Abstract
Pathological gambling (PG) affects about 0.2–2% of adults and the impact extends to family members, employers and society as a whole. Recent research has identified similarities in the pathophysiologies of PG and substance use disorders (SUDs). As such, findings regarding SUDs provide a framework for investigating PG. The aims of the manuscript are two-fold. First, we will briefly revivew neural systems implicated in PG. Cortico-limbic circuitry involving the ventral striatum, ventromedial prefrontal cortex, anterior cingulate cortex, and dorsolateral prefrontal cortex are discussed as are the neurotransmitters norepinephrine, serotonin, dopamine, opioids, glutamate, and gamma-aminobutyric acid (GABA). This background will provide a framework for reviewing the psychopharmacological treatments that have been tested for efficacy and safety in treating PG. Of medications, the strongest data suggest the efficacy and tolerability of opioid antagonists in the treatment of PG, and other agents have varying degree of empirical support. As behavioral therapies have also shown efficacy, they will be briefly considered as well. Future research is needed to understand how treatments work in PG and for whom specific treatments might work best.
Keywords: Neuropsychopharmacology, Pathological Gambling, Psychopharmacology, Treatment
Background
Gambling is defined as placing something of value at risk with the hope of gaining something of greater value. A significant portion of the adult population has gambled within the past year. However, the majority of gamblers do not develop gambling problems. Subsyndromal levels of gambling associate with substance use (e.g., tobacco and alcohol) and other psychiatric disorders [1–4] making gambling important to understand within a public health framework.
According to the American Psychiatric Association, “The essential feature of Pathological Gambling is persistent and recurrent maladaptive gambling behavior (Criterion A) that disrupts personal, family, or vocational pursuits. The diagnosis is not made if the gambling behavior is better accounted for by a Manic Episode (Criterion B)” [5]. PG is defined as having five or more of the A criteria and the B exclusionary criterion of the PG-related gambling behavior not being better accounted for by manic episodes. Multiple definitions have been applied to forms of gambling not meeting the threshold of PG. For example, the term problem gambling has been applied to individuals meeting 3–4 inclusionary criteria, although the terms have at times also been applied (perhaps less frequently) to individuals meeting 1–4 criteria, and sometimes the term is also used inclusive of PG. Non-problem or sub-syndromal (sometimes defined as sub-clinical or sub-threshold) gambling has been ascribed to individuals meeting anywhere from 0–4 inclusionary criteria, with the term at-risk gambling at times ascribed to individuals meeting 1–2 inclusionary criteria. The term recreational gambling has been applied to individuals not meeting any of the listed A criteria, or alternatively up to two inclusionary criteria. The field would benefit from more precise and uniformly agreed-upon definitions for levels of gambling not meeting the threshold for PG. Furthermore, the most appropriate threshold for defining PG has also been debated, with the numbers of inclusionary criteria needed for diagnosis changing from the Diagnostic and Statistical Manual of Mental Disorders III (DSM-III) to DSM-IV and currently being discussed in anticipation of DSM-V.
PG shares criteria with those for substance use disorders (SUDs), particularly with substance dependence. PG shares criteria with substance dependence with ones targeting preoccupation, tolerance, withdrawal, repeated unsuccessful attempts to cut down or stop, and interference in major areas of life functioning. Unlike substance dependence which requires only three out of seven criteria for diagnosis, PG requires five out of 10 with the added B criterion of not being better accounted for by a manic episode.
PG is currently classified in the DSM-IV as an Impulse Control Disorder (ICD) Not Elsewhere Classified. It shares this classification with kleptomania, trichotillomania, and pyromania, among others. In DSM-V, proposals have been forwarded to categorize PG with SUDs as addictions [6]. In addition to the inclusionary criteria that PG shares with SUDs, there exist other commonalities. Both disorders involve appetitive urge states, often immediately preceding engagement in the addictive behavior [7]. Similarities in the biologies of the disorders have been identified, with non-substance and substance addictions involving motivational neural pathway differences involving such structures as the ventral striatum and orbitofrontal cortex [8] and dopaminergic, noradrenergic, serotonergic and other neurochemical pathways. Given these shared features, researchers have tested hypotheses about PG based on knowledge of SUDs.
Epidemiology
Prevalence
Several national studies have been performed to estimate the prevalence rates of PG. Each study has limitations, such as the instruments used, the recencies with which the studies were conducted, response rates, and sampling errors. Prevalence estimates typically range from 0.2%–2%. The first national survey was performed in 1976 by Kallick et al., who conducted a telephone survey of 1749 adults, generating a lifetime prevalence estimate of 0.8% [9]. The next national survey was not done for another 20 years. The National Gambling Impact Study (NGIS) sampled over 2400 US residents and found a similar lifetime estimate of 0.8% [10]. Two years after the NGIS, Welte et al., conducted another survey and found a lifetime estimate of 2.0% [11].
The largest survey conducted to date was performed between 2001 and 2005. The National Epidemiological Survey of Alcohol and Related Conditions (NESARC) was a longitudinal study assessing a broad range of psychiatric and other disorders and behaviors. Over 43,000 respondents were surveyed in person. The study found a lifetime prevalence estimate of 0.4% and past-year prevalence estimate of 0.2% [12].
This survey further revealed that women were more likely to gamble to relieve a dysphoric mood or escape problems while men were motivated by preoccupations and economic incentives. Types of gambling also differed between men and women. Women were found to prefer casino gambling (especially non-strategic forms like electronic gambling machine (“slot machine”) gambling) while men were found to prefer sports gambling and other strategic forms. This information can be important in treatment development for PG; e.g., some medications may better target dysphoria and perhaps be more efficacious for women.
Biological and Pathophysiological Mechanisms
Overview
PG and other ICDs may be considered “behavioral” or non-substance addictions. There is considerable evidence, particularly in the case of PG, that these disorders share neurobiological links with SUDs [1,4,8]. SUDs have been studied more extensively than PG, and knowledge about SUDs provides a foundation for studies of behavioral addictions like PG.
Brain imaging modalities have been used to develop a better understanding of the pathophysiologies of addictions. In the past decade, there have been significant advances in understanding the biological underpinnings of PG and other ICDs. Multiple imaging modalities (e.g., functional magnetic resonance imaging (fMRI) and positron emission tomography (PET)) have been utilized to study ICDs and in some cases to compare and contrast them with substance addictions. A better understanding of the neural correlates of PG should lead to better treatment and prevention programs.
Neural Biology
Similar to the cravings in cocaine dependence, gambling urges in PG may immediately precede engagement in the addictive behavior. The ventral striatum (VS-an area of the brain with dopaminergic innervation) and the ventromedial prefrontal cortex (vmPFC-an area of the brain associated with reward processing and impulse control and including the medial portion of the orbitofrontal cortex (OFC)) have been implicated in urges in PG and cravings in cocaine dependence [8,13,14]. The striatum, which includes the caudate nucleus and putamen, is involved in both behavioral and physiological responses to rewards. It has been postulated that differences in VS function may predispose to addictions [15]. In PG, diminished VS activation has been observed during fMRI studies using tasks involving reward processing including during simulated gambling [16,17]. Similarly, individuals with alcohol dependence show diminished VS activation during reward processing, particularly during anticipatory phases [18,19].
The vmPFC, which may function together with the VS, has also been implicated in the processing of monetary rewards and in risk-reward decision-making [13]. The vmPFC has been implicated in multiple studies of SUDs, PG, and other ICDs. For example, individuals with PG as compared to those without show diminished vmPFC activation during simulated gambling and performance of a cognitive control (Stroop) task performance [20,21]. Individuals with SUDs with or without PG also show relatively diminished vmPFC activation during performance of the Iowa Gambling Task, testing risk-reward decision-making [8]. These findings suggest that addicted individuals display abnormalities in brain regions like the vmPFC that are involved in processing rewards and making advantageous decisions.
The anterior cingulate cortex (ACC), implicated frequently in cognitive control, and the insula, implicated in interoceptive processing, may contribute importantly to PG. For example, the ACC has been implicated in loss-chasing [22] and both the ACC and insula contribute to the processing of near-misses [23]. The ACC is involved in cognitive control, while the insula is linked to awareness of somatic states, including as related to decision making [24,25]. They may mediate emotional responses to pain and both areas showed enhanced activity when the opioid system was blocked by opioid receptor antagonists [26]. Endogenous opioid systems may contribute to hedonic aspects of rewards, and differences in aversive processing linked to opioid systems and brain circuitry involving the ACC and insula may theoretically result from addictions or make certain people more prone to developing addictions.
Activation of the dorsolateral prefrontal cortex (dlPFC) has been observed in individuals with PG who are exposed to gambling cues [27,28]. This activation may represent preferential attention given to gambling cues. While increased activation was observed in the dlPFC, diminished activation was noted in striatal and vmPFC regions. Two different studies have noted that prefrontal cortex activation is elevated when choosing delayed rewards over immediate rewards, suggesting that brain regions involved in executive decision-making may contribute to less impulsive behavior [29,30].
The individual brain regions listed above function as circuits relating to motivated behaviors [31,32]. Specifically, circuitry involving parallel cortico-striato-thalamo-cortical loops have been hypothesized to contribute to PG, SUDs and other psychiatric disorders involving motivated behaviors [33]. Developmental differences may influence function of these circuits and be expressed in behaviors related to PG and SUDs. Consistently, in a study of children, adolescents and adults, brain regions including the VS, ACC and PFC (including vmPFC) accounted for risk-taking in a developmentally sensitive fashion [34].
Neurotransmitters and Hormones
Norepinephrine/Noradrenaline
Norepinephrine is a catecholamine structurally related to epinephrine. Norepinephrine is released in response to stress. It affects the response of the sympathetic nervous system, one involved in the “fight or flight” reactions. Norepinephrine can be synthesized from dopamine and can have systemic (central and peripheral) effects [35,36]. Gambling may involve a norepinephrine-related arousal which can mimic a “high” feeling. The increased activation of the sympathetic nervous system may alter the mood of the gambler and reinforce gambling behavior. Gambling behavior has been associated with elevated heart and respiratory rates, suggesting a link to the autonomic arousal systems targeted by norepinephrine [37–39]. In addition to arousal, adrenergic agents may influence higher order executive functions, attention, and reward processing. Epinephrine (which is derived from norepinephrine) is associated with mental focus and attention. Norepinephrine may regulate mood, physical and mental arousal. Increased levels of norepinephrine can also raise heart rate and blood pressure.
PG subjects, as compared to control subjects, show increased noradrenergic function as evidenced by higher 3-methoxy-4-hydroxyphenylglycol (MHPG) and norepinephrine levels [40]. Noradrenergic function has been linked to sensation seeking behavior in PG. Over-activity of the noradrenergic system in PG may reinforce and/or maintain gambling behavior through influences on arousal. Individuals with PG, particularly men, often report excitement as an important reason for gambling.
To further examine the neuroendocrine response among problem gamblers, studies have examined the sympathoadrenal system, hypothalamic-pituitary-adrenal axis (HPA-axis), and pituitary hormones. When gambling for actual money, greater activation of the HPA-axis and sympathoadrenergic system is seen, with a greater increase in problem gamblers compared to control subjects [39]. This is demonstrated by higher heart rate levels among problem gamblers when gambling for real money compared to gambling for points. Additionally, epinephrine and norepinephrine levels among problem gamblers increased during actual gambling sessions though it should be noted that problem gamblers had high levels of epinephrine at the start of the gambling sessions, possibly indicating an anticipatory response among problem gamblers.
Together these findings suggest that problem gamblers may experience an acute stress state during actual gambling sessions that may lead to increased release of catecholamines and pituitary-adrenal hormones [41,42]. Cortisol levels of problem gamblers increase during gambling and this increase is similar to the increased cortisol levels of people exposed to acute stressors [38]. The levels of cortisol in problem gamblers may remain elevated for a long period of time once the stressor is removed (or gambling ceases). Further study into the relationship between stress and PG is needed.
Serotonin
Serotonin or 5-Hydroxytryptamine (5-HT) is a monoamine neurotransmitter biochemically derived from tryptophan. Serotonin is manufactured in the brain where it performs its primary functions but approximately 90% of serotonin in people is found in the digestive tract and in blood platelets. Serotonin may contribute to feelings of well-being. Serotonin influences multiple physiological and psychological aspects of brain function including processes related to mood, sexual desire and function, appetite, memory and learning, social behavior, and impulse control [43].
Serotonin contributes to rewarded and non-rewarded behaviors and in inhibiting behavior following punishment or an aversive event. Reductions in serotonin levels have been linked to continuation of aversive behaviors in healthy subjects [44]. Serotonin also contributes to learning from and coping with aversive events. Serotonin depletion can enhance neural responses to punishments in the ACC [45].
Abnormal serotonin function has been linked to poor impulse control. Low levels of the serotonin metabolite 5-hydroxy-indole acetic acid (5-HIAA) has been observed in PG [46]. Impulsive violent offenders who committed their crimes impulsively, rather than premeditating their actions, have shown lower levels of 5-HIAA in their cerebrospinal fluid (CSF) [47]. Administration of the drug metachlorophenylpiperzaine (m-CPP), a metabolite of trazodone and a partial agonist at serotonin 5HT1 and 5HT2 receptors, is associated with experiencing a subjective “high” in individuals with PG and those with alcohol dependence, whereas healthy control subjects typically report an aversive response [48]. In PG, increased prolactin response is also seen following m-CPP administration [48].
Consistent with this information, investigators have studied selective serotonin reuptake inhibitors – or SSRIs (drugs with antidepressant, anxiolytic and anti-obsessional/compulsive actions) - in the treatment of PG. These studies have had mixed results and are reviewed below.
Dopamine
Dopamine is a catecholaminergic neurotransmitter. The brain has five types of dopamine receptors — D1, D2, D3, D4, and D5—and their variants (e.g., related to alternative splicing). Dopamine is produced in several areas of the brain including the substantia nigra and the ventral tegmental area. Dopamine cannot cross the blood brain barrier so medications influencing dopamine function have included dopamine precursors such as L-Dopa. Dopamine receptors can also be targeted via directly acting drugs (e.g., with agonists like ropinirole or pramipexole) or by drugs that influence dopamine degradation or reuptake. Dopamine is a precursor for adrenaline and noradrenaline [49,50].
The dopaminergic mesolimbic pathway, which links the ventral tegmental area to the nucleus accumbens/ventral striatum, influences rewarding and reinforcing behaviors and has been widely implicated in addictions [32,51]. Addicted individuals may seek “rewards” (drugs or gambling) that may release dopamine and trigger feelings of pleasure. Increased activation of the dopaminergic pathways of the midbrain have been found in studies examining reward probability and magnitude [52]. Probability and reward magnitude are two key components of gambling, and, as such, if these dopaminergic neurons are showing sustained higher levels of activation during periods of higher uncertainty of reward and higher variability in size of reward, such mechanisms may contribute to PG. Midbrain dopamine function has been linked to impulsivity and may also contribute to PG through this mechanism [53].
Endorphins (endogenous opioid peptides)
Endorphins, opioidergic peptides produced by the body, have been implicated in pleasure and reward processing. The opioid system consists of several types of receptors (μ, δ and κ) and peptides (β-endorphin, enkephalins and dynorphins). How these contribute to PG is relatively poorly understood [54]. Mu- and δ-opioid receptor ligands may produce rewarding effects, while κ-opioid receptor ligands may have aversive effects [55]. Endorphins, much like exogenous opioids (e.g. morphine), may produce a rush or feeling of exhilaration, and endorphin release may be elicited by pain, love, danger, or other stresses [56]. Opioid function may influence dopamine neurotransmission in the mesolimbic pathway extending from the ventral tegmental area to the nucleus accumbens or ventral striatum [57]. Brain regions involved in affective, reward and aversive processing (e.g., ACC, insula) often express high levels of opioid receptors [26]. Differences in β-endorphin levels have been found in sub-groups of gamblers (horse racing versus poker machines) and may represent important considerations in treatment development for PG [58]. Gambling or gambling-like activities (e.g., Pachinko) may lead to the release of endogenous opioids [37]. There exist agonists and antagonists able to target specific opioid receptors, allowing for investigators to examine their roles in addictive processes.
Glutamate
Glutamate, one of the 20 amino acids involved in building proteins and the most abundant excitatory neurotransmitter, has been implicated in motivational processes and drug addiction [31,59]. Glutamate is involved in learning and memory and may activate different types of glutamate receptors including N-methyl-D-aspartate (NMDA) receptors expressed in brain regions comprising reward circuitry [60]. Levels of glutamate within the nucleus accumbens mediate reward-seeking behavior [60]. Repetitive behaviors closely followed by rewards increase extracellular glutamate levels [61]. In one study, CSF levels of glutamic and aspartic acid, both of which bind to NMDA receptors, were elevated among PG as compared to control subjects [62]. Targeting glutamate systems may be helpful in treating addictions.
N-acetyl cysteine, a cysteine pro-drug and amino acid, can increase extracellular levels of glutamate and has shown preliminary efficacy in treating substance addictions [63,64]. N-acetyl cysteine may stimulate inhibitory metabotropic glutamate receptors, possibly causing a reduction in synaptic release of glutamate. Studies in rat populations show N-acetyl cysteine is effective in reducing reward-seeking behavior, and preliminary data in PG are encouraging (see below) [65].
γ-aminobutyric acid (GABA- gamma-aminobutyric acid)
GABA, the major inhibitory neurotransmitter in the brain, is synthesized from glutamate. Similar to endorphins, GABA may influence dopaminergic neurons emerging from the ventral tegmental area (VTA) through GABAergic projections from the ventral palladum and nucleus accumbens (NAc). By inhibiting the dopamine releasing neurons, GABA may block rewarding aspects of addiction [66]. GABAb receptors have also been found in midbrain regions containing serotonergic neurons [67]. GABA can induce membranes to resist depolarization and allow membranes to return to resting states more quickly. GABAergic drugs may influence neuronal excitability [68]. GABAergic drugs have shown early promising results in treating addictions (e.g., tiagabine, a GABA reuptake inhibitor, in cocaine dependence) [69–72].
The Use of Animal Models to Study PG
Animal models have long been used to study the influences of abused drugs but have only recently been used to investigate PG. A rat gambling task (RGT) was developed based on the Iowa gambling task (IGT – a neuropsychological test widely used in assessing decision-making) [73]. Rats demonstrated preferences based on variations in patterns of large versus small immediate gains (food pellets) and intermittent losses (timeouts) of varying magnitudes. This task was then employed to investigate serotonergic and dopaminergic systems contributing to rat decision-making. The same group developed a separate task based on slot machines to investigate the neurochemical underpinnings of the near-miss phenomenon [74]. Rats appeared more likely to make commission errors (attempt to obtain a food pellet) on trials where two of the three lights would illuminate as compared to other “losing” conditions. Pro-dopaminergic drugs, particularly those targeting D2-like dopamine receptors, increased near-miss acquisition behaviors. These findings suggest that like in people, rat behaviors related to gambling may involve both dopaminergic and serotonergic contributions. The availability of these animal models of gambling behaviors should promote translational studies in PG, and may also be used to examine endophenotypic constructs linking PG and other psychiatric disorders [75].
Translating Neurobiological Understandings Into Effective Treatments
Knowing which regions of the brain, and which systems are involved, may guide the selection of medications to test for treatment development. Many pharmacological treatments for PG, as well as behavioral therapies, are based on work and trials in substance addictions and other psychiatric disorders. For example, opioid antagonists and glutamatergic agents have been tested in treating PG like they have in substance dependence and serotonin reuptake inhibitors (SRIs) have been tested in part based on findings in obsessive-compulsive disorder (OCD). As this article is dedicated to the work done on psychopharmacology of PG, we will briefly mention the work done with behavioral therapies before moving to the main focus of our article involving findings from randomized controlled pharmacological trials of treatments for PG.
Treatments
Behavioral Therapies and Interventions
Multiple behavioral therapies and approaches have been used in helping people manage their gambling problems. Gamblers Anonymous (GA), an abstinence based self-help program, is widely available throughout the world and attendance is associated with better treatment outcomes [76–78]. Behavioral therapies, including cognitive behavioral, aversive, and motivational therapies and brief interventions have all shown efficacy in controlled trials [79–87]. More limited empirical data exist for some other therapeutic modalities used in clinical settings (e.g., psychodynamic psychotherapy and family therapies). Meta-analyses investigating the efficacy of behavioral therapies for PG estimate large effects (effect sizes of 2.01 for treatment and 1.59 for follow-up) [88,89]. Few studies have investigated the combination of behavioral and pharmacological therapies. Studies of medications that employ a behavioral therapy platform have typically not identified a medication effect, possibly relating to the effectiveness of the behavioral therapies.
Pharmacotherapies
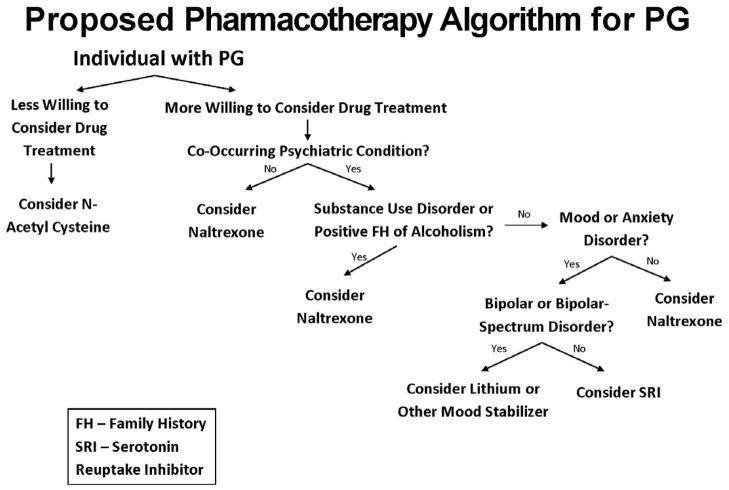
In part based on the neurobiological underpinnings of PG and pharmacotherapies efficacious in substance addictions and other psychiatric disorders, several families of medications (opioid antagonists, mood stabilizers, SRIs and glutamatergic drugs) have been investigated for their efficacies and tolerabilities in the treatment of PG. Data from these investigations, with a focus on placebo-controlled randomized controlled trials (RCTs), are presented, with a summary of data from these trials listed in Table 1. We also propose an empirically informed pharmacotherapy treatment algorithm based on existing data (Fig. 1).
Table 1.
| Clinical Trial Summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medication Type | Medication | Study | Design | Length of trial | Mean End-of-Study Daily Dose | N - Enrolled | N - Analysis | Summary Outcome |
| Serotonergic | Fluvoxamine | Hollander et al. 1998 | Single-blind, placebo-lead in | 8-week placebo lead in; 8-week fluvoxamine trial | 220± 79mg | 16 | 10 | Seven out of 10 defined as treatment responders (>25% reduction on PG-YBOCS) |
| Fluvoxamine versus placebo | Hollander et al. 2000 | Single-blind, placebo-lead in followed by double-blind randomization | Each subject completed 8 weeks of active fluvoxamine and 8 weeks of placebo | 195 ± 50mg | 15 | 10 | Fluvoxamine results in significantly greater improvement in PG-YBOCS and PG-CGI scores | |
| Fluvoxamine versus placebo | Blanco et al. 2002 | Randomized double-blind | 6 months active treatment with fluvoxamine or placebo | 200mg | 32 | 32 | Fluvoxamine not superior to placebo overall but was superior in males and younger participants | |
| Fluvoxamine versus Topiramate | Dannon et al. 2005 | Randomized blind-rater | 12 weeks | 200mg | 31 | 20 | Improvement on CGI significantly better for topiramate group but not for fluvoxamine group | |
| Sertraline | Saiz-Ruiz et al. 2005 | Double-blind, flexible-dose, placebo-controlled | 24 weeks | 95mg | 66 | 60 | N=23 sertraline (74%) and N=21 placebo (76%) considered responders (Criteria for Control of Pathological Gambling Questionnaire) | |
| Escitalopram | Grant & Potenza 2006 | Open-label with double-blind discontinuation | 12-week open-label treatment followed by 8-week double-blind discontinuation phase for responders | 25.4 ± 6.6 mg | 13 | 8 | Significant improvement on PG-YBOCS and HAM-A noted on subjects from the 12 week open-label study. Of the 3 subjects assigned to escitalopram discontinuation gambling symptoms remained abated | |
| Escitalopram & CBT versus CBT | Myrseth et al. 2011 | Randomized open-label | CBT group - 8 weeks CBT only/escitalopram group - 8 weeks escitalopram only followed by 8 weeks CBT & escitalopram | 20mg | 35 | 24 | Both treatments showed significant improvement over time but no significant between-group differences | |
| Paroxetine | Kim et al. 2002 | Double-blind, placebo-controlled, parallel-arm, flexible-dosing | 1 week placebo lead-in followed by 8 weeks active treatment with paroxetine or placebo | 51.7 ± 13.1mg | 53 | 45 | Statistically significant greater improvements on G-SAS and CGI for paroxetine group versus placebo | |
| Paroxetine | Grant et al. 2003 | Double-blind placebo controlled with placebo lead-in | 16 weeks | 60 mg | 76 | 71 | Both paroxetine and placebo group showed significant improvement on PG-CGI but no statistically significant difference between groups | |
| Dopaminergic | Bupropion versus Naltrexone | Dannon et al. 2005 | Randomized blind-rater | 12 weeks | Bupropion 423mg/Naltrexone 116mg | 36 | 25 | 9 (75%) of the bupropion group and 10 (76%) of naltrexone group considered responders (significant improvement on CGI) |
| Bupropion | Black et al. 2007 | Double-blind placebo-controlled | 2 weeks observation followed by 12 weeks active treatment | 324mg | 39 | 39 | Both groups showed significant improvement over time, but no statistically significant between-group differences found | |
| Olanzapine | McElroy et al. 2008 | Double-blind flexible-dose, placebo-controlled | 1 week placebo lead-in followed by 12 weeks active treatment | 8.9mg | 42 | 42 | No statistically significant differences between groups | |
| Olanzapine | Fong et al. 2008 | Double-blind placebo controlled | 7 weeks | 10mg | 23 | 21 | No statistically significant differences between groups | |
| Glutamatergic | N-Acetyl Cysteine | Grant et al. 2007 | Open-label followed by double-blind placebo-controlled discontinuation | 8 weeks open-label followed by 6 weeks of double-blind NAC or placebo | 1476.9 ± 311.3mg | 27 | 27 for open-label phase 13 for double-blind phase | 16 of 27 in the open-label phase met responder criteria (≥30% reduction in PG-YBOCS). In double-blind phase 83.3% of NAC remained as responders versus 28.6% of placebo |
| Topiramate | Berlin et al. 2011 | Double-blind placebo controlled | 14 weeks | 222.50 mg | 42 | 27 | Both groups showed significant improvement on the PG-YBOCS but no statistically significant differences between groups | |
| Amantadine | Thomas et al. 2010 | Double-blind, placebo-controlled, crossover open-extension involving individuals with Parkinson’s disease and co-occurring PG | 17 weeks | 100mg | 17 | 17 | Of the 12 patients assigned to amantadine, 7 reported no PG and the other 5 reported significant improvements | |
| Mood Stabilizers | Lithium | Hollander et al. 2005 | Double-blind placebo controlled trial involving individuals with PG and co-occurring bipolar-spectrum disorders | 10 weeks | 1150mg | 40 | 29 | Statistically significant greater improvements on PG-YBOCS, CARS-M and CGI for lithium group versus placebo |
| Lithium versus Valproate | Pallanti et al. 2002 | Single-blind (evaluator blinded) | 14 weeks | Lithium 795.6 ± 261.5mg & Valproate 873.7 ± 280.1mg | 42 | 31 | Both groups showed significant improvement on the PG-YBOCS but no statistically significant differences between groups | |
| Opioidergic | Naltrexone | Kim et al. 2001 | Double-blind placebo controlled with placebo lead-in | 1-week placebo lead-in followed by 11 weeks of active treatment | 188mg | 83 | 45 | Naltrexone treatment associated with significantly greater improvement on PG-CGI PT, PG-CGI MD, and G-SAS versus placebo |
| Naltrexone | Grant et al. 2008 | Double-blind placebo-controlled with four treatment arms-placebo and naltrexone at 50, 100, or 150mg/day | 18 weeks | 50/100/150 mg | 77 | 49 | Naltrexone versus placebo associated with significantly greater improvement on PG-YBOCS total scores as well as the urge/thought and behavior subscales, and CGI | |
| Naltrexone | Toneatto et al. 2009 | Double-blind placebo controlled with placebo lead-in individuals with co-occurring PG and alcohol use disorders, all of whom received behavioral therapy | 1 week placebo lead-in followed by 11 weeks of active treatment | 100 mg ± 59.4 mg | 52 | 38 | No statistically significant differences between groups | |
| Nalmefene | Grant et al. 2006 | Double-blind placebo controlled with four treatment arms - placebo, nalmefene at 25, 50 or 100mg/day | 16 weeks | 25/50/100mg | 207 | 73 | Nalmefene versus placebo associated with significantly greater improvement on PG-YBOCS total score as well as the urge/thought and behavior subscales, CGI, & G-SAS | |
| Nalmefene | Grant et al. 2010 | Double-blind placebo controlled with three treatment arms - placebo, nalmefene at 20 or 40mg/day | 1-week placebo lead-in followed by 3 weeks of active treatment | 20/40mg | 233 | 233 | ITT analysis revealed no significant differences between groups but post-hoc analysis (of those who received at least one week of targeted dose) showed greater improvement in the 40mg nalmefene group versus placebo | |
(PG – Pathological Gambling), (PG-YBOCS – Yale Brown Obsessive Compulsive Scale adapted for Pathological Gambling), (CGI – Clinical Global Impressions PT – patient rated/MD – MD rated/PG – Pathological Gambling), (G-SAS – Gambling Symptom Assessment Scale), (HAM-A – Hamilton Anxiety Scale), (CARS – Clinician-Administered Rating Scale for Mania), (ITT – Intent-to-Treat), (CBT – Cognitive Behavioral Therapy), (NAC – N-Acetyl-Cysteine)
Figure 1.
Meta-analysis Studies
A quantitative meta-analysis of pharmacological treatment trials conducted between 1966 and July 2006 has been performed [90]. To be included, the intervention had to be pharmacological, pertain to PG and report outcomes in relation to gambling behavior. Of 130 potential studies identified, only 16 met inclusionary criteria, leading to a pool of 597 subjects. The overall effect size, corrected for sample size, was 0.78 (P< 0.01) across conditions. Though the effect size still indicates that pharmacological treatments are effective compared to no treatment, the results from their earlier meta-analysis of psychological treatments had an effect size of 2.01 (P< 0.01) which would seem to indicate that psychological treatments are more effective. The authors noted this but cautioned that direct comparisons between the two types of approaches should be considered cautiously given that pharmacological studies employ placebo controls that are associated with high placebo response rates whereas psychological studies typically employ wait-list controls which are not as often associated with greater improvements, possibly because subjects realize they are not receiving an active treatment.
Serotonin
Clomipramine
Given data supporting a role for serotonin in PG and the efficacy of SRIs in the treatment of obsessive-compulsive disorder, several early clinical trials investigated SRIs in the treatment of PG. Selective SRIs (SSRIs) often have fewer adverse side effects than less selective agents like clomipramine and have thus received more attention, although an early trial of clomipramine involving one patient showed promise [91].
Fluvoxamine
The early promise seen in the trial involving clomipramine led to larger studies of other SRIs including fluvoxamine. In a pilot study, 16 PG subjects participated in a single-blind, placebo lead-in study. Ten patients remained through the fluvoxamine phase of the study, with a mean fluvoxamine dose at the end of the study of 220 mg/day (SD=79, minimum 100 mg/day for four weeks). The authors noted treatment responders tended to have lower mean doses (207 mg/day) than nonresponders (250 mg/day). Seven of the ten completers were considered treatment responders (Clinical Global Impressions (CGI) scores of 1 “very much improved” or 2 ”much improved” along with a greater than 25% reduction in Yale Brown Obsessive Compulsive Scale adapted for Pathological Gambling (PG-YBOCS) scores). The authors noted that two nonresponders had comorbid cyclothymia and suggested that the higher dose (250mg/day) of fluvoxamine may have exacerbated this condition, thereby leading to relapse of gambling behavior. Seven of 10 subjects achieved complete abstinence from gambling. Limitations included the small sample and single-blind nature [92].
In a subsequent double-blind randomized study of fluvoxamine, all subjects entered a single-blind, placebo lead-in, followed by random assignment to one of two arms - eight weeks of fluvoxamine treatment followed by eight weeks of placebo or the reverse order [93]. Fifteen subjects entered the study, of whom ten subjects completed the study, six of whom received placebo first followed by fluvoxamine; the remaining four subjects had the reverse order. All subjects started with a one-week placebo lead-in to assess compliance and early placebo response. Two subjects dropped out during the placebo lead-in with an additional three subjects discontinuing prior to week four of phase one. Dosing began at 50mg/day for the first week with 50mg/day increases in weeks two and three. Dosing was then titrated up in 50mg/day increments over the next five weeks based on clinical response and tolerability. The maximum dose was 250mg/day with a mean dose at end point of 195 ± 50mg/day. Side effects were mild and included those commonly associated with SSRIs such as gastrointestinal distress, sedation, mild anxiety, light-headedness, nausea, and sexual dysfunction. Adverse effects subsided upon discontinuation of study medication. Significant improvement on the PG-CGI scale was noted for fluvoxamine (40.6%) versus placebo (16.6%). Although subjects on fluvoxamine showed greater improvement on the PG-YBOCS score than those on placebo (33.4% versus 28%), the between-group results did not reach statistical significance. An early placebo response was observed, and in a post-hoc analysis, a significant phase x drug interaction was observed relating to a difference in fluvoxamine versus placebo in the second phase but not the first one.
A concurrent independent double-blind study enrolled 32 people with PG, of whom 15 were randomly assigned to a fluvoxamine arm and 17 to a placebo arm. All subjects were treated for six months with the dosing schedule of 100 mg/day for the first two weeks and then 200 mg/day for the remainder of the trial. The primary outcome measure was average amount of money spent gambling with a secondary measure of time spent gambling. Adverse effects included insomnia, headaches, dizziness, and nausea, with nausea being the only adverse effect reported more frequently in the fluvoxamine group than in the placebo group. Both groups showed improvement on both outcome measures, but those on fluvoxamine showed faster improvement than those on placebo. Between-treatment-group differences in outcome were not statistically significant, although differences were reported in males and younger patients. A high placebo response rate (59%) was also noted. The authors also noted high drop-out, particularly among those on fluvoxamine (12 over the course of the six months, versus seven from the placebo group over the course of the six months), although the between-group comparison on drop-out did not reach statistical significance [94].
Another clinical trial compared topiramate versus fluvoxamine in treating PG [95]. Topiramate has GABAergic agonist properties and will be discussed further when reviewing mood stabilizer and glutamatergic medication trials. Thirty-one patients were randomly assigned to one of the two groups (15 to topiramate and 16 to fluvoxamine). Although raters were blinded to assignment, the subjects and physicians were not. Topiramate and fluvoxamine were both titrated to 200mg/day by starting at 25mg/day, increasing to 50 mg/day on day three, and finally increasing in 50mg/day increments every three days until 200 mg/day was reached. Twelve of the fifteen patients from the topiramate group completed the 12-week study and eight out of sixteen receiving fluvoxamine completed. In the topiramate group, only two discontinued due to adverse effects (lack of concentration, dizziness, and vertigo) while five of the eight in the fluvoxamine group discontinued due to adverse effects (diarrhea, dizziness, headache, and loss of appetite). Of those on topiramate, nine reported full remission with an additional three reporting partial remission. CGI scores were significantly improved from baseline in this group. Of those receiving fluvoxamine, six reported full remission with the remaining two reporting partial remission. Limitations include lack of placebo control, subjects not being blind to treatment conditions, and use of self-report outcome measures. As noted in other trials involving fluvoxamine, drop-out rates were high for those on fluvoxamine.
In a subsequent multi-drug, long-term follow-up study, Dannon et al., followed 43 male PG patients who had responded fully to one of four medication treatments for their PG behavior. Six patients were treated with fluvoxamine, nine with topiramate, 18 with bupropion SR, and 10 with naltrexone [96]. All patients had responded fully to their prospective medication during the original trials. After those trials ended, responders from each trial were selected and followed for an additional six months following discontinuation of their medication. Three of six individuals treated with fluvoxamine, three out of nine treated with topiramate, seven out of 18 treated with bupropion SR, and four out of 10 treated with naltrexone relapsed. The authors acknowledge further trials are needed to verify the results given the open-label, uncontrolled nature of the study and the small samples studied.
Investigators have begun to explore the neurobiological factors predicting treatment outcome and the neurobiological mechanisms underlying effective behavioral change [97]. A 36-year-old man with PG underwent fMRI prior to and following treatment with fluvoxamine. The subject showed gambling-related improvement, with a decrease in his desire to gamble and abstinence from gambling during the follow-up period. The findings from the fMRI showed decrease activation in previously activated areas [left frontal (Brodmann’s area 6), right parietal (Brodmann’s areas 7,40), and left parietal (Brodmann’s areas 7, 19, and 40)] when exposed to gambling images [98]. Given the findings from an individual subject and the open-label nature of the study, the findings should be interpreted extremely cautiously.
Sertraline
Only one RCT involving sertraline has been published to date. Saiz-Ruiz et al., conducted a double blind, flexible-dose, placebo-controlled study involving 66 PG subjects [99]. Of the 66 randomly assigned to the 24-week trial, 31 in the sertraline group and 29 in the placebo group were included in their analysis of those who completed the baseline and at least one dose of medication. Dosage was started at 50mg/day during week one and increased up to 100 mg/day by week four, and then up to 150 mg/day at the end of week eight based on clinical response. Response to a lower dose would stop dosage increasing, as would intolerable side effects. The mean dose by the end of the study for sertraline was 95 mg/day. Adverse effects were comparable between sertraline and placebo and included dyspepsia, headache, dizziness, and diarrhea. None of those taking placebo and two on sertraline discontinued due to adverse effects (relating to diarrhea and hypertension). No statistically significant differences were observed in the primary outcome measure – the Criteria for Control of Pathological Gambling Questionnaire (CCPGQ) – nor in any secondary measures, including visual analog scales, amount of money spent gambling, frequency of gambling behavior or CGI scores. By the end of week two, 84% of those in the sertraline group and 69% in the placebo group were considered responders according to CCPGQ scores. By the end of the study, those numbers changed to 74% and 72%, respectively. Secondary measures revealed similar findings. As with most of the other clinical trials involving SSRIs, the high placebo response rate needs to be taken into consideration.
Escitalopram
PG often presents with co-occurring disorders. As SRIs are effective in treating anxiety and depression, they might be particularly helpful for individuals with these co-occurring conditions. In an open-label study followed by double-blind discontinuation, Grant and Potenza found a significant improvement in outcome measures in individuals with PG and co-occurring anxiety disorders [100]. Thirteen subjects were enrolled to a one-week placebo lead-in followed by 11 weeks of open-label escitalopram. Dosing started at 10 mg/day for two weeks and was titrated up to 20 mg/day by visit four and 30 mg/day by visit six, depending on tolerability and response. Mean dose at study end was 25.4 ± 6.6 mg/day. Side effects were minimal and included only one subject who discontinued due to anorgasmia. Only two other reports of mild headaches were noted. Those subjects who were defined as “responders” (>30% reduction on the PG-YBOCS) were invited to an additional eight-week double-blind discontinuation phase. Nine subjects completed the initial 12-week open-label phase of the study, of whom eight were considered responders. PG-YBOCS scores improved by 46.8% from baseline while Hamilton Anxiety Scale (HAM-A) scores improved by 82.8%. Of those responders, four agreed to continue on to the double-blind discontinuation phase, with assignment to placebo associated with worsening in both gambling and anxiety domains and assignment to active drug associated with more sustained improvement in both areas. Although promising, additional studies involving larger samples are warranted.
A second study involving escitalopram was performed using a behavioral therapy platform in a randomized trial involving two parallel treatment arms [101]. The first arm consisted of cognitive behavioral therapy (CBT) alone; the second arm consisted of eight weeks of escitalopram only followed by an additional eight weeks of escitalopram combined with CBT. Thirty subjects were randomized and began treatment (N=15 CBT and N=15 escitalopram and CBT). Outcome assessments involved self-reported scales administered by a clinician who was blinded to treatment condition. Assessments included the Gambling Symptom Assessment Scale (G-SAS) and PG 100-mm Visual Analogue Craving Scale (PG VAC). CBT consisted of eight weekly sessions and escitalopram was started at 5mg/day for the first week, increased to 20mg/day by the third week and held there for the last 14 weeks of treatment. At the 8-week post-treatment phase, 11 subjects from the CBT group remained and 13 from the escitalopram/CBT group remained. By the 16-week assessment, only one subject discontinued from the escitalopram/CBT group. Analyses were performed at both time-points as those in the CBT-only group had completed their treatment at the 8-week mark while those in the escitalopram/CBT group continued to the second arm of their treatment. Adverse effects were minimal and temporary with the exception of one participant who experienced manic features and was therefore discontinued from the medication. Effects included nausea and fatigue. The authors found that at the 8-week mark, there were no significant differences between treatment groups, although both groups reported improvement in G-SAS scores and on other self-reports of gambling behavior. Similar results were seen at the 16-week mark but with the CBT group reporting greater improvement across multiple measures than the combined escitalopram/CBT group. Limitations include the small sample size, lack of blinding to medication, and short time frame of treatment. Although no between-group differences were noted, both treatments groups improved over time. However, escitalopram did not appear to enhance CBT treatment outcome.
Paroxetine
Two RCTs have investigated the efficacy and tolerability of paroxetine in the treatment of PG. In the first study, 53 individuals with PG and without other Axis I disorders were enrolled in an 8-week double-blind, placebo-controlled, parallel-arm, flexible-dosing paroxetine trial [102]. Subjects were given a one-week placebo lead-in followed by eight weeks of double-blinded treatment with paroxetine, titrated from 20mg up to 60mg (in 10mg increments per week) depending on tolerability and efficacy. Adverse events were minimal and included nausea, headache, and sweating. One patient discontinued due to adverse effects. Mean dose at study end was 51.7 ± 13.1mg/day. Outcome measures were determined through scores on the CGI and G-SAS. Of the 53 who entered the placebo lead-in, 45 continued on to the medication phases (six were placebo responders and two dropped out). Forty-one patients completed all study visits with 45 being included in analyses (N=22 placebo, N=23 paroxetine). G-SAS scores decreased significantly for those in the paroxetine arm (52% reduction) versus those in the placebo arm (23% reduction). A similar significant improvement was noted on the CGI (47.8% for the paroxetine arm versus 4.5% for the placebo arm). Limitations to this study include the short duration of medication, a high percentage of women enrolled (67%), and exclusion of Axis I disorders.
A 16-week, multi-center, randomized, double-blind, placebo-controlled trial of paroxetine was next performed at five study sites across two countries [103]. Subjects with other Axis I disorders were also excluded in this study. A one-week placebo lead-in was employed with placebo responders dropped from to the study prior to the opportunity to receive active medication. The primary outcome measure was the PG-CGI, with secondary measures including the PG-YBOCS and G-SAS. Eighty-three patients were consented with seven identified as placebo responders (defined as 30% or greater reduction in PG-YBOCS score). Twenty-one out of 36 patients assigned to paroxetine, and 24 out of 40 patients assigned to placebo, completed all study visits. The main analyses included 34 from the paroxetine group and 37 from the placebo group who completed at least one post-baseline visit. Dosing started at 10 mg/day for week one, increased to 20 mg/day for week two and increased in weekly increments of 10 mg/day up to 60 mg depending on tolerability and response. Adverse events were comparable between paroxetine and placebo groups, with the most common reported adverse effects being dry mouth, headache, and nausea. Six individuals from the paroxetine group and one from the placebo group discontinued due to adverse effects. Unlike the initial paroxetine RCT, no treatment-group-related outcome differences were found. Both placebo and active medication groups had significant improvement in scores across all measures, with those assigned to paroxetine having larger improvements week to week, but not at a statistically significant level.
Dopamine
Bupropion
Bupropion, a dopamine and norepinephrine reuptake inhibitor, was compared to naltrexone in a blind-rater study comparing 19 PG subjects taking naltrexone and 17 subjects taking bupropion over a 12-week period in a parallel fashion [104]. Bupropion SR was started at 150 mg/day for the first week then increased to 300 mg/day for an additional three weeks. Those not responding or responding only partially to that dose were then increased to 450 mg/day. Naltrexone was started at 25 mg/day for the first four days and then increased to 100 mg/day in two divided doses for three weeks. Those not responding or only partially responding to that dose were increased to 150 mg/day. Twelve out of 17 of the patients receiving sustained-release bupropion completed the study compared to 13 out of 19 receiving naltrexone. Nine individuals in the bupropion group were considered full responders as compared to 10 in the naltrexone group. The remaining participants were all considered partial responders. A full responder was defined as absence of gambling behavior for two weeks and improvement on the CGI. Partial response was defined as a decrease in the frequency of gambling behavior and amount of money spent gambling. Individuals in the bupropion group typically increased doses to the highest level to obtain results while those taking naltrexone often responded to more moderate doses. Limitations of the study include no placebo control group, open-label nature, small sample sizes, and exclusion of other Axis I or II disorders (other than PG), limiting the ability to apply these findings to the greater population of PG subjects, many of whom have additional co-morbidities.
In the first and only RCT to date examining bupropion, Black et al., conducted a 12-week, double-blind, placebo-controlled trial involving non-depressed PG subjects [105]. Those meeting inclusion criteria were entered into a two-week observation period before beginning the medication phase. Dosing started at 75mg/day with titrations up to 375mg/day with no increase greater than 150mg per week. The minimum dose for inclusion was 150mg/day, with the average dose by study end being 324mg/day. Efficacy and tolerability were assessed at multiple time-points during the trial, and subjects were evaluated at one, three, and six months. The primary outcome measure was the PG-YBOCS, with secondary measures including the G-SAS and CGI. Thirty-nine subjects were randomized (18 to bupropion) and 22 completed the study (eight from the bupropion group). Eight dropped out before a post-baseline assessment could be obtained, and 17 withdrew during the trial. No significant between-treatment-group difference was found for dropping out. Both treatment groups were balanced with respect to baseline severity of PG, CGI severity scores, Hamilton Depression Rating Scale (HDRS) scores, GSAS scores, and ADHD ratings. Subjects in both groups improved their PG-YBOCS scores with the bupropion group mean score decreasing by 0.71 per week and the placebo group mean score decreasing by 0.81 per week. However, no statistically significant difference was observed between placebo and bupropion. Final scores for the bupropion group decreased by study end a total of 9.2 points while the placebo group showed a decrease of 9.7 points. In addition, bupropion was not superior to placebo on any of the secondary measures, with both groups showing similar improvements across the CGI, GSAS, and ADHD rating scales. Although significant improvement was seen in subjects, no statistical difference was found between bupropion and placebo. Adverse effects in the bupropion group did not differ significantly from the placebo group, although numerically higher frequencies of headache, nervousness, stomach discomfort, and dry mouth were reported. Limitations to this study include frequent non-completion, high placebo response, exclusion of comorbidities (especially substance abuse/dependence), short duration of medication dosing, and small sample size.
Modafinil
One study to date involving PG subjects has investigated modafinil, a stimulant drug with pro-dopaminergic and pro-noradrenergic properties. A double-blind, placebo-controlled study examining modafinil in high-impulsivity (HI) subjects versus low-impulsivity (LI) subjects was conducted based on promising reports of modafinil in targeting impulsivity in subjects with ADHD and cocaine abuse/dependence [106]. The results suggested bi-directional influences based on impulsivity status. The authors predicted that modafinil (200mg) would decrease the priming and reinforcing effect of the slot machine and this effect would be more pronounced in HI versus LI subjects. Main outcome measures included betting behavior, self-report on pleasurable effects of gambling, desire to gamble, and physiological responses to gambling. In addition, post-game inhibitory control was assessed using the Stop-Signal Task (SST). All subjects recruited into the study played a slot-machine-type game in a mock-bar setting and were explicitly informed that this was not a treatment study. Twenty subjects (10 per group) were separated into HI and LI groups according to their scores on the Eysenck Impulsivity Questionnaire (EIQ). A score of <9 was considered LI and a score ≥9 was considered HI. Each subject participated in two slot-machine-playing days, receiving in a random-order double-blinded fashion three hours before playing placebo on one day and modafinil on another. Modafinil decreased motivation to gamble and risky decision making and improved inhibitory control in HI subjects but had opposite influences on LI subjects. Modafinil did not alter self-reported pleasure during game playing. Modafinil significantly decreased mean bet size to a similar degree in both HI and LI subjects. The drug appeared to be well tolerated among both groups with no significant side effects reported. The limitations on this study include small sample size, the complex pharmacology of modafinil, and the exclusion of co-morbidities, which reduces the generalizability of the findings to the population as a whole. As with several other studies, the authors believe the heterogeneity of PG necessitates multiple medications be available to treat the differing people with PG.
Olanzapine
Olanzapine, a drug with antagonistic properties at dopamine and serotonin receptors, has been evaluated in a 12 week double-blind, placebo-controlled, flexible-dose study [107]. The study also included a placebo wash out period of a week, whereby responders, defined as 50% or greater reduction in PG-YBOCS score, were disqualified from continuing on into the 12 week trial and one week discontinuation phases. The dosage schedule began at 2.5mg per day and could be increased by 2.5mg/day every seven days, to a maximum dose of 15mg/day. The average dose for patients during the study was 8.9mg/day. Forty-two subjects were randomized, 40 had at least one post-randomization measure and 25 completed all phases, of which 15 received placebo and 10 olanzapine. There were no statistically significant between-group differences in the reasons for discontinuation. Adverse events were mild to moderate in nature with the most commonly reported being somnolence and increased appetite, which led to a significantly greater weight gain among those on olanzapine than on placebo. The primary outcome measure was the PG-YBOCS, with secondary measures of frequency of gambling episodes, time spent gambling, and CGI scores. The results showed no significant difference across all outcome measures in olanzapine versus placebo. All subjects had improvement across all measures with no discernible difference between those on placebo and those on olanzapine. Limitations of this study were sample size and ineligibility of subjects with Bipolar I disorder.
A concurrent double-blind, placebo-controlled, seven week RCT investigated olanzapine for treating PG involving video poker gambling [108]. Twenty-three participants were enrolled and 21 completed the study. Dosage was started at 2.5mg/day and increased weekly in increments of 2.5mg/day to a final dose of 10mg/day for the last four weeks. Subjects met with the study team on a weekly basis and were encouraged to attend GA. Primary outcome measures were self-report measures of craving on the Brecksville Gambling Craving Scale (BGCS) and the Desire to Gamble Scale (DGS) and gambling-related clinical improvement on the CGI-PG. There were no significant differences between those on placebo and those on olanzapine in gambling-related craving or clinical improvement, although some reductions in gambling behaviors were seen on both groups over time. No serious adverse effects were reported but two subjects in the olanzapine group discontinued due to fatigue and sedation. Limitations include a small sample size, short duration of trial, possible influences of meeting weekly with a clinician (though no formal psychosocial treatment was given), exclusion of co-occurring disorders, and sole involvement of patients who reported video poker as their main addiction.
Together these two trials do not support a role for olanzapine in the treatment of PG, consistent with other findings that show drugs that antagonize dopamine D2-like receptors (e.g. haloperidol) have not been associated with improvement in PG and may instead exacerbate features [109].
Haloperidol
The influences of haloperidol, a D2-like dopamine receptor antagonist, on PG subjects during slot machine gambling were examined [109]. Twenty non-treatment-seeking PG subjects and 18 matched healthy controls were studied. Each subject attended two sessions, one week apart. One session involved administration of haloperidol (3 mg) and one placebo in a double-blind fashion. Each subject gambled in a mock-bar setting 2.75 hours after drug administration. They gambled for 15 minutes or until their $200 in credits were exhausted. The Addiction Research Center Inventory (ARCI) and short form of the Profile of Mood States (POMS) were administered before they took the capsule, and again immediately before gambling. A visual analog scale was used to measure subjective ratings of pleasure at these times as well as right after the gambling episode. The Lexical Salience Task (LST) was administered right after the slot machine episode and blood pressure was measured at 30-minute intervals throughout the session. Haloperidol appeared to increase enjoyment, excitement, and involvement and motivation to gamble for PG subjects with no appreciable effect on controls. Blood pressure rose during gambling in both groups under both conditions, with the haloperidol augmenting the effect for both groups. In PG subjects, haloperidol also enhanced the salience to gambling words relative to neutral words on the LST. This study suggests that D2-like dopamine receptor antagonism may promote gambling-related thoughts and behaviors in PG, and, together with dopamine agonist associations with PG in Parkinson’s disease (PD), suggest that dopamine function may contribute to PG psychopathology in a complex manner and may complicate development of pharmacotherapies targeting D2-like dopamine systems.
Glutamate
N-Acetyl Cysteine
One clinical trial to date has investigated in PG N-Acetyl Cysteine (NAC), a nutraceutical with glutamatergic properties [110]. An eight-week open-label trial with “responders” then randomized to a six week double-blind, placebo-controlled trial was conducted. Dosage started at 600mg/day for two weeks, increased to 1200mg/day for another two weeks, and increased once more to 1800 mg/day for the last four weeks, unless clinical improvement was noted at a lower dose. The mean ± SD effective dose of NAC at the end of the open-label period was 1476.9 ± 311.3mg/day. Adverse events during this phase were few with several reports of flatulence. The primary outcome measure for the open-label period was a 30% reduction on the PG-YBOCS. Those defined as “responders” during the open-label phase were then randomized to placebo-controlled discontinuation. Twenty-seven subjects entered the first open-label phase of the study. Of those, 23 completed the eight week open-label period and showed an average decrease on the PG-YBOCS of 41.9%. Sixteen of these subjects met the a priori definition of a “responder,” of which 13 agreed to randomization in the six-week double-blind, placebo-controlled discontinuation phase. Of note, those subjects who were currently taking a psychotropic medication (not an exclusionary criteria as long as they were stable on the medications for a period of three months prior to enrollment, and no changes were made to their medication schedule while enrolled), showed no response to the open-label NAC. Of the 13 entering discontinuation, six were assigned to NAC and seven to placebo. Of those assigned to NAC, 83.3% still met responder criteria by the end of the double-blind phase, while only 28.6% of those on placebo met criteria. Limitations to this study include the fact that only responders were randomized into the double-blind portion of the study, small sample size, and short duration of treatment.
Topiramate
Topiramate, a glutamatergic antagonist and pro-GABAergic drug that has shown promise in disorders characterized by impulsivity and cravings/urges, has been investigated in the treatment of PG in a 14-week double-blinded, placebo-controlled RCT [111]. Individuals with Axis I disorders other than PG were excluded. Subjects were titrated up to 300 mg/day over the first six weeks and then maintained on their final dosage for another seven weeks, with a one-week taper upon discontinuation. Subjects had to maintain a steady dose of 50mg/day to remain in the study and the mean dose at study end was 222.50 mg/day. The primary outcome measure was change in the obsessions subscale of the PG-YBOCS. Secondary measures included the full PG-YBOCS, G-SAS, and CGI scores. Forty-two subjects were randomized to topiramate or placebo, and 27 completed the study. Of those discontinuing, nine were from the placebo group and six from the topiramate group. Across all measures except the Barratt Impulsiveness Scale (BIS-11) where a trend difference was reported, there were no significant differences seen between those on topiramate versus placebo. Both groups showed significant decreases in their PG-YBOCS scores on the whole and obsessions subscale, and 20 subjects achieved a score of 1 or 2 on the CGI. A total of 28 participants across both groups reported adverse events, although all were mild to moderate in intensity. The most commonly reported adverse effects were fatigue, headache, nausea, and shoulder pain. A total of 101 adverse effects were reported across both groups, with a numerical majority (52) coming from the placebo group. Limitations include the small sample size, exclusion of comorbidities, and short trial duration.
Amantadine
Amantadine, a drug with glutamatergic antagonist properties at the N-methyl d-aspartate (NMDA) receptor and dopaminergic, serotonergic and noradrenergic influences, has been evaluated in treating PG in individuals with PD [112]. Seventeen patients with PG and PD were entered into a 17-week double-blind, placebo-controlled, crossover open-extension study. Four weeks of baseline were followed by eight weeks of amantadine/placebo crossover with one week of discontinuation and four weeks of follow-up. The dosage schedule was titrated from 50mg/bid to 100mg/bid over a 16-day period. The primary gambling outcome measures were the G-SAS and PG-YBOCS. Five patients dropped out. Of the remaining 12 subjects, seven had full remission of gambling behavior with the remaining five showing reduced scores on the G-SAS and PG-YBOCS. G-SAS and PG-YBOCS scores showed significant reduction (80%) after two weeks of treatment with amantadine, whereas no changes were seen during treatment with placebo. Limitations include short treatment schedule, only PG with PD were subjected to the treatment, small sample size, and all subjects were concurrently on anti-parkinsonian medications.
Following-up a study assessing the prevalence of ICDs in PD patients [113], the relationship between amantadine use and ICDs was evaluated [114]. Amantadine use associated with ICDs (17.6% vs. 12.4%) and specifically with problem/pathological gambling (7.4% vs. 4.2%), even after controlling for variables including dopamine agonist and levodopa use. Thus, amantadine use in targeting PG in PD should be considered cautiously and the extent to which amantadine might be efficacious in treating non-PD PG warrants direct investigation.
Mood Stabilizers
Lithium
A placebo-controlled RCT enrolling individuals with PG and bipolar-spectrum disorders (bipolar II, bipolar disorder not otherwise specified, or cyclothymia, with Bipolar I being excluded) investigated sustained-release lithium carbonate [115]. The dosing regimen began with one tablet (300mg/day) for the first four days, then two tablets (600mg/day) for the next four days, followed by three tablets (900 mg/day) according to tolerability and effectiveness. Targeted serum lithium levels ranged from 0.6–1.2 meq/liter, and once the dose for each subject was determined, they were maintained on that dose for the remainder of the trial. The mean dosage at the end of the study was 1150mg/day with a mean blood lithium level of 0.87 meq/liter. Subjects unable to maintain doses or comply with instructions were dropped. Primary outcome measures were the PG-YBOCS and CGI. Forty subjects were enrolled with 18 of them assigned to the lithium group. Six lithium and five placebo subjects dropped out of the study before completing, with no differences between the groups. Ten out of the 12 lithium-treated subjects were rated as responders (>35% reduction in PG-YBOCS score and a score of 1 or 2 on the CGI) versus five of 17 in the placebo group. The lithium group showed a significant improvement over the placebo group in their PG-YBOCS scores (68.8% reduction versus 32.2% reduction). Significant differences were also found between the lithium group and the placebo group on gambling behavior and thoughts with the lithium group having less severe thoughts/urges and gambling behavior by study end. Similar results were seen in the CGI scores, with the lithium group having greater improvement from baseline than the placebo group. Overall, there was a significant main effect of treatment outcome as well as a significant drug-by-time interaction. The lithium-treated group also showed improvement in their Barratt Impulsiveness Scale nonplanning impulsivity subscale scores compared to the placebo group, perhaps indicating this treatment may work by targeting this aspect of impulsivity.
In a study investigating the effects of lithium on brain activity in PG subjects with co-occurring bipolar disorder, Pallanti et al., reported that lithium may alleviate some symptoms of PG by elevating the relative glucose metabolic rate (rGMR) in the caudate nucleus [116,117]. Twenty-one patients with PG and co-occurring bipolar disorder and 21 matched comparison subjects underwent baseline PET scans. Sixteen PG patients entered a double-blind, placebo-control, randomized trial of lithium, as described in the paragraph above. Four out of the five assigned to the lithium group and three out of eleven assigned to the placebo group were classified as responders. After 10 weeks of treatment, these patients had a second PET scan. The treatment had no effect on the rGMR in the putamen and thalamus, but did increase rGMR in the ventral caudate. Compared to the controls at baseline, there was a lower GMR in PG patients, and lithium treatment was associated with increases to levels more in line with the control subjects at baseline. Limitations to this study include the small sample size, presence of bipolar disorder limiting the generalizability of the findings, and lack of subjects completing an assessment of their cognitive processing related to gambling while undergoing the PET scan.
Lithium was compared to valproate in a single-blind (evaluator blinded) trial [118]. Forty-two patients were enrolled, of whom, 15 receiving lithium and 16 patients receiving valproate completed. The lithium-treated group received doses of 600mg/day for days one through four, 900 mg/day for days five through nine, and then 1200mg/day if tolerated. The valproate-treated group received doses of 600 mg/day for days one through five, and the dose was then titrated up to 1500 mg/day as tolerated. Mean dose of lithium at study end was 795.6 ± 261.5mg/day and mean dose of valproate was 873.7 ± 280.1mg/day. Primary outcome measures included PG-YBOCS and CGI measures with secondary measures including the Mania Rating Scale (MRS), and the Hamilton Rating Scale for Depression (HAM-D). Both groups showed improvement after the first week, with the lithium group’s changes approaching significance. CGI scores improved over the course of four to 14 weeks. PG-YBOCS scores improved on average 30.1% for those on lithium, and 35.9% for those on valproate. Limitations include small sample size, single-blind design, lack of placebo control, and exclusion of co-morbidities commonly associated with PG (substance use and bipolar disorders).
Opioid Antagonists
Naltrexone
Naltrexone, an antagonist at mu and kappa opioid receptors, has been shown in RCTs to be efficacious in treating substance addictions including alcohol and opioid dependence. Several RCTs suggest it is also efficacious in treating PG.
An early RCT investigated naltrexone in an 11-week, double-blind, placebo-controlled trial with a one week single-blind placebo lead-in [119]. The primary outcome measures were the G-SAS (improvement of 50% or more after the placebo lead-in excluded subjects from continuing), the CGI-PT (patient rated) and CGI-MD (clinician rated). Naltrexone was started at 25mg/day for the first two days then increased to 50mg/day for the rest of the first week. The dosage was then titrated up until improvement was seen or 250mg/day was reached (rate of increase limited to 50mg/week). Eighty-three subjects were enrolled and 38 were terminated from the study prior to being treated with 100mg/day naltrexone for two weeks (22 were considered placebo responders, five had abnormal hepatic transaminase levels, and the rest for varying reasons). Forty-five subjects (N=25 placebo, N=20 naltrexone) were included in the data analysis. Thirty-six subjects completed all study visits with nine more completing at least six visits. Naltrexone demonstrated superiority over placebo. On the CGI, those on naltrexone showed 55% very much improved and 20% much improved versus 12% on both for placebo. The average dose of naltrexone at study end was 188mg/day. Side effects were common to both groups with no significant between-group differences with the exception of dry mouth (40% versus 0%), vivid dreams (40% versus 4%), and decreased libido (35% versus 8%). Of those taking naltrexone, there were four subjects who developed elevated liver transaminase levels, and this occurred in individuals who were concurrently on an analgesic. A significant placebo effect was also observed. Limitations include the high dropout frequency for placebo responders, small sample sizes, high drop-out frequency among the naltrexone group during the double-blind phase (mostly due to elevated transaminase levels common to higher doses of naltrexone), and short duration of trial.
A subsequent RCT investigated the efficacy and tolerability of naltrexone in an 18-week, double-blind, placebo-controlled trial involving four conditions – naltrexone at 50mg/day, 100mg/day, 150mg/day, and placebo [120]. To guard against nausea, all subjects were given ondansetron (4mg/day) for the first three days of their medication regime. To further protect the blinded nature of the study, a third investigator saw the subjects at their week two visit to assess side effects and improvement. This investigator did not have contact with study participants at any other visit. Due to their findings from the previous trial, only subjects who reported gambling due to urges or cravings were enrolled. Individuals with co-occurring disorders (with the exception of bipolar, substance abuse, and psychotic disorders) were included. The primary outcome measure was the PG-YBOCS, with secondary measures including the G-SAS and CGI. Seventy-seven subjects entered a one-week placebo lead-in and those not considered responders (greater than 50% reduction on the G-SAS of which six were considered responders) were randomized into one of the four treatment groups in blocks of eight for 17 weeks (19 in the placebo group, 58 in one of the three naltrexone groups). Dosing for naltrexone started at 25mg/day for two days, and then increased to 50mg/day. At week three, those in the 100 or 150mg/day group were raised to their respective levels. There were no significant differences between groups in relation to reported adverse events. Most adverse events were mild to moderate in nature and occurred within the first week of treatment. Adverse events included nausea, diarrhea, insomnia, dry mouth, and constipation. There were no significant differences among the three groups receiving naltrexone on baseline characteristics, treatment completion, compliance, treatment effects, or side effects, so the results were combined and presented as group comparisons between naltrexone and placebo. Thirteen of the placebo group and 36 of the naltrexone group completed the study with no significant between-group differences noted for those who did and did not complete. On the primary outcome measure (PG-YBOCS), those on naltrexone had an average decrease of 43% while those on placebo only had an average decrease of 31%. Of those assigned to naltrexone, 23 were able to abstain from gambling for at least a month compared to just two from the placebo group. Statistically significant differences were seen on the urge and behavior subscales of the PG-YBOCS as well as on the G-SAS. Limitations to this study include no long-term follow-up beyond the 18 weeks of medication, exclusion of those with severe mental illness comorbidities, and a placebo effect common in pharmacological treatment trials for PG.
An additional trial involving naltrexone combined pharmacological and behavioral therapies and involved subjects with co-occurring alcohol dependence and PG [121]. Fifty-two subjects (placebo n=25, naltrexone n=27) enrolled in the trial that involved a one-week placebo lead-in followed by 11-week double-blind, placebo-controlled trial of naltrexone with concurrent CBT. Placebo response was defined as 50% or greater reduction in gambling behavior or alcohol consumption. Dosing of naltrexone was initiated at 25 mg/day for three days and then increased to 50 mg/day for the next 11 days. Thereafter, the dose was maintained at 50 mg/day or was increased by increments of 50 mg until therapeutic effect was noted by the patient and physician. Dose increases could occur every two weeks to a maximum of 250 mg/day. The mean dose by study end was 100 mg ± 59.4 mg. The most common adverse effects reported were nausea, dry mouth, fatigue, and headaches. In addition to the medication/placebo, all subjects received CBT of up to seven sessions for both alcohol dependence and PG. Primary outcome measures were number of days drinking, number of days gambling, number of drinks/drinking day, and amount spent gambling/gambling day. All but one subject from the naltrexone group completed the medication portion of the trial. Those considered completers (attendance at 12-month follow-up assessment) included 19 from the original 27 in the naltrexone group and 19 from the original 25 in the placebo group. Eight and six failed to complete the study from the naltrexone and placebo groups, respectively. Although significant time effects were noted among completers, no statistically significant between-group differences were seen on any of the four behavioral measures. Limitations include no clinician rated assessments of improvement (i.e. PG-YBOCS, G-SAS, CGI, etc.), short duration of study, small sample size, and primarily male (93%) sample. It should also be noted that all subjects received cognitive behavioral counseling and any improvement cannot be attributed solely to the medication or to the counseling.
Nalmefene
Nalmefene, an antagonist at the mu, delta and kappa opioid receptors, also has shown promising results in the treatment of substance addictions, particularly alcohol dependence. However, unlike naltrexone, nalmefene is considered devoid of hepatotoxicity. Nalmefene was initially investigated in the treatment of PG in a 16-week, randomized, double-blind, placebo-controlled trial at 15 sites throughout the United States [122]. Two hundred and seven subjects were enrolled. The primary outcome measure was the PG-YBOCS with secondary measures including the CGI, G-SAS, and Sheehan Disability Scale (SDS). After screening, subjects were randomly assigned to one of four groups – nalmefene at 25mg/day, 50mg/day, 100mg/day, or placebo. Fifty-one subjects were assigned to placebo with the remainder assigned to one of the nalmefene groups. All subjects began at 25mg/day nalmefene or placebo and those assigned to the higher doses had their dosage raised at week two. Although 207 subjects began the treatment study, dropouts included 27 from the placebo group and 107 from the nalmefene group, leaving 49 individuals in the nalmefene groups and 24 individuals in the placebo group for comparison. Discontinuation was more frequent at higher doses of nalmefene compared to placebo or 25mg/day of nalmefene. Reasons for discontinuation included adverse events and loss to follow-up. Adverse events were mild to moderate in nature, occurred primarily in the first week of treatment, and most commonly included nausea, insomnia, constipation, and dizziness. There was no significant difference in the outcome measures between the three nalmefene groups so those data were combined and compared as a group against the placebo group. On the primary measure, those on nalmefene had better outcomes from those on placebo (placebo – 18.9% reduction on PG-YBOCS compared to 36.3% reduction for combined nalmefene groups). Responders, defined as demonstrating a score of 1 “very much improved” or 2 “much improved” on the CGI at the last evaluation point, were more frequent in the group assigned to receive 25mg/day of nalmefene group, in which 59% were responders as compared to 34% on placebo. Limitations to this study include the short duration of treatment, exclusion of individuals with psychiatric co-morbidities, frequent dropout, and possible compromise of blinding related to adverse effects.
A subsequent confirmatory study involved a double-blind, placebo-controlled RCT of nalmefene with a single-blind placebo lead-in phase [123]. Two-hundred and thirty-three participants from 25 outpatient centers throughout the United States were enrolled into the single-blind placebo lead-in phase. Patients who maintained a score of 15 or greater on the PG-YBOCS were then randomized into one of three groups for the double-blind phase – nalmefene at 20mg/day (n=77), nalmefene at 40mg/day (n=82), or placebo (n=74). Subjects assigned to nalmefene were started at 5mg/day and increased to 20mg/day for week two, then 40mg/day at week three for those assigned to that arm. The primary outcome measure was the total score on the PG-YBOCS with secondary measures including the subscales on the PG-YBOCS, G-SAS, and SDS. Responders were defined as those who had a decrease of 35% or greater on the PG-YBOCS total score. Adverse events were similar to those in the initial study of nalmefene. In an intent-to-treat (ITT) analysis, 46.8% of those on 20mg/day, 56.1% of those on 40mg/day, and 59.5% of those on placebo were considered responders, no between-group differences observed. However, the ITT group included 43 participants who discontinued prior to receiving active medication. Using only individuals who had a full week of their assigned dose (73% of those assigned to nalmefene) revealed a significant difference in mean PG-YBOCS scores between the 40mg/day nalmefene group and those on placebo. Limitations of this study include the exclusion of comorbidities, short trial duration, and frequent dropout.
Data from placebo-controlled RCTs of naltrexone and nalmefene were analyzed to identify factors associated with treatment outcome [124]. In alcohol dependence, opiate antagonist treatment outcome is better amongst those with a family history of alcoholism and strong cravings for alcohol [125,126]. Similarly, treatment outcome to naltrexone or nalmefene in PG was associated with a family history of alcoholism and, amongst those receiving high doses of opioid antagonist (nalmefene 50 or 100mg, naltrexone 100 or 150mg), strong gambling urges at treatment onset. The variable most strongly associated with placebo response was age, with younger individuals more likely to respond to placebo.
Conclusions and Future Directions
Multiple neurotransmitter systems, including serotonergic, dopaminergic, noradrenergic, glutamatergic and opioidergic, have been implicated in the pathophysiology of PG. Consistently, brain imaging studies have implicated multiple brain regions and circuits, particularly ventral cortical-striatal areas. RCTs investigating the efficacy and tolerability of pharmacological treatments have demonstrated multiple promising leads, with opioid antagonists demonstrating the greatest empirical support. Nonetheless, few studies have examined how opioids and opioid receptor function relates to gambling and PG, and individual differences in opioid receptor systems may have important treatment implications. For example, allelic variants of the gene encoding mu-opioid receptor gene have been linked to naltrexone treatment outcome in alcoholism, and these warrant investigation in PG, particularly with respect to treatment with naltrexone or nalmefene [127,128]. Existing data suggest that co-occurring disorders may be used to guide the selection of pharmacotherapies (Figure 1). Although promising, multiple aspects still warrant additional testing. Additionally, treatments based on other important individual differences (e.g., such as facets of impulsivity or compulsivity or stress responsiveness) also should be considered as measures that can guide treatment selection or as targets for treatment development.
Multiple knowledge gaps exist. For example, the frequent placebo response observed is poorly understood, may complicate treatment outcome, and warrants study. Treatments that combine pharmacological and behavioral approaches may help optimize treatment outcome. Additional measures (genetic, neuroimaging) might help identify factors that can be used to select appropriate treatments on an individual basis and may also better define brain mechanisms predicting and mediating the effectiveness of specific treatments. Imaging trials using PET ligands may also help identify novel treatment targets in PG. Using these approaches, the lives of many people currently struggling with PG may be improved.
Acknowledgments
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, and Psyadon pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
This research was supported by NIH grants R01 DA019039, P20 DA027844, RC1 DA028279, and by a grant from the National Center for Responsible Gaming and its Institute for Research on Gambling Disorders.
Footnotes
Conflicts of Interest: The authors report that they have no financial conflicts of interest with respect to the content of this manuscript.
Mr. Bullock reports no biomedical financial interests or other conflicts of interest.
References
- 1.Wareham JD, Potenza MN. Pathological gambling and substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):242–7. doi: 10.3109/00952991003721118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(5):564–74. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- 3.Aragay N, Roca A, Garcia B, Marqueta C, Guijarro S, Delgado L, et al. Pathological gambling in a psychiatric sample. Compr Psychiatry. 2011 doi: 10.1016/j.comppsych.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Petry NM. Gambling and substance use disorders: current status and future directions. Am J Addict. 2007;16(1):1–9. doi: 10.1080/10550490601077668. [DOI] [PubMed] [Google Scholar]
- 5.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: Author; 2000. Text Revision. [Google Scholar]
- 6.Holden C. Psychiatry. Behavioral addictions debut in proposed DSM-V. Science. 2010;327(5968):935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- 7.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 8.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75(1):63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallick M, Suits D, Dielman T, Hybels J. A Survey of American Gambling Behaviors and Behavior. Ann Arbor: Research Report Series, Survey Research Center, Institute for Social Research; 1979. [Google Scholar]
- 10.Gerstein D, Hoffman J, Larison C, Engelman L, Murphy S, Palmer A, et al. Gambling Impact and Behavior Study: Report to National Gambling Impact Study Commission. Chicago, Ill: National Opinion Research Center; 1999. [Google Scholar]
- 11.Welte JW, Barnes GM, Tidwell MC, Hoffman JH. Gambling and problem gambling across the lifespan. J Gambl Stud. 2011;27(1):49–61. doi: 10.1007/s10899-010-9195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai RA, Desai MM, Potenza MN. Gambling, health and age: data from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Addict Behav. 2007;21(4):431–40. doi: 10.1037/0893-164X.21.4.431. [DOI] [PubMed] [Google Scholar]
- 13.Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann N Y Acad Sci. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8(2):147–8. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26(51):13213–7. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum Brain Mapp. 2010;31(3):410–23. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue G, Lu Z, Levin IP, Bechara A. An fMRI study of risk-taking following wins and losses: implications for the gambler’s fallacy. Hum Brain Mapp. 2011;32(2):271–81. doi: 10.1002/hbm.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100(1–2):17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66(8):734–42. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160(11):1990–4. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 21.Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):828–36. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- 22.Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD. Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry. 2008;63(3):293–300. doi: 10.1016/j.biopsych.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61(3):481–90. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(Pt 8):1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- 25.Domenech P, Dreher JC. Decision threshold modulation in the human brain. J Neurosci. 2010;30(43):14305–17. doi: 10.1523/JNEUROSCI.2371-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrovic P, Pleger B, Seymour B, Kloppel S, De Martino B, Critchley H, et al. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci. 2008;28(42):10509–16. doi: 10.1523/JNEUROSCI.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biol Psychiatry. 2005;58(10):787–95. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 28.Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15(4):491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 30.Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62(4):593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 33.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35(3):591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–55. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 35.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 36.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–8. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara K, Yanagisawa A, Kagota Y, Gomi A, Nemoto K, Moriya E, et al. Physiological changes in Pachinko players; beta-endorphin, catecholamines, immune system substances and heart rate. Appl Human Sci. 1999;18(2):37–42. doi: 10.2114/jpa.18.37. [DOI] [PubMed] [Google Scholar]
- 38.Meyer G, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, Exton MS. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biol Psychiatry. 2000;48(9):948–53. doi: 10.1016/s0006-3223(00)00888-x. [DOI] [PubMed] [Google Scholar]
- 39.Meyer G, Schwertfeger J, Exton MS, Janssen OE, Knapp W, Stadler MA, et al. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29(10):1272–80. doi: 10.1016/j.psyneuen.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, et al. Pathological gambling. A psychobiological study. Arch Gen Psychiatry. 1988;45(4):369–73. doi: 10.1001/archpsyc.1988.01800280085011. [DOI] [PubMed] [Google Scholar]
- 41.Benschop RJ, Jacobs R, Sommer B, Schurmeyer TH, Raab JR, Schmidt RE, et al. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB J. 1996;10(4):517–24. doi: 10.1096/fasebj.10.4.8647351. [DOI] [PubMed] [Google Scholar]
- 42.Benschop RJ, Nieuwenhuis EE, Tromp EA, Godaert GL, Ballieux RE, van Doornen LJ. Effects of beta-adrenergic blockade on immunologic and cardiovascular changes induced by mental stress. Circulation. 1994;89(2):762–9. doi: 10.1161/01.cir.89.2.762. [DOI] [PubMed] [Google Scholar]
- 43.Müller C, Jacobs B, editors. Handbook of the behavioral neurobiology of serotonin. 1. London: Academic Press; 2010. [Google Scholar]
- 44.Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci. 2009;29(38):11993–9. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, et al. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30(6):1138–47. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- 46.Nordin C, Eklundh T. Altered CSF 5-HIAA Disposition in Pathologic Male Gamblers. CNS Spectr. 1999;4(12):25–33. doi: 10.1017/s1092852900006799. [DOI] [PubMed] [Google Scholar]
- 47.Linnoila M, Virkkunen M. Biologic correlates of suicidal risk and aggressive behavioral traits. J Clin Psychopharmacol. 1992;12(2 Suppl):19S–20S. [PubMed] [Google Scholar]
- 48.Pallanti S, Bernardi S, Quercioli L, DeCaria C, Hollander E. Serotonin dysfunction in pathological gamblers: increased prolactin response to oral m-CPP versus placebo. CNS Spectr. 2006;11(12):956–64. doi: 10.1017/s1092852900015145. [DOI] [PubMed] [Google Scholar]
- 49.Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 50.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–69. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- 51.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 (Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 52.Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29(14):4531–41. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329(5991):532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herz A, Spanagel R. Endogenous opioids and addiction. Harwood, Germany: Taylor & Francis; 1995. (The pharmacology of opioids) [Google Scholar]
- 55.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129(2):99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 56.Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25(4):235–51. [PubMed] [Google Scholar]
- 57.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89(6):2046–50. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaszczynski AP, Winter SW, McConaghy N. Plasma endorphin levels in pathological gambling. Journal of Gambling Studies. 1986;2(1):3–14. [Google Scholar]
- 59.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 60.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 (Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nordin C, Gupta RC, Sjodin I. Cerebrospinal fluid amino acids in pathological gamblers and healthy controls. Neuropsychobiology. 2007;56(2–3):152–8. doi: 10.1159/000115782. [DOI] [PubMed] [Google Scholar]
- 63.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 64.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25(27):6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 66.Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol. 2010;58:315–71. doi: 10.1016/S1054-3589(10)58013-X. [DOI] [PubMed] [Google Scholar]
- 67.Wirtshafter D, Sheppard AC. Localization of GABA(B) receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56(1):1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 68.Egebjerg J, Schousboe A, Krogsgaard-Larsen P, editors. Glutamate and gaba receptors and transporters: structure, function and pharmacology London. New York: Taylor & Francis; 2002. [Google Scholar]
- 69.Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64(12):1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 70.Myrick H, Henderson S, Brady KT, Malcom R, Measom M. Divalproex loading in the treatment of cocaine dependence. J Psychoactive Drugs. 2001;33(3):283–7. doi: 10.1080/02791072.2001.10400575. [DOI] [PubMed] [Google Scholar]
- 71.Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91(2–3):141–8. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 73.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34(10):2329–43. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- 74.Winstanley CA, Cocker PJ, Rogers RD. Dopamine modulates reward expectancy during performance of a slot machine task in rats: evidence for a ‘near-miss’ effect. Neuropsychopharmacology. 2011;36(5):913–25. doi: 10.1038/npp.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernando AB, Robbins TW. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 76.Petry NM. Gamblers anonymous and cognitive-behavioral therapies for pathological gamblers. J Gambl Stud. 2005;21(1):27–33. doi: 10.1007/s10899-004-1919-5. [DOI] [PubMed] [Google Scholar]
- 77.Petry NM, Ammerman Y, Bohl J, Doersch A, Gay H, Kadden R, et al. Cognitive-behavioral therapy for pathological gamblers. J Consult Clin Psychol. 2006;74(3):555–67. doi: 10.1037/0022-006X.74.3.555. [DOI] [PubMed] [Google Scholar]
- 78.Petry NM. Patterns and correlates of Gamblers Anonymous attendance in pathological gamblers seeking professional treatment. Addict Behav. 2003;28(6):1049–62. doi: 10.1016/s0306-4603(02)00233-2. [DOI] [PubMed] [Google Scholar]
- 79.Champine RB, Petry NM. Pathological gamblers respond equally well to cognitive-behavioral therapy regardless of other mental health treatment status. Am J Addict. 2010;19(6):550–6. doi: 10.1111/j.1521-0391.2010.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hodgins DC, Peden N. Cognitive-behavioral treatment for impulse control disorders. Rev Bras Psiquiatr. 2008;30 (Suppl 1):S31–40. doi: 10.1590/s1516-44462006005000055. [DOI] [PubMed] [Google Scholar]
- 81.Tavares H, Zilberman ML, el-Guebaly N. Are there cognitive and behavioural approaches specific to the treatment of pathological gambling? Can J Psychiatry. 2003;48(1):22–7. doi: 10.1177/070674370304800105. [DOI] [PubMed] [Google Scholar]
- 82.Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21(6):835–42. doi: 10.1016/0306-4603(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 83.Grant JE, Donahue CB, Odlaug BL, Kim SW. A 6-month follow-up of imaginal desensitization plus motivational interviewing in the treatment of pathological gambling. Ann Clin Psychiatry. 2011;23(1):3–10. [PMC free article] [PubMed] [Google Scholar]
- 84.McConaghy N, Armstrong MS, Blaszczynski A, Allcock C. Controlled comparison of aversive therapy and imaginal desensitization in compulsive gambling. Br J Psychiatry. 1983;142:366–72. doi: 10.1192/bjp.142.4.366. [DOI] [PubMed] [Google Scholar]
- 85.McConaghy N, Blaszczynski A, Frankova A. Comparison of imaginal desensitisation with other behavioural treatments of pathological gambling. A two-to nine-year follow-up. Br J Psychiatry. 1991;159:390–3. doi: 10.1192/bjp.159.3.390. [DOI] [PubMed] [Google Scholar]
- 86.Petry NM, Weinstock J, Ledgerwood DM, Morasco B. A randomized trial of brief interventions for problem and pathological gamblers. J Consult Clin Psychol. 2008;76(2):318–28. doi: 10.1037/0022-006X.76.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brewer JA, Grant JE, Potenza MN. The Treatment of Pathologic Gambling. Addictive Disorders & Their Treatment. 2008;7(1):1–13. 0.1097/ADT.0b013e31803155c2. [Google Scholar]
- 88.Pallesen S, Mitsem M, Kvale G, Johnsen BH, Molde H. Outcome of psychological treatments of pathological gambling: a review and meta-analysis. Addiction. 2005;100(10):1412–22. doi: 10.1111/j.1360-0443.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 89.Gooding P, Tarrier N. A systematic review and meta-analysis of cognitive-behavioural interventions to reduce problem gambling: hedging our bets? Behav Res Ther. 2009;47(7):592–607. doi: 10.1016/j.brat.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Pallesen S, Molde H, Arnestad HM, Laberg JC, Skutle A, Iversen E, et al. Outcome of pharmacological treatments of pathological gambling: a review and meta-analysis. J Clin Psychopharmacol. 2007;27(4):357–64. doi: 10.1097/jcp.013e3180dcc304d. [DOI] [PubMed] [Google Scholar]
- 91.Hollander E, Frenkel M, Decaria C, Trungold S, Stein DJ. Treatment of pathological gambling with clomipramine. Am J Psychiatry. 1992;149(5):710–1. doi: 10.1176/ajp.149.5.710b. [DOI] [PubMed] [Google Scholar]
- 92.Hollander E, DeCaria CM, Mari E, Wong CM, Mosovich S, Grossman R, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(12):1781–3. doi: 10.1176/ajp.155.12.1781. [DOI] [PubMed] [Google Scholar]
- 93.Hollander E, DeCaria CM, Finkell JN, Begaz T, Wong CM, Cartwright C. A randomized double-blind fluvoxamine/placebo crossover trial in pathologic gambling. Biol Psychiatry. 2000;47(9):813–7. doi: 10.1016/s0006-3223(00)00241-9. [DOI] [PubMed] [Google Scholar]
- 94.Blanco C, Petkova E, Ibanez A, Saiz-Ruiz J. A pilot placebo-controlled study of fluvoxamine for pathological gambling. Ann Clin Psychiatry. 2002;14(1):9–15. doi: 10.1023/a:1015215809770. [DOI] [PubMed] [Google Scholar]
- 95.Dannon PN, Lowengrub K, Gonopolski Y, Musin E, Kotler M. Topiramate versus fluvoxamine in the treatment of pathological gambling: a randomized, blind-rater comparison study. Clin Neuropharmacol. 2005;28(1):6–10. doi: 10.1097/01.wnf.0000152623.46474.07. [DOI] [PubMed] [Google Scholar]
- 96.Dannon PN, Lowengrub K, Musin E, Gonopolsky Y, Kotler M. 12-month follow-up study of drug treatment in pathological gamblers: a primary outcome study. J Clin Psychopharmacol. 2007;27(6):620–4. doi: 10.1097/jcp.0b013e31815a4400. [DOI] [PubMed] [Google Scholar]
- 97.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung SK, You IH, Cho GH, Chung GH, Shin YC, Kim DJ, et al. Changes of functional MRI findings in a patient whose pathological gambling improved with fluvoxamine. Yonsei Med J. 2009;50(3):441–4. doi: 10.3349/ymj.2009.50.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saiz-Ruiz J, Blanco C, Ibanez A, Masramon X, Gomez MM, Madrigal M, et al. Sertraline treatment of pathological gambling: a pilot study. J Clin Psychiatry. 2005;66(1):28–33. doi: 10.4088/jcp.v66n0104. [DOI] [PubMed] [Google Scholar]
- 100.Grant JE, Potenza MN. Escitalopram treatment of pathological gambling with co-occurring anxiety: an open-label pilot study with double-blind discontinuation. Int Clin Psychopharmacol. 2006;21(4):203–9. doi: 10.1097/00004850-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Myrseth H, Molde H, Støylen I, Johnsen B, Holsten F, Pallesen S. A pilot study of CBT versus escitalopram combined with CBT in the treatment of pathological gamblers. International Gambling Studies. 2011;11(01):121–41. [Google Scholar]
- 102.Kim SW, Grant JE, Adson DE, Shin YC, Zaninelli R. A double-blind placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling. J Clin Psychiatry. 2002;63(6):501–7. doi: 10.4088/jcp.v63n0606. [DOI] [PubMed] [Google Scholar]
- 103.Grant JE, Kim SW, Potenza MN, Blanco C, Ibanez A, Stevens L, et al. Paroxetine treatment of pathological gambling: a multi-centre randomized controlled trial. Int Clin Psychopharmacol. 2003;18(4):243–9. doi: 10.1097/00004850-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Dannon PN, Lowengrub K, Musin E, Gonopolski Y, Kotler M. Sustained-release bupropion versus naltrexone in the treatment of pathological gambling: a preliminary blind-rater study. J Clin Psychopharmacol. 2005;25(6):593–6. doi: 10.1097/01.jcp.0000186867.90289.ed. [DOI] [PubMed] [Google Scholar]
- 105.Black DW, Arndt S, Coryell WH, Argo T, Forbush KT, Shaw MC, et al. Bupropion in the treatment of pathological gambling: a randomized, double-blind, placebo-controlled, flexible-dose study. J Clin Psychopharmacol. 2007;27(2):143–50. doi: 10.1097/01.jcp.0000264985.25109.25. [DOI] [PubMed] [Google Scholar]
- 106.Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660–71. doi: 10.1177/0269881108091072. [DOI] [PubMed] [Google Scholar]
- 107.McElroy SL, Nelson EB, Welge JA, Kaehler L, Keck PE., Jr Olanzapine in the treatment of pathological gambling: a negative randomized placebo-controlled trial. J Clin Psychiatry. 2008;69(3):433–40. doi: 10.4088/jcp.v69n0314. [DOI] [PubMed] [Google Scholar]
- 108.Fong T, Kalechstein A, Bernhard B, Rosenthal R, Rugle L. A double-blind, placebo-controlled trial of olanzapine for the treatment of video poker pathological gamblers. Pharmacol Biochem Behav. 2008;89(3):298–303. doi: 10.1016/j.pbb.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 109.Zack M, Poulos CX. A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers. Neuropsychopharmacology. 2007;32(8):1678–86. doi: 10.1038/sj.npp.1301295. [DOI] [PubMed] [Google Scholar]
- 110.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652–7. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Berlin HA, Braun A, Simeon D, Koran LM, Potenza MN, McElroy SL, et al. A double-blind, placebo-controlled trial of topiramate for pathological gambling. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.560964. [DOI] [PubMed] [Google Scholar]
- 112.Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68(3):400–4. doi: 10.1002/ana.22029. [DOI] [PubMed] [Google Scholar]
- 113.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–95. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 114.Weintraub D, Sohr M, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010;68(6):963–8. doi: 10.1002/ana.22164. [DOI] [PubMed] [Google Scholar]
- 115.Hollander E, Pallanti S, Allen A, Sood E, Baldini Rossi N. Does sustained-release lithium reduce impulsive gambling and affective instability versus placebo in pathological gamblers with bipolar spectrum disorders? Am J Psychiatry. 2005;162(1):137–45. doi: 10.1176/appi.ajp.162.1.137. [DOI] [PubMed] [Google Scholar]
- 116.Pallanti S, Haznedar MM, Hollander E, Licalzi EM, Bernardi S, Newmark R, et al. Basal Ganglia activity in pathological gambling: a fluorodeoxyglucose-positron emission tomography study. Neuropsychobiology. 2010;62(2):132–8. doi: 10.1159/000317286. [DOI] [PubMed] [Google Scholar]
- 117.Hollander E, Buchsbaum MS, Haznedar MM, Berenguer J, Berlin HA, Chaplin W, et al. FDG-PET study in pathological gamblers. 1. Lithium increases orbitofrontal, dorsolateral and cingulate metabolism. Neuropsychobiology. 2008;58(1):37–47. doi: 10.1159/000154478. [DOI] [PubMed] [Google Scholar]
- 118.Pallanti S, Quercioli L, Sood E, Hollander E. Lithium and valproate treatment of pathological gambling: a randomized single-blind study. J Clin Psychiatry. 2002;63(7):559–64. doi: 10.4088/jcp.v63n0704. [DOI] [PubMed] [Google Scholar]
- 119.Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914–21. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- 120.Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783–9. doi: 10.4088/jcp.v69n0511. [DOI] [PubMed] [Google Scholar]
- 121.Toneatto T, Brands B, Selby P. A randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of concurrent alcohol use disorder and pathological gambling. Am J Addict. 2009;18(3):219–25. doi: 10.1080/10550490902787007. [DOI] [PubMed] [Google Scholar]
- 122.Grant JE, Potenza MN, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303–12. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- 123.Grant JE, Odlaug BL, Potenza MN, Hollander E, Kim SW. Nalmefene in the treatment of pathological gambling: multicentre, double-blind, placebo-controlled study. Br J Psychiatry. 2010;197(4):330–1. doi: 10.1192/bjp.bp.110.078105. [DOI] [PubMed] [Google Scholar]
- 124.Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl) 2008;200(4):521–7. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, et al. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10(3):258–68. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- 126.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162(8):1423–31. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 127.Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, et al. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31(4):555–63. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 128.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]