Abstract
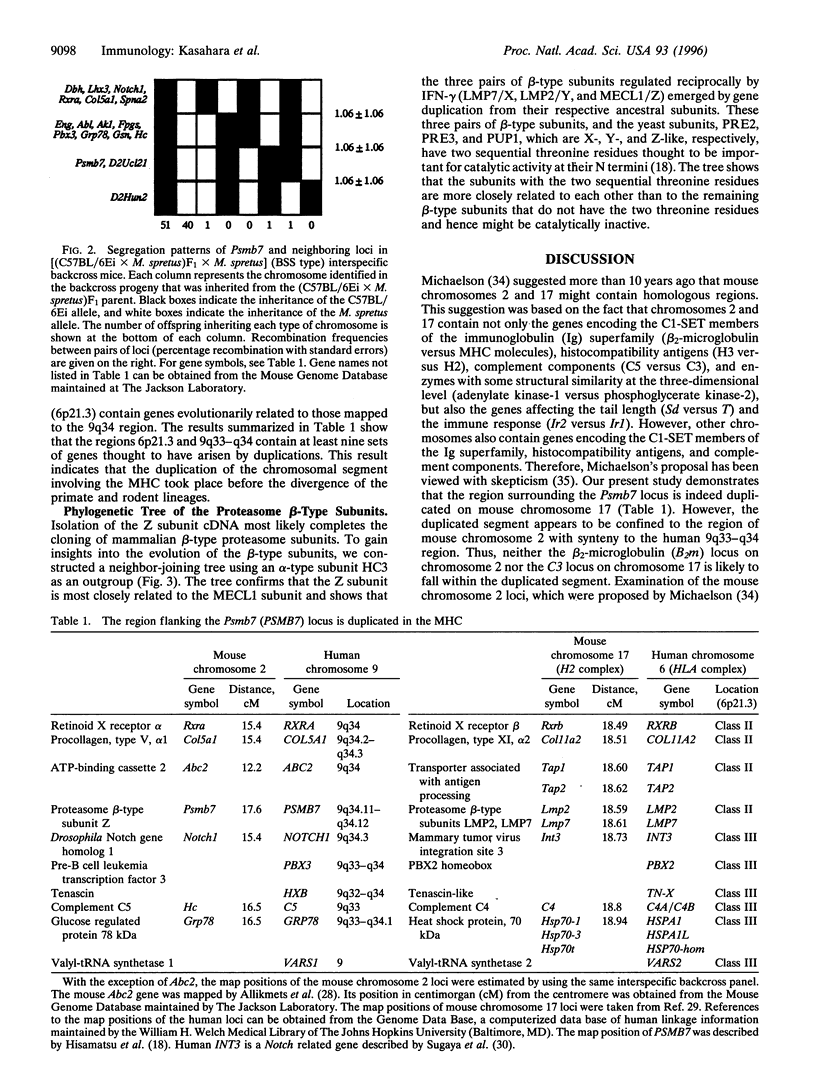
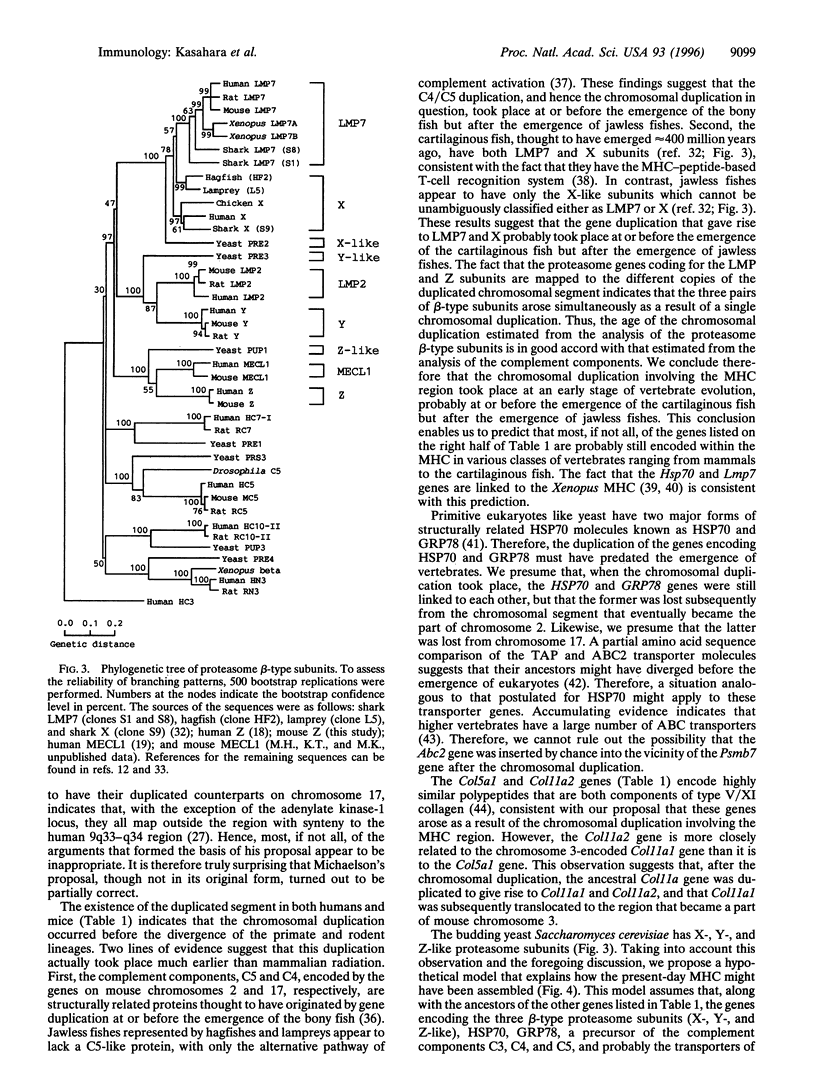
Proteasomes are the multi-subunit protease thought to play a key role in the generation of peptides presented by major histocompatibility complex (MHC) class I molecules. When cells are stimulated with interferon gamma, two MHC-encoded subunits, low molecular mass polypeptide (LMP) 2 and LMP7, and the MECL1 subunit encoded outside the MHC are incorporated into the proteasomal complex, presumably by displacing the housekeeping subunits designated Y, X, and Z, respectively. These changes in the subunit composition appear to facilitate class I-mediated antigen presentation, presumably by altering the cleavage specificities of the proteasome. Here we show that the mouse gene encoding the Z subunit (Psmb7) maps to the paracentromeric region of chromosome 2. Inspection of the mouse loci adjacent to the Psmb7 locus provides evidence that the paracentromeric region of chromosome 2 and the MHC region on chromosome 17 most likely arose as a result of a duplication that took place at an early stage of vertebrate evolution. The traces of this duplication are also evident in the homologous human chromosome regions (6p21.3 and 9q33-q34). These observations have implications in understanding the genomic organization of the present-day MHC and offer insights into the origin of the MHC.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama K., Yokota K., Kagawa S., Shimbara N., Tamura T., Akioka H., Nothwang H. G., Noda C., Tanaka K., Ichihara A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science. 1994 Aug 26;265(5176):1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- Allikmets R., Gerrard B., Glavac D., Ravnik-Glavac M., Jenkins N. A., Gilbert D. J., Copeland N. G., Modi W., Dean M. Characterization and mapping of three new mammalian ATP-binding transporter genes from an EST database. Mamm Genome. 1995 Feb;6(2):114–117. doi: 10.1007/BF00303254. [DOI] [PubMed] [Google Scholar]
- Belich M. P., Glynne R. J., Senger G., Sheer D., Trowsdale J. Proteasome components with reciprocal expression to that of the MHC-encoded LMP proteins. Curr Biol. 1994 Sep 1;4(9):769–776. doi: 10.1016/s0960-9822(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Ziegelhoffer T., Craig E. A. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994 Jan;38(1):1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- Dean M., Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995 Dec;5(6):779–785. doi: 10.1016/0959-437x(95)80011-s. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Brown M. G., Finley D., Monaco J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993 Sep 16;365(6443):262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Fenteany G., Standaert R. F., Lane W. S., Choi S., Corey E. J., Schreiber S. L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995 May 5;268(5211):726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Flajnik M. F., Canel C., Kramer J., Kasahara M. Evolution of the major histocompatibility complex: molecular cloning of major histocompatibility complex class I from the amphibian Xenopus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):537–541. doi: 10.1073/pnas.88.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik M. F., Canel C., Kramer J., Kasahara M. Which came first, MHC class I or class II? Immunogenetics. 1991;33(5-6):295–300. doi: 10.1007/BF00216688. [DOI] [PubMed] [Google Scholar]
- Forejt J., Artzt K., Barlow D. P., Hamvas R. M., Lindahl K. F., Lyon M. F., Klein J., Silver L. M. Mouse chromosome 17. Mamm Genome. 1994;5(Spec No):S238–S258. [PubMed] [Google Scholar]
- Früh K., Gossen M., Wang K., Bujard H., Peterson P. A., Yang Y. Displacement of housekeeping proteasome subunits by MHC-encoded LMPs: a newly discovered mechanism for modulating the multicatalytic proteinase complex. EMBO J. 1994 Jul 15;13(14):3236–3244. doi: 10.1002/j.1460-2075.1994.tb06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Goldberg A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993 Sep 16;365(6443):264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Spies T., Goldberg A. L. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9213–9217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995 Apr 28;268(5210):522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Heemels M. T., Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- Heinemeyer W., Tröndle N., Albrecht G., Wolf D. H. PRE5 and PRE6, the last missing genes encoding 20S proteasome subunits from yeast? Indication for a set of 14 different subunits in the eukaryotic proteasome core. Biochemistry. 1994 Oct 11;33(40):12229–12237. doi: 10.1021/bi00206a028. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hisamatsu H., Shimbara N., Saito Y., Kristensen P., Hendil K. B., Fujiwara T., Takahashi E., Tanahashi N., Tamura T., Ichihara A. Newly identified pair of proteasomal subunits regulated reciprocally by interferon gamma. J Exp Med. 1996 Apr 1;183(4):1807–1816. doi: 10.1084/jem.183.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil E., Namikawa C., Nonaka M., Greenberg A. S., Flajnik M. F., Ishibashi T., Kasahara M. Isolation of low molecular mass polypeptide complementary DNA clones from primitive vertebrates. Implications for the origin of MHC class I-restricted antigen presentation. J Immunol. 1996 Jun 1;156(11):4245–4253. [PubMed] [Google Scholar]
- Kandil E., Noguchi M., Ishibashi T., Kasahara M. Structural and phylogenetic analysis of the MHC class I-like Fc receptor gene. J Immunol. 1995 Jun 1;154(11):5907–5918. [PubMed] [Google Scholar]
- Kasahara M., Flajnik M. F., Ishibashi T., Natori T. Evolution of the major histocompatibility complex: a current overview. Transpl Immunol. 1995 Mar;3(1):1–20. doi: 10.1016/0966-3274(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Vazquez M., Sato K., McKinney E. C., Flajnik M. F. Evolution of the major histocompatibility complex: isolation of class II A cDNA clones from the cartilaginous fish. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6688–6692. doi: 10.1073/pnas.89.15.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen F., Solheim J., Kristensen T., Kolstø A. B., Prydz H. A tight cluster of five unrelated human genes on chromosome 16q22.1. Hum Mol Genet. 1993 Oct;2(10):1589–1595. doi: 10.1093/hmg/2.10.1589. [DOI] [PubMed] [Google Scholar]
- Luciani M. F., Denizot F., Savary S., Mattei M. G., Chimini G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics. 1994 May 1;21(1):150–159. doi: 10.1006/geno.1994.1237. [DOI] [PubMed] [Google Scholar]
- Lundin L. G. Evolution of the vertebrate genome as reflected in paralogous chromosomal regions in man and the house mouse. Genomics. 1993 Apr;16(1):1–19. doi: 10.1006/geno.1993.1133. [DOI] [PubMed] [Google Scholar]
- Löwe J., Stock D., Jap B., Zwickl P., Baumeister W., Huber R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995 Apr 28;268(5210):533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- Michaelson J. Genetics of beta-2 microglobulin in the mouse. Immunogenetics. 1983;17(3):219–260. doi: 10.1007/BF00364409. [DOI] [PubMed] [Google Scholar]
- Mizuki N., Kasahara M. Mouse submandibular glands express an androgen-regulated transcript encoding an acidic epididymal glycoprotein-like molecule. Mol Cell Endocrinol. 1992 Nov;89(1-2):25–32. doi: 10.1016/0303-7207(92)90207-m. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. Structure and function of genes in the MHC class II region. Curr Opin Immunol. 1993 Feb;5(1):17–20. doi: 10.1016/0952-7915(93)90075-4. [DOI] [PubMed] [Google Scholar]
- Morshauser R. C., Wang H., Flynn G. C., Zuiderweg E. R. The peptide-binding domain of the chaperone protein Hsc70 has an unusual secondary structure topology. Biochemistry. 1995 May 16;34(19):6261–6266. doi: 10.1021/bi00019a001. [DOI] [PubMed] [Google Scholar]
- Nadeau J. H., Kosowsky M. Mouse map of paralogous genes. Mamm Genome. 1991;1(Spec No):S433–S460. doi: 10.1007/BF00656503. [DOI] [PubMed] [Google Scholar]
- Namikawa C., Salter-Cid L., Flajnik M. F., Kato Y., Nonaka M., Sasaki M. Isolation of Xenopus LMP-7 homologues. Striking allelic diversity and linkage to MHC. J Immunol. 1995 Aug 15;155(4):1964–1971. [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Nonaka M., Fujii T., Kaidoh T., Natsuume-Sakai S., Nonaka M., Yamaguchi N., Takahashi M. Purification of a lamprey complement protein homologous to the third component of the mammalian complement system. J Immunol. 1984 Dec;133(6):3242–3249. [PubMed] [Google Scholar]
- Nonaka M., Takahashi M. Complete complementary DNA sequence of the third component of complement of lamprey. Implication for the evolution of thioester containing proteins. J Immunol. 1992 May 15;148(10):3290–3295. [PubMed] [Google Scholar]
- Parham P. Antigen processing. Transporters of delight. Nature. 1990 Dec 20;348(6303):674–675. doi: 10.1038/348674a0. [DOI] [PubMed] [Google Scholar]
- Peters J. M. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci. 1994 Sep;19(9):377–382. doi: 10.1016/0968-0004(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Rippmann F., Taylor W. R., Rothbard J. B., Green N. M. A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J. 1991 May;10(5):1053–1059. doi: 10.1002/j.1460-2075.1991.tb08044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L. B., Nadeau J. H., Turner R., Frankel W. N., Letts V. A., Eppig J. T., Ko M. S., Thurston S. J., Birkenmeier E. H. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm Genome. 1994 May;5(5):253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- Rubin D. M., Finley D. Proteolysis. The proteasome: a protein-degrading organelle? Curr Biol. 1995 Aug 1;5(8):854–858. doi: 10.1016/s0960-9822(95)00172-2. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salter-Cid L., Flajnik M. F. Evolution and developmental regulation of the major histocompatibility complex. Crit Rev Immunol. 1995;15(1):31–75. doi: 10.1615/critrevimmunol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Salter-Cid L., Kasahara M., Flajnik M. F. Hsp70 genes are linked to the Xenopus major histocompatibility complex. Immunogenetics. 1994;39(1):1–7. doi: 10.1007/BF00171790. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Flajnik M. F., Du Pasquier L., Katagiri M., Kasahara M. Evolution of the MHC: isolation of class II beta-chain cDNA clones from the amphibian Xenopus laevis. J Immunol. 1993 Apr 1;150(7):2831–2843. [PubMed] [Google Scholar]
- Seemüller E., Lupas A., Stock D., Löwe J., Huber R., Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995 Apr 28;268(5210):579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- Siracusa L. D., Abbott C. M. Mouse chromosome 2. Mamm Genome. 1994;5(Spec No):S22–S39. [PubMed] [Google Scholar]
- Sugaya K., Fukagawa T., Matsumoto K., Mita K., Takahashi E., Ando A., Inoko H., Ikemura T. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics. 1994 Sep 15;23(2):408–419. doi: 10.1006/geno.1994.1517. [DOI] [PubMed] [Google Scholar]
- Tanahashi N., Tsurumi C., Tamura T., Tanaka K. Molecular structure of 20S and 26S proteasomes. Enzyme Protein. 1993;47(4-6):241–251. doi: 10.1159/000468683. [DOI] [PubMed] [Google Scholar]
- Trowsdale J. "Both man & bird & beast": comparative organization of MHC genes. Immunogenetics. 1995;41(1):1–17. doi: 10.1007/BF00188427. [DOI] [PubMed] [Google Scholar]



