80] suggests that cortical neurons may, to some degree, be directly injured. In this case, our data suggests that this injury does not involve 12/15-LOX mediated events. Alternatively, 12/15-LOX may be involved, but the timing of upregulation is such that 12/15-LOX mediated cell death occurs early in the development of the injury, prior to demise, thus precluding these cells from our analyses.
12/15-LOX in systemic inflammation/infection
In addition to H-I injury, 12/15-LOX is involved in the inflammatory response to certain pathogenic infections [81–83] and in response to stimulation by bacterial wall component lipopolysaccharide (LPS) [84, 85]. In our study, given the number of PVL cases with infection alone or in the setting of H-I, we cannot rule out the possibility that the increase in 12/15-LOX positive cells in PVL was, in part, a response to infection.
Conclusion
In this study, we present evidence of a 12/15-LOX mediated pathway in OL cell death and/or damage in PVL during the second half of gestation. 12/15-LOX expression in PVL likely represents a cell-specific inflammatory response to the pathogenic mechanisms involved in PVL, i.e., H-I and systemic inflammation/infection. While this pathway has been identified as a potential target for therapy in animal and cell culture, confirmation of the 12/15-LOX pathway directly in the human neonate in this study offers potential new avenues for therapeutic targets that can help ameliorate or reduce damage and/or loss to the OLs.
Acknowledgments
We would like to acknowledge the following people for their guidance and input during the course of this study: Dr. Hannah C. Kinney for assistance with pathological concerns and questions regarding tissue and database; Dr. Rebecca D. Folkerth for continual support in obtaining tissue through the autopsy services at Boston Children’s Hospital and Brigham and Women’s Hospital; Dr. Joseph J. Volpe for guidance on issues relating to neonatal brain injury and clinical implications of our research; and Dr. Paul A. Rosenberg for initial guidance into the lipoxygenase field in PVL. We would like to especially acknowledge Dr. Hannah C. Kinney for her critical review and thoughtful input into the writing of this manuscript. This work was supported by the National Institutes of Health (PO1-NS38475) (RLH), Cerebral Palsy International Research Foundation (RLH), and the National Institutes of Health (R01 NS049430) (KVL)
BIBLIOGRAPHY
- 1.Volpe JJ. Neurology of the Newborn. 5. Philadelphia: WB Saunders Company; 2008. [Google Scholar]
- 2.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29:423–40. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney HC, Volpe JJ. Perinatal Panencephalopathy in the Premature Infant: Is It Due to Hypoxia-Ischemia? In: Haddad GG, Ping YS, editors. Brain Hypoxia and Ischemia. New York: Humana Press; 2009. [Google Scholar]
- 5.Kinney HC, Volpe JJ. Modeling the encephalopathy of prematurity in animals: The important role of translational research. Neurol Res Int. 2012:295–389. doi: 10.1155/2012/295389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–31. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andiman SE, Haynes RL, Trachtenberg FL, Billiards SS, Folkerth RD, Volpe JJ, Kinney HC. The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathol. 2010;20:803–14. doi: 10.1111/j.1750-3639.2010.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–61. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 10.Back SA, Luo NL, Mallinson RA, O’Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr, Murdoch GH, Montine TJ. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–20. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 11.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, Baud O, Gressens P. Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol. 2011;70:698–712. doi: 10.1002/ana.22518. [DOI] [PubMed] [Google Scholar]
- 13.Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, Ferriero DM, Guillet R, Gunn AJ, Hagberg H, Hirtz D, Inder TE, Jacobs SE, Jenkins D, Juul S, Laptook AR, Lucey JF, Maze M, Palmer C, Papile L, Pfister RH, Robertson NJ, Rutherford M, Shankaran S, Silverstein FS, Soll RF, Thoresen M, Walsh WF Eunice Kennedy Shriver National Institute of Child H, Human Development Hypothermia Workshop S. Moderators: Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–858. e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passemard S, Sokolowska P, Schwendimann L, Gressens P. VIP-induced neuroprotection of the developing brain. Curr Pharm Des. 2011;17:1036–9. doi: 10.2174/138161211795589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011;29:583–91. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Fan X, van Bel F. Pharmacological neuroprotection after perinatal asphyxia. J Matern Fetal Neonatal Med. 2010;23:17–9. doi: 10.3109/14767058.2010.505052. [DOI] [PubMed] [Google Scholar]
- 17.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–40. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 19.Mallard C, Wang X. Infection-induced vulnerability of perinatal brain injury. Neurol Res Int. 2012;2012:102153. doi: 10.1155/2012/102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. MRDD Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 21.Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–9. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–63. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 23.Adibhatla RM, Hatcher JF, Dempsey RJ. Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–54. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- 24.Rordorf G, Uemura Y, Bonventre JV. Characterization of phospholipase A2 (PLA2) activity in gerbil brain: enhanced activities of cytosolic, mitochondrial, and microsomal forms after ischemia and reperfusion. J Neurosci. 1991;11:1829–36. doi: 10.1523/JNEUROSCI.11-06-01829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer RM, Stephenson DT, Roberts EF, Clemens JA. Cytosolic phospholipase A2 (cPLA2) and lipid mediator release in the brain. J Lipid Mediat Cell Signal. 1996;14:3–7. doi: 10.1016/0929-7855(96)01501-5. [DOI] [PubMed] [Google Scholar]
- 26.Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer RM. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–35. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 27.Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–38. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–56. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal. 2011;14:1889–903. doi: 10.1089/ars.2010.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–64. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Leyen K. Lipoxygenase: An Emerging Target for Stroke Therapy. CNS Neurological Disorders-Drug Targets. 2012 doi: 10.2174/18715273112119990053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schewe T, Rapoport SM, Kuhn H. Enzymology and physiology of reticulocyte lipoxygenase: comparison with other lipoxygenases. Adv Enzymol Relat Areas Mol Biol. 1986;58:191–272. doi: 10.1002/9780470123041.ch6. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20:2049–58. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 36.van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–9. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009;87:1997–2005. doi: 10.1002/jnr.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–62. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan R, Witztum J, Montaner J, Holman T, Lo EH, van Leyen K. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of Neurology. 2012 doi: 10.1002/ana.23734. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004;14:265–74. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes RL, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC. Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol. 2009;118:391–9. doi: 10.1007/s00401-009-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–67. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 45.Love S, Louis DN, Ellision DW. Greenfield’s Neuropathology. Vol. 8. Oxford University Press; 2008. [Google Scholar]
- 46.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 47.Nishiyama M, Watanabe T, Ueda N, Tsukamoto H, Watanabe K. Arachidonate 12-lipoxygenase is localized in neurons, glial cells, and endothelial cells of the canine brain. J Histochem Cytochem. 1993;41:111–7. doi: 10.1177/41.1.8417106. [DOI] [PubMed] [Google Scholar]
- 48.Bendani MK, Palluy O, Cook-Moreau J, Beneytout JL, Rigaud M, Vallat JM. Localization of 12-lipoxygenase mRNA in cultured oligodendrocytes and astrocytes by in situ reverse transcriptase and polymerase chain reaction. Neurosci Lett. 1995;189:159–62. doi: 10.1016/0304-3940(95)11482-c. [DOI] [PubMed] [Google Scholar]
- 49.Sun L, Yang L, Xu YW, Liang H, Han J, Zhao RJ, Cheng Y. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: Inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res. 2012;1473:227–35. doi: 10.1016/j.brainres.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 50.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–43. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, Bolshakov VY. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci. 2003;23:11427–35. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piomelli D, Shapiro E, Feinmark SJ, Schwartz JH. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. J Neurosci. 1987;7:3675–86. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T, Fujita T, Nakatsuka T, Kumamoto E. Phospholipase A2 activation enhances inhibitory synaptic transmission in rat substantia gelatinosa neurons. J Neurophysiol. 2008;99:1274–84. doi: 10.1152/jn.01292.2007. [DOI] [PubMed] [Google Scholar]
- 54.Kinney HC. The encephalopathy of prematurity: one pediatric neuropathologist’s perspective. Semin Pediatr Neurol. 2009;16:179–90. doi: 10.1016/j.spen.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28:1236–45. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13:1441–53. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yonezawa M, Back SA, Gan X, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor. J Neurochem. 1996;67:566–73. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- 59.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26. doi: 10.1523/JNEUROSCI.23-13-05816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Folkerth RD, Haynes RL, Borenstein NS, Belliveau RA, Trachtenberg F, Rosenberg PA, Volpe JJ, Kinney HC. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–9. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 62.Baud O, Haynes RL, Wang H, Folkerth RD, Li J, Volpe JJ, Rosenberg PA. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. Eur J Neurosci. 2004;20:29–40. doi: 10.1111/j.0953-816X.2004.03451.x. [DOI] [PubMed] [Google Scholar]
- 63.Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Ann N Y Acad Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–84. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 65.Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–63. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–30. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 69.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–87. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 70.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 71.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278:43508–15. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Wang H, Li J, Dong L, Xu P, Chen W, Neve RL, Volpe JJ, Rosenberg PA. Intracellular zinc release and ERK phosphorylation are required upstream of 12-lipoxygenase activation in peroxynitrite toxicity to mature rat oligodendrocytes. J Biol Chem. 2006;281:9460–70. doi: 10.1074/jbc.M510650200. [DOI] [PubMed] [Google Scholar]
- 74.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 75.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 76.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 77.Nosarti C, Al-Asady MHS, Franfou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volume. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 78.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–25. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 80.Folkerth RD, Trachtenberg FL, Haynes RL. Oxidative injury in the cerebral cortex and subplate neurons in periventricular leukomalacia. J Neuropathol Exp Neurol. 2008;67:677–86. doi: 10.1097/NEN.0b013e31817e5c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Enteroaggregative Escherichia coli promotes transepithelial migration of neutrophils through a conserved 12-lipoxygenase pathway. Cell Microbiol. 2012;14:120–32. doi: 10.1111/j.1462-5822.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A2 mediate the ability of Salmonella enterica serotype typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76:3614–27. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy H, Cogan T, Humphrey T. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect. 201;13:42–8. doi: 10.1016/j.micinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Wuest SJ, Crucet M, Gemperle C, Loretz C, Hersberger M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis. 2012;225:121–7. doi: 10.1016/j.atherosclerosis.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 85.Middleton MK, Rubinstein T, Pure E. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol. 2006;176:265–74. doi: 10.4049/jimmunol.176.1.265. [DOI] [PubMed] [Google Scholar]