Abstract
Objective
To study the effect of hyaluronic acid (HA) on local anaesthetic chondrotoxicity in vitro.
Methods
Chondrocytes were harvested from bovine femoral condyle cartilage and isolated using collagenase-containing media. At 24 hours after seeding 15 000 cells per well onto a 96-well plate, chondrocytes were treated with media (DMEM/F12 + ITS), PBS, 1:1 lidocaine (2%):PBS, 1:1 bupivacaine (0.5%):PBS, 1:1 lidocaine (2%):HA, 1:1 bupivacaine (0. 5%):HA, or 1:1 HA:PBS for one hour. Following treatment, groups had conditions removed and 24-hour incubation. Cell viability was assessed using PrestoBlue and confirmed visually using fluorescence microscopy.
Results
Media-treated groups had a mean of 1.55×104 cells/well (sem 783). All treated cells showed statistically significant reduced viability when compared with media alone (all p < 0.003). Cells treated with bupivacaine + HA (6.70×103 cells/well (sem 1.10×103)) survived significantly more than bupivacaine (2.44×103 cells/well (sem 830)) (p < 0.001). Lidocaine + HA (1.45×103 cells/well (sem 596)) was not significantly more cytotoxic than lidocaine (2.24×103 cells/well (sem 341)) (p = 0.999). There was no statistical difference between the chondrotoxicities of PBS (8.49×103 cells/well (sem 730) cells/well) and HA (4.75×103 cells/well (sem 886)) (p = 0.294).
Conclusions
HA co-administration reduced anaesthetic cytotoxicity with bupivacaine but not lidocaine, suggesting different mechanisms of injury between the two. Co-administered intra-articular injections of HA with bupivacaine, but not lidocaine, may protect articular chondrocytes from local anaesthetic-associated death.
Cite this article: Bone Joint Res 2013;2:270–5.
Keywords: Hyaluronic acid, Local anaesthetic, Osteoarthritis, Intra-articular injections, Chondrocytes
Article focus
To confirm whether or not co-treatments of local anaesthetics and hyaluronic acid viscosupplements are toxic to chondrocytes in monolayer
Reiterate the risks of using local anaesthetics intra-articularly with regards to the health of chondrocytes
Key messages
Co-treatment of local anaesthetics in hyaluronic acid significantly improves chondrocyte viability when treated with bupivacaine, but not with lidocaine
Co-treating local anaesthetics with hyaluronic acid improves chondrocyte viability to a point that it is not significantly different than treating chondrocytes with media alone
Strengths and limitations
Two measures of chondrocyte viability (Strength)
Used clinically used commercial products (Strength)
This study used bovine chondrocytes in monolayer (Limitation)
Introduction
Osteoarthritis (OA) is a debilitating and widespread disease, affecting 80% of individuals over the age of 75 years.1 Clinical consequences of OA include loss of mobility and pain. Local anaesthetics, such as lidocaine and bupivacaine, are commonly used intra-articularly for therapeutic purposes and post-operatively in pain management for OA. Unfortunately, local anaesthetics have been found to be cytotoxic in a variety of cell types, including chondrocytes.2,3 As such, local anaesthetics delivered intra-articularly could worsen the severity of osteoarthritis.4
Recently, hyaluronic acid (HA) supplementation has been suggested as a symptomatic treatment for osteoarthritis. HA is a synthetic glycosaminoglycan normally present in healthy synovial fluid. While HA supplementation has only been approved for use in the knee, similar HA treatments have been used in the treatment of OA in joints other than the knee, such as the ankle and shoulder.5
Injecting HA locally into the joint is one option for maintaining normal biomechanics in the joint.6 Furthermore, the literature has suggested that intra-articular injections of HA in osteo-arthritic patients make the local environment of the knee joint closer in composition to healthy knees and may aid in normal biomechanics.7 Beyond the lubrication effect of HA, there is basic scientific evidence suggesting a direct effect on chondrocytes through the CD44 receptor.8,9 There is data to support that HA suppresses matrix metalloproteinases (MMP) and A Disintegrin And Metalloproteinase with Thrombospondin MotifS (ADAMTS).8 HA supplementation, however, has not been shown to be able to change the natural history of osteoarthritis.5
Clinically, local anaesthetics can be co-injected during HA treatment to manage the pain associated with these injections. The literature has not previously looked at the effect of HA in combination with local anaesthetics on articular chondrocytes. The goal of our study was to assess the effect of co-treating articular chondrocytes with a single-dose of HA and local anaesthetics. We hypothesise that the addition of HA to local anaesthetics will reduce their toxicity in chondrocytes compared with treatment with only local anaesthetics. In this study, bovine articular chondrocytes in monolayer received treatments of 1% lidocaine and 0.25% bupivacaine, shown to be cytotoxic, co-incubated with the commercially available viscosupplement Supartz (Smith & Nephew, London, United Kingdom).
Materials and Methods
Chondrocyte harvest
Articular chondrocytes were harvested by removing cartilage from weight-bearing portions of bovine femoral condyles (Rancho Veal, Petaluma, California). Cartilage was minced into 3-mm3 cubes and washed in sterile phosphate buffered saline (PBS; Hyclone, Logan, Utah) treated with 250 μg/ml Amphotericin B (MP Biomedicals, Solon, Ohio) and Penicillin-Streptomycin-Glutamine antibiotic (Pen-Strep; Hyclone). Chondrocytes were isolated by digesting cartilage in digestion media consisting of 500 ml 1:1 Dulbecco’s Modified Eagle Media (DMEM):F-12 (Hyclone), 50 ml Fetal Bovine Serum (FBS) (Axenia BioLogix, Sacramento, California), and 100 mg collagenase-P (Roche, Mannheim, Germany). The digested cartilage was filtered and the remaining chondrocytes in suspension were centrifuged at 500 g for 10 minutes. After discarding the supernatant and re-suspending the chondrocytes in a mixture of 10% FBS Media (500 ml 1:1 DMEM:F12, 50 ml FBS, 5 ml 100×Amphotericin B, 5 ml Pen-Strep), cells were counted using a haemacytometer.
Chondrocytes were then plated on flat-bottomed clear 75-cm2 flasks at a concentration of 7.5×105 cells/ml and allowed to grow to confluence. At 24 hours following plating, cells were treated with insulin transferrin selenium (ITS)-supplemented media until use. ITS media was prepared by combining 500 ml 1:1 DMEM:F12, 25 mg L-ascorbic acid 2-phospate sesquimagnesium salt hydrate (Sigma-Aldrich, St. Louis, Missouri), 5 ml 100×Pen-Strep antibiotic, 5 ml Amphotericin B, 500 mg bovine serum albumin (BSA) (Sigma Aldirich), 5 ml 10 mg/ml sodium pyruvate (Mediatech, Manassas, Virginia), 5 ml 1M HEPES Buffer (University of California, San Francisco Cell Culture Facility, San Francisco, California), and 5 ml of ITS premix (BD Biosciences, San Jose, California).
Seeding
Chondrocytes were removed from flasks, counted, and transferred to 96-well plates for treatment. A plate was seeded with 15 000 chondrocytes in each well. Each plate consisted of five conditions, described below, in replicates of six and a standard curve. The standard curve was prepared by serial dilution, starting with a density of 30 000 chondrocytes per well. The appropriate volume for each was calculated using the concentration found by methods described in the previous section. Additional ITS Media was added to each of the occupied wells to bring their total volume to 100 μl. Each plate was incubated at 37°C in standard cell culture conditions for 48 hours to allow the cells to rest and adhere to the wells.
Treatment
After 48 hours, the media was removed from the wells and the chondrocytes were treated with 70 μl of one of the seven following conditions: 1) ITS media; 2) PBS; 3) 1% lidocaine diluted from 2% stock solution (20 mg/ml lidocaine HCl, 6 mg/ml sodium chloride; APP Pharmaceuticals, Schaumburg, Illinois) and PBS; 4) 0.25% bupivacaine diluted from 0.5% stock (5 mg/ml bupivacaine HCl, 8.1 mg/ml sodium chloride; Hospira Inc., Lake Forest, Illinois) and PBS; 5) 1:1 2% Lidocaine and Supartz (Smith & Nephew); 6) 1:1 0.5% bupivacaine and Supartz; or 7) 1:1 Supartz and PBS. Following treatment, the conditions were removed from the wells and replaced with 70 μl of ITS media. Chondrocytes were allowed to recover by incubating for 24 hours at 37°C.
Quantification
After 24 hours, media was removed with from all wells and replaced with 50 μl of a 1:10 solution of PrestoBlue (Invitrogen, Frederick, Maryland) in 10% FBS media (500 ml 1:1 DMEM:F12, 50 ml FBS, 5 ml 100× Amphotecerin B, 5 ml Pen-Strep). Briefly, PrestoBlue is a non-cytotoxic cell viability fluorescence assay. The reagent measures viability by testing the cell’s ability to reduce nicotinamide adenine dinucleotide (NAD+). Following ten minutes of incubation at 37°C, the plate was read on the Synergy2 plate reader machine (BioTek Instruments Inc., Winooski, Virginia) with an excitation frequency of 535 nm and emission frequency of 595 nm, producing a fluorescence intensity read out in arbitrary units. The mean of the blank well readouts on each plate was calculated and subtracted from each experimental well. Fluorescence values were converted number of chondrocytes using the standard curve.
LIVE/DEAD stain
In order to confirm the results from the PrestoBlue staining, chondrocytes were visualised to qualitatively assess chondrocyte viability. Following staining with LIVE/DEAD Viability/Cytotoxicity Kit for Mammalian Cells (Invitrogen), representative images of chondrocytes under each treatment were taken using fluorescence microscopy. The stain was prepared by adding 5 μl calcein AM and 20 μl ethidium bromide homodimer-1 to 10 ml of 1× PBS. 100 μl of the LIVE/DEAD stain was added to each well and allowed to incubate at room temperature and protected from light for 35 minutes. Employing fluorescence microscopy, live cells were visualised using a 5× and 10× objective under a fluorescein isothiocyanate (FITC) filter (approximately 494 nm) and dead cells were visualised under a rhodamine filter (approximately 517 nm).
Statistical analysis
Statistical analysis was conducted using the computer software R (The R Foundation for Statistical Computing; University of Vienna, Vienna, Austria). Data were analysed for statistically significant differences between all conditions using analysis of variance (ANOVA). A post-hoc Tukey’s Honestly Significant Difference (HSD) test was conducted to make pair-wise comparisons between conditions in order to find statistical significance, indicated by p-values < 0.05.
Results
Fluorescence assay for cell viability
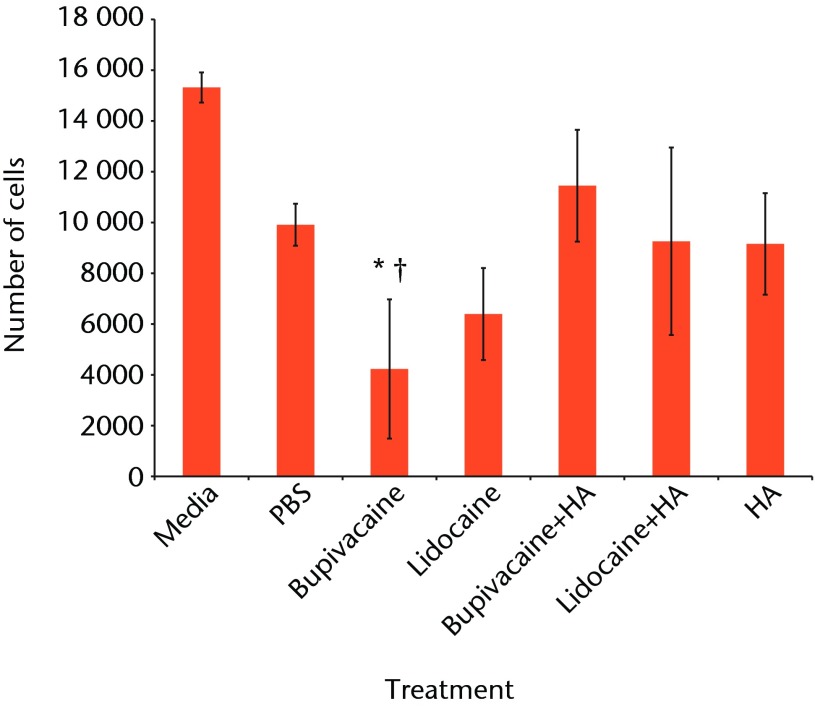
The fluorescence using the PrestoBlue reagent was converted to number of chondrocytes using a standard curve. Combining results from the two experiments, the mean number of cells for each condition is given in Table I and Figure 1.
Fig. 1.
Bar chart showing the mean chondrocyte viability by treatment group. The error bars denote the standard error of the mean (sem). All treatments showed significantly reduced viability compared with media-only controls (all < 0.003). †, statistically significant difference between the bupivacaine-only and bupivacaine and hyaluronic acid (HA) groups (p = 0.027) (PBS, phosphate buffered saline).
Table I.
Number of cells for each treatment
| Condition* | Mean (sem) number of cells (×103) | p-value (vs media only)† | ||
|---|---|---|---|---|
| Media only | 15.3 (0.594) | - | ||
| PBS | 9.91 (0.826) | 0.0028522 | ||
| 0.25% bupivacaine | 4.23 (2.74) | < 0.0000001 | ||
| 1% lidocaine | 6.39 (1.81) | < 0.0000001 | ||
| Bupivacaine:HA (1:1) | 11.4 (2.2) | 0.0001237 | ||
| Lidocaine:HA (1:1) | 9.26 (3.69) | < 0.0000001 | ||
| HA:PBS (1:1) | 9.16 (2.00) | 0.0000036 | ||
* PBS, phosphate buffered saline; HA, hyaluronic acid † post-hoc Tukey’s Honestly Significant Difference test
ANOVA comparison of all conditions against one another indicated a statistically significant difference within the data (p < 0.001). A post-hoc Tukey’s HSD test was conducted to test pair-wise differences in comparison with the media-only controls. All conditions resulted in significantly lower viability compared with the media-only controls.
Treating chondrocytes with bupivacaine and HA significantly increased cell viability when compared with bupivacaine only (p = 0.027). The lidocaine and HA treatment did not significantly increase chondrocyte viability compared with the lidocaine-only treatment (p = 0.999).
LIVE/DEAD staining and visualisation
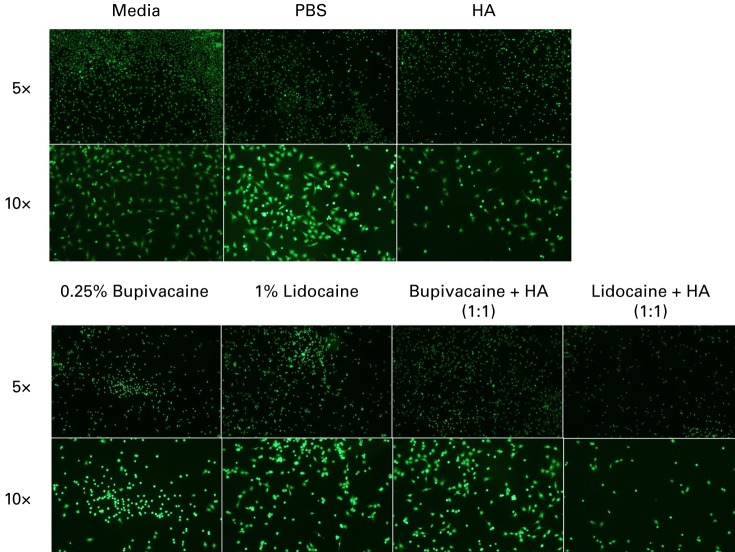
Chondrocyte viability was also assessed microscopically by LIVE/DEAD staining. Representative pictures of LIVE-stained chondrocytes at both 5× and 10× magnification are shown in Figure 2.
Fig. 2.
Representative LIVE staining at 5× and 10× magnification of chondrocytes treated with each condition, stained using 4 mM Calcien AM and visualised using fluorescence microscopy under a fluorescein isothiocyanate (FITC) filter. Chondrocytes co-treated with bupivacaine and hyaluronic acid (HA) showed improved density when compared with bupivacaine-only treated chondrocytes. There was no noticeable increase in cell density between cells treated with lidocaine and cells co-treated with lidocaine and HA (PBS, phosphate buffered saline).
At 5× magnification, the media only, PBS and HA groups show high, even live cell distribution and low dead signal. 1% lidocaine and 0.25% bupivacaine show lower live cell density, while also showing increased dead cell staining at 5× magnification. Under the 10× objective, the remaining live chondrocytes under these two conditions have a more ball-like shape.
As observed on microscopy, bupivacaine and HA co-treated groups show an increase in live stained chondrocytes at 5×. However, there is still a high density of dead staining chondrocytes as well, especially compared with the control groups. Comparing the LIVE/DEAD pictures from the 1% lidocaine treated and the lidocaine-HA co-treated chondrocytes, there does not appear to be a change in the number of LIVE/DEAD cells or in the shape of these cells.
Discussion
This study investigated the effect of co-treating bovine articular chondrocytes in monolayer with a combination of HA and local anesthetics. Clinically, HA injections can alleviate the symptoms of osteoarthritis in some patients. Intra-articular local anesthetics are commonly used in the clinic and post-operatively. However, literature supports the toxic effects of local anesthetics on articular cartilage.4,10-16 Chu et al10 showed that bupivacaine was toxic to bovine articular chondrocytes in monolayer. Miziyaki et al14 showed that treating bovine articular chondrocytes in monolayer with lidocaine caused high cell death.
Intra-articular HA injections have been shown to be a safe treatment both in basic science research and clinically.5-7 In our study, chondrocytes co-treated with HA and bupivacaine were significantly more viable than those treated with bupivacaine alone (p = 0.027). However, the lidocaine-HA co-treatment group does not appear to be significantly different than lidocaine alone. This suggests that the mechanism by which cytotoxicity is caused by lidocaine and bupivacaine may be different from one another.
The exact mechanism underlying chondrocyte death following treatment with local anaesthetics remains largely unknown. Our experiments confirmed that 24 hours after treatment and with incubation at 37°C, 1% lidocaine and 0.25% bupivacaine caused a significant reduction in chondrocyte viability (both p < 0.001). The data from this study does not allow us to make any conclusions in regard to the mechanism responsible for following local aneasthetic treatment. Several authors have proposed mechanisms by which local anesthetic cytotoxicity can occur. Miyazaki et al14 observed decreased glycosaminoglycan production with increasing concentration of lidocaine using a dimethyl blue assay. However, these authors did not propose how lidocaine modulated normal cellular function in a way that caused proteoglycan production to decrease.14 Dragoo et al12 suggested that preservatives and lower pH caused by drug formulations containing epinephrine led to chondrocyte death following treatment with lidocaine and bupivacaine. Bogatch et al15 suggested from their study that chemical incompatibility between local anaesthetics and cell culture media or synovial fluid was responsible for a decrease in chondrocyte viability. Grishko et al16 correlated chondrocyte death with mitochondrial dysfunction in a dose dependent manner, especially 120 hours after treatment. None of these proposed mechanisms address why HA co-treatment would mitigate the effect of local anaesthetics.
There have also been investigations into the role that molecular weight plays in the efficacy and biological activity of HA in the joint. Intra-articular HA injections are categorised as either low- or high-molecular-weight formulations. There is variation in the literature as to the ranges that qualify a particular molecular weight as being high or low. Supartz (Smith & Nephew), the HA product used in this study, is considered to be high-molecular-weight, and contains HA with molecular weights ranging from about 0.6 million Daltons (Da) to 1.2 million Da.17 A clinical double-blinded study has demonstrated that high-molecular-weight formulations of HA have a moderate improvement in efficacy over time compared with low-molecular-weight.18 The basic scientific literature might support this finding, as it has been suggested that high-molecular-weight HA has anti-inflammatory effects and more favourable viscoelastic properties.18 Wang et al19 were able to demonstrate that high-molecular-weight HA reduced the inflammatory activity of fibroblast synoviocytes derived from patients with early-stage osteoarthritis. Masuko et al8 suggested that HA does have anti-inflammatory activity through the CD44 receptor. HA has been shown not to interfere with the analgesic effects of bupivacaine, extending bupivacaine’s effects rather than neutralising or eliminating the drug.20 More work needs to be done to understand the mechanism of local anaesthetic toxicity, before trying to explain how the biological activity of HA can rescue chondrocytes during treatment. Because this study used one formulation, it remains unclear what effect, if any, molecular weight of HA would have on mitigating local anaesthetic cytotoxicity.
Our study shows that co-treating articular chondrocytes in monolayer with HA and local anaesthetics does not reduce the viability of these chondrocytes, and may help mitigate bupivacaine toxicity. Additionally, the differing responses to HA co-treatment between bupivacaine and lidocaine suggest that their respective cytotoxicity can potentially be attributed to different mechanisms. Future studies should confirm these results in different cell sources, ideally from osteo-arthritic human patients, and for chondrocytes still in vivo.
Conclusions
Despite the scientific evidence that HA does have a positive effect on chondrocytes, there are no studies that have shown a clinical effect when used for osteoarthritis. More broadly, it is our hope that clinicians will consider more carefully non-surgical treatment and diagnosis. It may be that HA is better suited as an acute treatment following injury or inflammation. At the same time, clinical evidence of local anaesthetic toxicity is limited, suggesting that the in vivo effects of anaesthetics are missed or tempered by the normal articular environment. In clinical practice, the presence of normal synovial fluid, which is rich in HA, may mitigate some of this anaesthetic toxicity. In applying our growing foundation of knowledge to clinical practice, the risks and benefits of all our treatments must be weighted among individual patient factors. Understanding situations in which the normal joint environment is compromised, such as after haemarthrosis or injury, will help us to guide treatments to maximise benefit while minimising risks.
Funding Statement
The authors would like to acknowledge Smith & Nephew for providing the Supartz® used in this study. The authors would also like to thank the following funding sources for their support of this project: UCSF Department of Orthopaedic Surgery, Northern California Institute of Research and Education.
Footnotes
Author contributions:T. S. Onur: Data collection, Data analysis, Performed experiments, Wrote paper
C. S. Sitron: Data collection, Data analysis, Performed experiments, Wrote paper
A. Dang: Writing and editing the paper
ICMJE Conflict of Interest:None declared
References
- 1.Arden N, Nevitt M. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3–25 [DOI] [PubMed] [Google Scholar]
- 2.Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of local anesthetics on articular cartilage. Am J Sports Med 2011;39:2245–2253 [DOI] [PubMed] [Google Scholar]
- 3.Rahnama R, Wang M, Dang AC, Kim HT, Kuo AC. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Joint Surg [Am] 2013;95-A:132–137 [DOI] [PubMed] [Google Scholar]
- 4.Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg [Am] 2008;90-A:986–991 [DOI] [PubMed] [Google Scholar]
- 5.Altman RD. Status of hyaluronan supplementation therapy in osteoarthritis. Curr Rheumatol Rep 2003;5:7–14 [DOI] [PubMed] [Google Scholar]
- 6.Doral MN, Bilge O, Batmaz G, et al. Treatment of osteochondral lesions of the talus with microfracture technique and postoperative hyaluronan injection. Knee Surg Sports Traumatol Arthrosc 2011;20:1398–1403 [DOI] [PubMed] [Google Scholar]
- 7.Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res 2001;390:173–181 [DOI] [PubMed] [Google Scholar]
- 8.Masuko K, Murata M, Yudoh K, Kato T, Nakamura H. Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med 2009;2:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campo GM, Avenoso A, D’Ascola A, et al. Hyaluronan in part mediates IL-1beta-induced inflammation in mouse chondrocytes by up-regulating CD44 receptors. Gene 2012;494:24–35 [DOI] [PubMed] [Google Scholar]
- 10.Chu CR, Izzo NJ, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy 2006;22:693–699 [DOI] [PubMed] [Google Scholar]
- 11.Dragoo JL, Braun HJ, Kim HJ, Phan HD, Golish SR. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med 2012;40:794–799 [DOI] [PubMed] [Google Scholar]
- 12.Dragoo JL, Korotkova T, Kim HJ, Jagadish A. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med 2010;38:1154–1159 [DOI] [PubMed] [Google Scholar]
- 13.Braun HJ, Wilcox-Fogel N, Kim HJ, et al. The effect of local anesthetic and corticosteroid combinations on chondrocyte viability. Knee Surg Sports Traumatol Arthrosc 2011;20:1689–1695 [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, Kobayashi S, Takeno K, et al. Lidocaine cytotoxicity to the bovine articular chondrocytes in vitro: changes in cell viability and proteoglycan metabolism. Knee Surg Sports Traumatol Arthrosc 2011;19:1198–1205 [DOI] [PubMed] [Google Scholar]
- 15.Bogatch MT, Ferachi DG, Kyle B, et al. Is chemical incompatibility responsible for chondrocyte death induced by local anesthetics? Am J Sports Med 2010;38:520–526 [DOI] [PubMed] [Google Scholar]
- 16.Grishko V, Xu M, Wilson G, Pearsall AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg [Am] 2010;92-A:609–618 [DOI] [PubMed] [Google Scholar]
- 17.Curran MP. Hyaluronic acid (Supartz®): a review of its use in osteoarthritis of the knee. Drugs Aging 2010;27:925–941 [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis 2012;71:1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Lin Y, Chiang B, Lin Y, Hou S. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage 2006;14:1237–1247 [DOI] [PubMed] [Google Scholar]
- 20.Dollo G, Malinovsky J, Péron A, et al. Prolongation of epidural bupivacaine effects with hyaluronic acid in rabbits. Int J Pharm 2004;272:109–119 [DOI] [PubMed] [Google Scholar]