Abstract
Graft-versus-host disease (GVHD) is a major complication associated with allogeneic bone marrow transplantation (BMT). Recent advances in the treatment of lymphoid malignancies with BMT include exploring mechanisms that can inhibit GVHD while maintaining graft-versus-leukemic (GVL) effects. In this issue of EJI, Yu et al have demonstrated efficient separation of GVHD and GVL by abrogating c-rel in T cells. Intrinsic c-Rel deficiency in T cells resulted in complete protection against GVHD in both major and minor histocompatibility mismatched murine models of BMT. Protection against GVHD was associated with decreased presence of Th1 and Th17 cells with a concomitant increase in Treg cells. Interestingly, intrinsic defect of c-Rel also resulted in decreased expression of the Th1-associated chemokine receptor cxcr3. Finally lack of c-Rel maintained GVL effects with significant tumor clearance in murine recipients. These data suggest that specific targeting of the T cell specific transcription factor c-Rel can inhibit GVHD while maintaining GVL effects.
Keywords: c-Rel, GVHD, GVL
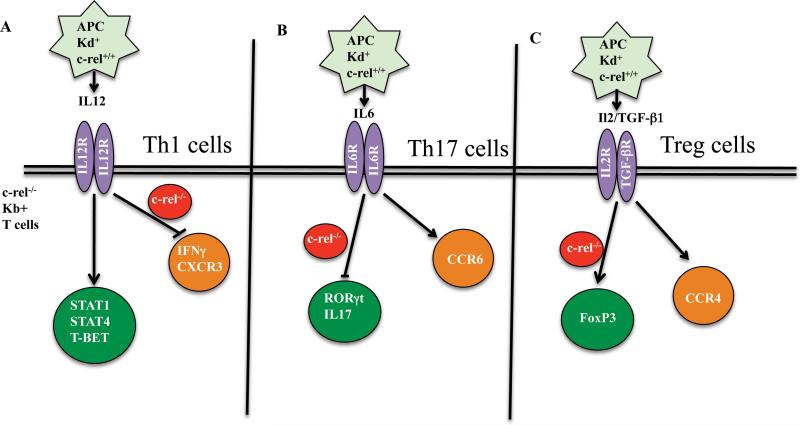
Members of the transcription factor family Rel/NF-κB are key regulators in the development of innate and adaptive immune responses[1]. c-Rel belongs to the family of NF-κB transcription factors, is selectively expressed by lymphoid and myeloid cells, and plays an important role in T cell mediated effector functions. Early studies indicate that lack of c-Rel results in dampened lymphocyte proliferation in response to polyclonal stimulation due to reduced autocrine IL-2 production[2]. Subsequent work on c-Rel further showed its importance in IL-12p40 production by macrophages [3]and IL-12p35 and p19 productions by dendritic cells[4]. Given its ubiquitous function in both innate and adaptive immunity, the role of c-Rel in infectious disease models has been widely studied[5, 6]. However, limited information exists regarding the potential importance of c-Rel in allogeneic BMT. In this issue of EJI, Yu et al[7] have shown for the first time the importance of c-Rel in the development of GVHD and its unique role in promoting GVL effects (Figure 1).
Figure. The potential role of c-Rel in T helper cell subset signaling in BMT.
Checkpoints at which c-Rel is involved in T cell differentiation is shown. (A) During alloreactivity, absence of c-Rel in donor T cells results in a significant decrease in IFNγ production and CXCR3 expression in Th1 cells, (B) c-Rel deficiency also inhibits Th17 transcription factor RORγt expression and IL17 cytokine production, (C) c-Rel deficiency enhances FoxP3 expression and iTreg cell number and prevalence.
GVHD is a major complication associated with allogeneic BMT and arises due to the presence of inflammatory host antigen presenting cells that stimulate the donor T cells in the graft. By using a murine model that sufficiently depicts the clinical setting, Yu et al have sequentially studied the role of c-Rel in both complete MHC mismatch and minor mismatch models of BMT. In the complete mismatch model, bone marrow and T cells from C57BL/6 mice were transplanted into lethally irradiated BALB/c mice. The donor T cells were obtained from c-rel−/− recipients whereas the host was sufficient in c-Rel expression. The authors found that lack of c-Rel in donor T cells resulted in the rescue of mice from GVHD as compared to control cohorts. Next, using a minor MHC mismatch BMT model, the authors transplanted lethally irradiated BALB.b mice with bone marrow and T cells from C57BL/6 mice. Donor T cells with sufficient c-Rel expression caused severe GVHD which was attenuated in cohorts receiving c-rel−/− donor T cells. These results indicate that c-Rel inhibition can be beneficial in abrogating GVHD post-BMT. Despite previous reports where the lack of T cell function in c-Rel deficiency was attributed to dampened priming of antigen-presenting-cells (APC)[8], Yu et al.'s report shows that intrinsic deficiency of c-Rel in T cells is the main cause of the reduced clinical symptoms in GVHD.
The authors subsequently explored the immunobiological phenotype associated with the lack of clinical symptoms of GVHD. Using in vitro and in vivo experiments, they show that c-Rel deficiency resulted in significantly reduced T cell proliferation with an increased sensitivity to apoptosis in the CD4+ T cell compartment. These data are in accordance with other reports where c-Rel was critical for T cell proliferation to infectious disease antigens (in particular, Th1-type T cell proliferation). While these observations are in line with previously published murine reports[8], the fundamental importance of these data lies in the myeloablative regimen that is used as conditioning in murine recipients prior to BMT. Following BMT, it is well known that an increase in cytokines such IL-7 and IL-15 occur in both murine and human recipients. It has been suggested that the increase in these cytokines results in an increase in donor alloreactive T cell proliferation and hence in the occurrence of GVHD. The data presented here suggests a new and important role for IL-2 and c-Rel transcription factor above and beyond the previously described roles of IL-7 and IL-15. For the first time, the data presented here suggest that IL-2 mediated survival and expansion of T cells post-BMT is as crucial as the other T cell supporting cytokines, including IL-7 and IL-15. These data collectively suggest that c-Rel may be crucial in inhibiting downstream signaling of these cytokines by inhibiting STAT5 signaling. Indeed, c-Rel has been shown to selectively bind to enhancer regions of STAT5 protein [9], thereby facilitating IL-2 mediated T cell proliferation.
In addition to decreased T cell proliferation, lack of c-Rel also impacts the differentiation of T cells into Th1 and Th17 cell lineage. With respect to Th1 cells, intrinsic c-Rel deficiency does not affect Tbet expression but does impact the production of IFN-γ [8]. Moreover, lack of c-Rel impacts production of IL-12 in macrophages, which further attenuates the differentiation of T cells into Th1 phenotype[5]. In line with these observations, Yu et al show that lack of c-Rel indeed inhibits Th1 cell development post-BMT. These observations are of crucial importance in the setting of BMT given the increasing realization that Th1 cells play a major role in both acute and chronic GVHD[10]. Characterization of patients with both acute and chronic forms of GVHD has shown increased numbers of IFN-γ producing T cells and is directly correlated to aggressive disease phenotype[11]. Taken together, while c-Rel does not inhibit Tbet, its relative importance in IFN-γ production may indicate its novel role in dampening GVHD responses.
Another interesting observation in the current report is the dampened expression of Th1 chemokine receptors on post-BMT lymphocytes. The authors have sequentially studied lymphocyte populations in the known GVHD target organs such as the liver and skin. Since c-Rel modulates Th1 signaling downstream of T-bet and given the importance of T-bet in regulating CXCR3[12], the down regulation of CXCR3 in c-rel−/− T cells suggest that c-Rel may play other inhibitory roles in Th1 differentiation (in addition to the previously reported mechanisms by which c-Rel regulates IFN-γ production).
The importance of Th17 cells in GVHD is still under investigation, although some studies indicate that the presence of Th17 cells is sufficient but not important for disease occurrence[13, 14]. For instance, murine recipients that received donor T cells lacking IL17 succumbed to GVHD in a similar fashion to WT cohorts. However, IL17 deficiency dampened production of inflammatory cytokines during the early stages of GVHD disease development. Hence, IL17 is critical in allowing for the development of robust Th1 responses and in GVHD development[14]. Moreover, an intrinsic defect in T cell STAT3 signaling (the critical signaling molecule involved in Th17 cells) completely abrogates GVHD[15]. These data suggest that inhibition of Th17 cells during GVHD would improve disease outcome. c-Rel is critical for the differentiation of T helper cells into Th17 cell lineage and c-Rel deficient mice are incapable of mounting specific Th17 cell responses[16, 17]. It has been suggested that c-Rel activates two distinct RORγ promoters, which in turn control RORγT and RORγ expression[17]. Given its importance in both Th1 and Th17 cell differentiation, Yu et al's data further confirms this prior observation because minimal Th17 cell polarization was noted in c-Rel deficient recipients. However, Yu et al observed a significant increase in Th17 cell associated chemokine receptor in the lungs of murine recipients but with minimal tissue damage. This lack of tissue damage could be attributed to the decreased IL-17 production in these murine recipients. Taken together, these data suggest that c-Rel may have an identical regulatory function with respect to cytokine production in both Th1 and Th17 cells. However, c-Rel regulation of chemokine receptors may be specific to Th1 cells with no effect on Th17 cell expression of CCR6. Indeed, further studies are needed in order to delineate this important role of c-Rel in chemokine expression in the various T helper subsets.
Next, the authors investigated the importance of c-Rel in Treg cell prevalence post-BMT. C-Rel has been implicated in being important for thymic derived Treg cell (nTregs) development[18] [19]while its role in peripheral induced Treg (iTreg) generation is still controversial. Some reports suggest that iTreg generation is unaltered in c-rel−/− T cells while others have noted severe attenuation of iTreg generation in c-rel−/− T cells. However, the deficiency in iTreg generation can be rescued by TGF-β[20]. In light of these reports, it appears that the generation of iTregs are predominantly dependent on TGF-β signaling which is not altered by c-Rel deficiency. Neverthless, the signaling networks between c-Rel and TGF-β in iTregs remains to be further investigated. With respect to thymic derived Tregs, multiple mechanisms by which c-Rel regulates nTregs have been shown where c-Rel directly interacts with the cis regulatory elements of the Foxp3 gene, which in turn results in the expression of FoxP3. The enhanced number of Treg cells post BMT observed by Yu et al hence suggests that these Tregs are of peripheral origin and induced from the adoptively transferred conventional CD4+ T cells. Also, given that the TGF-β1 signaling is not perturbed by c-Rel, blocking c-Rel in a clinical setting might result in an increase in induced Treg numbers which may allow for not only the prevention but also the treatment of GVHD[21] [22] [23]
Finally, Yu et al have addressed the most important problem associated with allogeneic BMT. The authors show that crel−/− recipients while protected from GVHD showed robust anti-tumor effect. The greatest challenge in BMT includes preserving GVL effects with minimal GVHD damage to the host. Yu et al have elegantly shown that it is indeed possible to delineate GVL and GVHD effects by targeting specific transcription factor expression in allogeneic T cells. There may be numerous mechanisms by which this could occur. First, decreased T cell proliferation in combination with decreased chemokine receptor expression may redirect effector T cells to the tumor microenvironment thereby protecting other GVHD target organs. Second, since c-Rel does not alter Th2 signaling unlike NF-κB; an intact Th2 differentiation may suffice for the induction of optimal GVT effects in the murine model. Indeed, a recent study has evaluated the importance of Th2 cells in BMT for patients with lymphomas[24]. Third, c-Rel blockade may employ a similar mechanism to other NF-κB inhibitors such as bortezomib, which is known to cause tumor regression through increased tumor apoptosis[25]. Although the data presented here can culminate in increased excitement in the field of BMT treatment, caution needs to be applied with respect to Treg generation in this model. Since tumors are notorious in escaping the immune system through inhibitory molecules such as PDL-1 and TGF-β1[26], one needs to be aware of the plasticity of effector T cells and their preference to up regulate FoxP3 in the presence of tolerogenic signals[27].
In summary, the report by Yu et al shows tor the first time the importance of c-Rel in GVHD and GVL immunopathology. Moreover, the authors have delineated the importance of intrinsic importance of c-Rel in T cells as compared to the host or donor derived APCs in BMT. These data give rise to several interesting immunological and clinical questions with respect to c-Rel's role during allo-inflammation. By efficiently blocking c-Rel in T cells, the authors show that a GVL effect can be maintained in the absence of robust Th1 and Th17 cell production. This may be the key missing link in GVL biology: curbing the T cell compartment may enhance other cell types such as NK cells to elicit anti-tumor responses. These data also suggest that the ability of c-Rel to inhibit Th1 chemokine factors may be the key function whereby Th1 cell trafficking to GVHD target organs is severely curbed. Moreover, since these data were generated from a clinically relevant murine model of disease, translating these findings into a clinical trial will be of potential interest. Known NF-κB inhibitors, such as PS1145 and bortezomib [28], can modulate immunity but have been associated with gut toxicity; it is certainly possible that T cell specific c-Rel inhibitors may provide an improved therapeutic window relative to these existing, less selective agents. In conclusion, continued understanding of the c-Rel pathway and its inhibitors in modulating GVHD and GVL will allow for enhanced treatment of human tumors post allogeneic bone marrow transplantation.
Acknowledgements
The author would like to thank Dr. William G. Telford and Dr. Daniel H. Fowler, Experimental Transplantation Immunology Branch, NCI, NIH, for critical reading of the manuscript. This work is supported by the Intramural Research of NCI, NIH.
Footnotes
Conflict of Interest
The author does not have any conflict of interest to declare.
Bibliography
- 1.Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 2.Liou HC, Jin Z, Tumang J, Andjelic S, Smith KA, Liou ML. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int Immunol. 1999;11:361–371. doi: 10.1093/intimm/11.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci U S A. 2000;97:12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grumont R, Hochrein H, O'Keeffe M, Gugasyan R, White C, Caminschi I, Cook W, Gerondakis S. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194:1021–1032. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason N, Aliberti J, Caamano JC, Liou HC, Hunter CA. Cutting edge: identification of c-Rel-dependent and -independent pathways of IL-12 production during infectious and inflammatory stimuli. J Immunol. 2002;168:2590–2594. doi: 10.4049/jimmunol.168.6.2590. [DOI] [PubMed] [Google Scholar]
- 6.Mason NJ, Liou HC, Hunter CA. T cell-intrinsic expression of c-Rel regulates Th1 cell responses essential for resistance to Toxoplasma gondii. J Immunol. 2004;172:3704–3711. doi: 10.4049/jimmunol.172.6.3704. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Wang D, Kaosaard K, Liu C, Fu J, Haarberg K, Anasetti C, Beg AA, Yu XZ. c-Rel is an essential transcription factor for the development of acute graft-versus-host disease in mice. Eur J Immunol. 43 doi: 10.1002/eji.201243282. DOI 10.1002/eji.201243282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun SC, Maggirwar SB, Harhaj EW, Uhlik M. Binding of c-Rel to STAT5 target sequences in HTLV-I-transformed T cells. Oncogene. 1999;18:1401–1409. doi: 10.1038/sj.onc.1202430. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu XZ. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORgammat in mice. Blood. 2011;118:5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korholz D, Kunst D, Hempel L, Sohngen D, Heyll A, Bonig H, Gobel U, Zintl F, Burdach S. Decreased interleukin 10 and increased interferon-gamma production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:691–695. doi: 10.1038/sj.bmt.1700718. [DOI] [PubMed] [Google Scholar]
- 12.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 13.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O'Shea JJ, Fowler DH. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S, Daley S, Shannon MF. The NF-kappaB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol. 2011;187:4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 17.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, Gerondakis S. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori S. c-Rel: a pioneer in directing regulatory T-cell lineage commitment? Eur J Immunol. 2010;40:664–667. doi: 10.1002/eji.201040372. [DOI] [PubMed] [Google Scholar]
- 21.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ, Ritz J. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Miehlke S, Bornhauser M, Schneider T, Zeitz M, Stein H, Thiel E, Duchmann R, Uharek L. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 23.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, Riley JL, Levine BL, June CH, Fong T, Warner NL, Fowler DH. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler DH, Mossoba ME, Steinberg SM, Halverson DC, Stroncek D, Khuu HM, Hakim FT, Castiello L, Sabatino M, Leitman SF, Mariotti J, Gea-Banacloche JC, Sportes C, Hardy NM, Hickstein DD, Pavletic SZ, Rowley S, Goy A, Donato M, Korngold R, Pecora A, Levine BL, June CH, Gress RE, Bishop MR. Phase 2 clinical trial of rapamycin-resistant donor CD4+ Th2/Th1 (T-Rapa) cells after low-intensity allogeneic hematopoietic cell transplantation. Blood. 2013;121:2864–2874. doi: 10.1182/blood-2012-08-446872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Welniak LA, Panoskaltsis-Mortari A, O'Shaughnessy MJ, Liu H, Barao I, Riordan W, Sitcheran R, Wysocki C, Serody JS, Blazar BR, Sayers TJ, Murphy WJ. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 27.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]