Abstract
The presence of elevated glucose concentrations in diabetes is a metabolic change that leads to an increase in the amount of non-enzymatic glycation that occurs for serum proteins. One protein that is affected by this process is the main serum protein, human serum albumin (HSA), which is also an important carrier agent for many drugs and fatty acids in the circulatory system. Sulfonylureas drugs, used to treat type 2 diabetes, are known to have significant binding to HSA. This study employed ultrafiltration and high-performance affinity chromatography to examine the effects of HSA glycation on the interactions of several sulfonylurea drugs (i.e., acetohexamide, tolbutamide and gliclazide) with fatty acids, whose concentrations in serum are also affected by diabetes. Similar overall changes in binding were noted for these drugs with normal HSA or glycated HSA and in the presence of the fatty acids. For most of the tested drugs, the addition of physiological levels of the fatty acids to normal HSA and glycated HSA produced weaker binding. At low fatty acid concentrations, many of these systems followed a direct competition model while others involved a mixed-mode interaction. In some cases, there was a change in the interaction mechanism between normal HSA and glycated HSA, as seen with linoleic acid. Systems with only direct competition also gave notable changes in the affinities of fatty acids at their sites of drug competition when comparing normal HSA and glycated HSA. This research demonstrated the importance of considering how changes in the concentrations and types of metabolites (e.g., in this case, glucose and fatty acids) can alter the function of a protein such as HSA and its ability to interact with drugs or other agents.
Keywords: Diabetes, drug-protein binding, fatty acids, glycation, high-performance affinity chromatography, human serum albumin, sulfonylurea drugs, ultrafiltration
INTRODUCTION
Diabetes has been estimated to affect 285 million people worldwide and 24 million in the U.S. [1,2]. The high blood glucose concentrations that are characteristic of this metabolic disease are caused by a deficiency in insulin production or an impaired efficiency in insulin action [1–3]. These elevated concentrations of glucose are often combined with other factors, such as high blood pressure and altered lipid levels, which can lead to cardiovascular disease, kidney or liver damage, and blindness [1–4]. Many of these complications are associated with the non-enzymatic glycation of proteins [5].
Glycation is a process that is enhanced during diabetes. This process begins with the reaction of a free amine group on a protein with an aldehyde group on a reducing sugar, such as glucose, to form a reversible Schiff base. A slow rearrangement of this Schiff base can produce a more stable fructosamine residue, or Amadori product [6–8]. Numerous studies have indicated that glycation can produce structural and functional modifications in proteins with relatively long half-lives, such as collagen, lens crystalline, and hemoglobin [4,5,8–10]. Glycation can also occur with human serum albumin (HSA), the main protein in serum [6,7,9–14]. For modern affinity-based detection methods, the reported reference range for glycated HSA in normal individuals is typically 0.6–3%, with this amount increasing by two- to five-fold during type 2 diabetes [14,15].
The glycation of HSA has been of recent interest due to the importance of this protein as a carrier agent for many drugs and endogenous compounds in the circulatory system [10–14]. Sulfonylurea drugs are a set of pharmaceutical agents that are known to have significant binding to HSA [11,14,16–25]. These drugs are used to treat type 2 diabetes by stimulating the secretion of insulin [16]. Some common sulfonylurea drugs are acetohexamide, tolbutamide and gliclazide (see structures in Figure 1). These drugs are all highly bound in blood to HSA and have been shown to have significant interactions at a set of high affinity sites that include both of the major drug interaction sites of this protein: Sudlow sites I and II, as shown in Figure 2 [16–23]. In addition, recent studies have found that glycation can alter the binding of sulfonylurea drugs with HSA [20–25]. The association equilibrium constants for the high affinity interactions of these drugs with normal HSA, and with HSA that has glycation levels similar to those seen in diabetes, have been reported to be in the range of 1.3–4.2 × 105 M−1 for acetohexamide, 0.87–1.2 × 105 M−1 for tolbutamide, and 0.7–1.0 × 105 M−1 for gliclazide at pH 7.4 and 37 °C [19,21–23].
Figure 1.
Structures of the sulfonylurea drugs and fatty acids that were examined in this study.
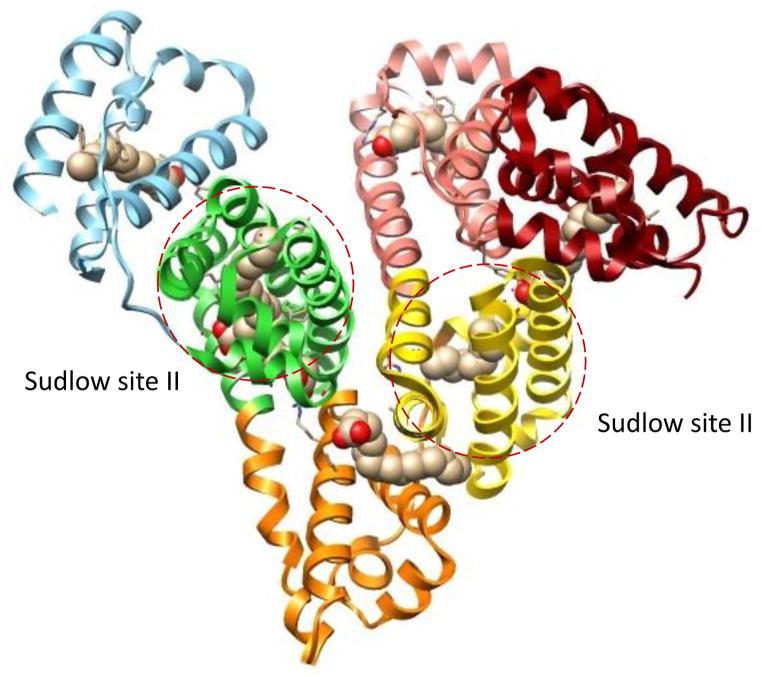
Figure 2.
Structure of HSA, including the regions that bind palmitic acid and the major drug binding sites of this protein (i.e., Sudlow sites I and II). Each subdomain of HSA is shown in a different color and the atoms in the palmitic acid are shown with the carbon in tan and the oxygen in red. This structure was generated using Protein Data Bank (PDB) file ID: 1E7H [33].
Another metabolic change that occurs during diabetes is the presence of elevated concentrations of free fatty acids in serum [26–28]. Several studies have noted that increased levels of free fatty acids can contribute to the pathogenesis of hypertension, cardiovascular risk factors, and conditions such as atherosclerosis [29]. In addition, high glucose levels and elevated fatty acid concentrations in serum have been correlated with impaired sensitivity, increased inflammation, and oxidative stress, among other metabolic complications [30,31]. HSA binds to many fatty acids, some of which are shown in Figure 1 [32]. In addition, it is known that some fatty acids can have direct competition with drugs or lead to allosteric effects during the binding of drugs and other solutes with HSA [14,33–35]. There are a number of binding regions for fatty acids on HSA, as illustrated in Figure 2 [33,35–39], with the strongest of these interactions having association equilibrium constants in the range of 105 to 108 M−1 [33,39].
A recent report used a chromatographic high-throughput screening method to compare the overall effects of glycation and the presence of fatty acids on the binding of several sulfonylurea drugs with HSA [20]. It was found that there was a significant increase in retention (i.e., stronger binding) for drugs such as acetohexamide, tolbutamide and gliclazide in going from normal HSA to glycated HSA. It was also noted that the addition of low levels of fatty acids led to lower binding for these drugs with both normal HSA and glycated HSA. This current study examined these effects further by using both ultrafiltration and high-performance affinity chromatography (HPAC) [14,40,41]. Ultrafiltration was utilized to determine the overall effects of various fatty acids at physiological concentrations on the binding of acetohexamide, tolbutamide and gliclazide with normal HSA and glycated HSA. Experiments based on HPAC and competition experiments were used to examine these interactions in more detail, particularly in the presence of low concentrations of fatty acids. The results of this study were then utilized to provide further clues as to how the interactions of sulfonylurea drugs and fatty acids with HSA may change during metabolic diseases such as diabetes.
MATERIALS AND METHODS
Reagents
The HSA (essentially fatty acid free) and glycated HSA used in the chromatographic studies (3.6 mol hexose/mol HSA, essentially fatty acid free) [20] were from Sigma-Aldrich (St. Louis, MO, USA). The sulfonylurea drugs and fatty acids that were examined in this study (see summary in Figure 1) were also obtained from Sigma-Aldrich. The glycated HSA used in the ultrafiltration studies was prepared from normal HSA according to a previous method [20,21] and contained 3.4 mol hexose/mol HSA, as determined by using a fructosamine assay from Diazyme Laboratories (Poway, CA, USA). The modification levels that were present in the glycated HSA samples were representative of what might be present during uncontrolled or advanced diabetes [20–23]. Sterilized culture tubes (17 × 100 mm) and siliconized polypropylene microcentrifuge tubes (13 × 40 mm) were obtained from Fisher Scientific (Pittsburgh, PA, USA). Slide-A-Lyzer dialysis cassettes (7 kDa MW cutoff; 12–30 mL sample volume) were purchased from Thermo Scientific (Rockford, IL, USA). Econo-Pac 10 DG disposable desalting columns were acquired from Bio-Rad Laboratories (Hercules, CA, USA). Nucleosil Si-300 silica (7 μm particle diameter, 300 Å pore size) was obtained from Macherey-Nagel (Düren, Germany). Other chemicals were reagent grade or better. All buffers and aqueous solutions were prepared using water from a Nanopure water system (Barnstead, Dubuque, IA) and were filtered using 0.2 μm GNWP nylon filters from Millipore (Billerica, MA, USA).
Apparatus
Ultrafiltration was performed using Centrifree Micropartition devices (30 kDa MW cutoff, 0.15–1.0 mL sample capacity) from Amicon (Danvers, MA, USA) and a 5702RH temperature-controlled centrifuge from Eppendorf (New York, NY, USA) equipped with a fixed-angle centrifuge rotor from VWR (West Chester, PA, USA). Flow-injection analysis of the drug content in each filtrate was carried out by using a Jasco 2000 HPLC system (Easton, MD, USA) that contained a DG-2080-53 degasser, a PU-2080 pump, an AS-2057 autosampler equipped with a 100 μL sample loop (operated in the partial loop injection mode), and a UV-2075 absorbance detector. These system components were controlled by a Jasco LC-Net II/ADC system and using EZ Chrom SI software (Scientific Software, Pleasanton, CA, USA). The peaks detected in the injected samples were integrated using PeakFit 4.12 (SeaSolve Software, San Jose, CA, USA). Non-linear regression of the data was carried out by utilizing Data Fit 8.1.69 (Oakdale, PA, USA).
The system employed in the chromatographic measurements consisted of a Hitachi L-6000 pump (Pleasanton, CA, USA), a ThermoSeparations SpectraSystem AS3000 autosampler (Waltham, MA, USA) with a 10 μL sample loop, and a Waters 481 UV detector (Milford, MA, USA). Detection was performed at 248 nm for acetohexamide and at 226 nm for gliclazide and tolbutamide. The columns were kept at 37 (± 0.1) °C by using a water jacket from Fisher Scientific and a 2067 CH/P recirculating water bath from Forma Scientific (Marietta, OH, USA). Data were acquired using LabView 8.0 (National Instruments, Austin, TX, USA) and processed using PeakFit 4.12.
Ultrafiltration studies
All solutions of drugs, fatty acids and proteins used in the ultrafiltration studies were prepared in pH 7.4, 0.067 M potassium phosphate buffer. To study the effect of fatty acids on the binding of sulfonylurea drugs to HSA and glycated HSA, fatty acid solutions were prepared over the following ranges of concentrations, which included the typical physiological levels of these solutes and concentrations that can be found in serum during diabetes [42–44]: lauric acid (0–16 μM), linoleic acid (0–300 μM), myristic acid (0–220 μM), oleic acid (0–600 μM), palmitic acid (0–400 μM), and stearic acid (0–80 μM). The samples for these fatty acid studies were prepared by mixing 0.25 mL of a fatty acid solution with 0.5 mL of an HSA or glycated HSA solution, giving a final protein concentration of 260–290 μM. The fatty acid/protein mixture was incubated at 37°C in a water bath for 24 h, followed by the addition of 0.5 mL of a given drug at a final fixed concentration of 200 μM (Note: this concentration represents a common therapeutic level for acetohexamide and tolbutamide [45] and allowed for easy detection of the free drug fractions). Although sulfonylureas are weak acids with pKa values in the range of 5.2 to 6.2, all of the tested drug solutions had a pH that differed by less than 0.05 units from pH 7.4; these pH values were found to be quite stable and to change by less than 0.05 units over the entire course of the binding studies [21–23]. The drug/fatty acid/protein mixture was incubated at 37°C for 45 min in a water bath; the use of longer incubation times up to 90 min did not produce any significant change in the final results.
Prior to ultrafiltration, the membrane in each ultrafiltration device was rinsed three times with deionized water and pH 7.4, 0.067 M potassium phosphate buffer to reduce interferences by any contaminants or preservatives that may have been present. The ultrafiltration device was spun for at least 15 min at 1,500 × g after rinsing to avoid dilution errors due to the presence of any remaining rinse solution in the device; the volume of rinse solution that remained after this step was less than ~10 μL.
The ultrafiltration experiments were carried out by placing a 1 mL aliquot of each test mixture into a Centrifree micropartition device and spinning this mixture at 1,500 × g for 25 min at 37 °C. The filtrate was collected and analyzed for its drug content by injecting 5 μL aliquots in triplicate onto a flow-injection analysis system with no column present. These injections were made at 0.5 mL/min and in the presence of pH 7.4, 0.067 M potassium phosphate buffer. The eluting drug fractions were monitored at 257 nm for acetohexamide and at 227 nm for tolbutamide or gliclazide. A background correction for any contaminants or preservatives remaining in the filtrate from the ultrafiltration device was made by carrying out similar measurements on filtrates for 0.5 mL samples that contained only HSA or glycated HSA in pH 7.4, 0.067 M potassium phosphate buffer.
Chromatographic studies
The normal HSA and glycated HSA columns were prepared by converting Nucleosil Si-300 silica into a diol-bonded form and then using the Schiff base coupling method, as described previously [19–21]. As noted in previous work, the immobilization of HSA by this approach mainly occurs through the N-terminus or lysines that are not located at Sudlow sites I and II [43] and has been shown to provide columns containing immobilized normal HSA or glycated HSA that are good models for the soluble forms of these proteins [19–24]. These supports were found by a bicinchoninic acid (BCA) protein assay to contain 25 (± 2) mg HSA or 15.4 (± 0.7) mg glycated HSA per g silica, as determined in triplicate by using HSA as the standard and diol-bonded silica as the blank. The supports were packed into separate 2.0 cm × 2.1 mm I.D. columns at 3500 psi (24.1 MPa). A pH 7.4, 0.067 M potassium phosphate buffer was used as the packing solution, and the columns were stored in this buffer at 4°C when not in use. Control columns were prepared in the same manner but with no protein being added during the immobilization step. All columns were used over the course of a few months and were found to provide stable retention factors under these operating conditions [19–24].
The mobile phase used in the chromatographic-based competition studies contained pH 7.4, 0.067 M phosphate buffer or the same buffer plus a known concentration of the fatty acid to be tested. The fatty acid concentrations that were used were generally 100-fold lower than physiological levels or those seen in diabetes, which made it possible to monitor changes in the high affinity binding of the fatty acids during the chromatographic studies [20,42–44]. The samples contained 5 μM of the desired drug in the mobile phase, which was used as a tracer to probe any changes in retention for the drug at its binding sites on HSA [19,22–24]. Under the conditions used in these studies, approximately 82–99% of the observed drug retention in the absence of any fatty acids should have been due to interactions at Sudlow sites I and II [25]. These drug samples were injected in triplicate at 37°C and 0.5 mL/min. No significant changes in retention occurred when using slightly higher sample concentrations of the drugs, indicating that linear elution conditions were present, or when using slower flow rates for the chromatographic studies. Injections of 5 μM sodium nitrate (i.e., a non-retained solute for HSA columns [19–24], which was detected at 205 nm) were made to measure the void time of each column. Studies with the control column indicated that the tested drugs had no significant non-specific retention to the support (i.e., less than 3% of the total retention noted on the normal HSA or glycated HSA columns).
RESULTS AND DISCUSSION
Effects of fatty acids on the overall binding of sulfonylurea drugs to normal or glycated HSA
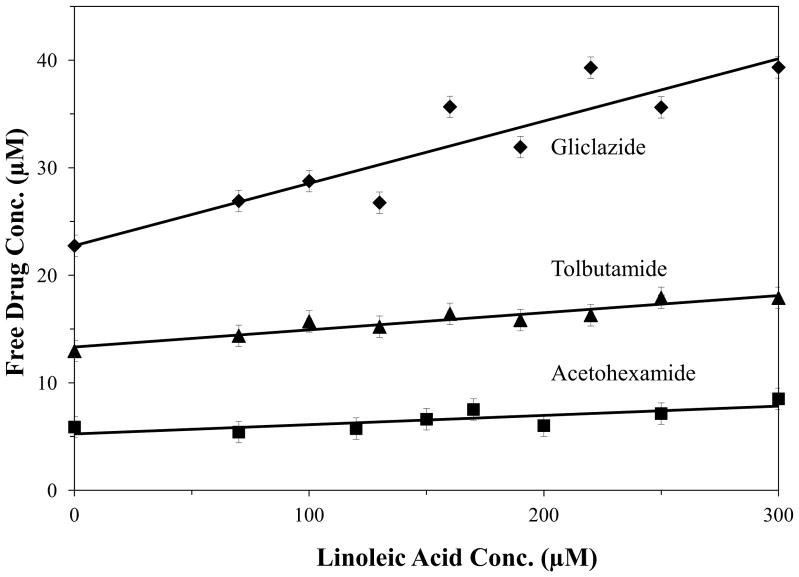
Ultrafiltration studies were used to first examine the overall effects of glycation versus fatty acids on the binding of sulfonylureas to HSA. This method was utilized to compare the changes in the free, or non-protein bound, fraction of each drug, which was used as an index of the total binding of these drugs with normal HSA or glycated HSA. Some typical experimental results are provided in Figure 3, which show how the measured free drug fractions changed for normal HSA as increasing concentrations of linoleic acid were added. Tables 1 and 2 summarize the changes in the free drug concentrations that were obtained in these studies with normal HSA or glycated HSA and in the absence or presence of various fatty acids that are commonly found in blood. In each case, both normal concentrations (Table 1) and elevated concentrations (Table 2) of these fatty acids, as found during diabetes, were tested.
Figure 3.
Effect of increasing the concentration of linoleic acid on the free drug concentration of acetohexamide (■), gliclazide (◆) and tolbutamide (▲) in the presence of fixed total concentrations of each drug and normal HSA. Each point represents the average of three measurements and the error bars represent a range of ± 1 S.D.
Table 1.
Effects of normal concentrations of fatty acids on the binding of sulfonylurea drugs to HSA and glycated HSA
| Fatty Acid | Change in Free Drug Concentrationa | ||
|---|---|---|---|
| Acetohexamide | Gliclazide | Tolbutamide | |
| Lauric acid (0.1–6 μM) | |||
| Normal HSA | 0.2–10% | 0.03–2.1%b | 0.04–2.7% |
| Glycated HSA | 0.04–2.6%b,c | 0.1–7% | 0.02–1.0%b,c |
| Linoleic acid (20–93 μM) | |||
| Normal HSA | 3.2–15% | 5–24% | 2.4–11% |
| Glycated HSA | 6–29%c | 5–23%c | 8–38% |
| Myristic acid (3–24 μM) | |||
| Normal HSA | 2.6–20% | 0.6–4.6%b | 0.04–0.3%b |
| Glycated HSA | 1.0–8%c | 0.3–2.5%c | 0.5–4.2%c |
| Oleic acid (33–182 μM) | |||
| Normal HSA | 17–93% | −1.5–8%a | 9–35%d |
| Glycated HSA | 0.4–2.1% | 9–21%b | 1.5–8% |
| Palmitic acid (25–171 μM) | |||
| Normal HSA | 4.0–27%d | 4.3–29% | 4.3–29% |
| Glycated HSA | 1.5–10%c | 1.3–9% | 5–36%c |
| Stearic acid (6–57 μM) | |||
| Normal HSA | 0.6–5% | 4.4–41% | 19–31%d |
| Glycated HSA | 0.6–5%c | 1.5–14% | 2.8–25%c |
All of the indicated changes are relative to the free concentration measured for a drug in the absence of any fatty acid at pH 7.4 and 37°C; the given values represent an increase in the free drug concentration, except for the case of gliclazide in the presence of oleic acid and normal HSA.
All changes in the free drug concentration, except those indicated by this footnote, were significantly different at the 95% confidence level when comparing the results measured at the upper end of the normal fatty acid concentration range versus the results obtained when no fatty acid was present.
All changes in the free drug concentration, except those indicated by this footnote, were significantly different at the 95% confidence level when comparing the results measured at the upper end of the normal fatty acid concentration range for normal HSA versus glycated HSA.
Non-linear regression or a combination of linear regions were used in this case because some curvature was noted in the corresponding plot of free drug concentration versus fatty acid concentration.
Table 2.
Effects of elevated concentrations of fatty acids on the binding of sulfonylurea drugs to HSA and glycated HSA
| Fatty Acid | Change in Free Drug Concentrationa | ||
|---|---|---|---|
| Acetohexamide | Gliclazide | Tolbutamide | |
| Lauric acid (up to 9 μM) | |||
| Normal HSA | 13% | 3.1% | 3.9% |
| Glycated HSA | 3.8%b | 10% | 1.5%b,c |
| Linoleic acid (up to 300 μM) | |||
| Normal HSA | 49% | 76% | 36% |
| Glycated HSA | 94% | 76%c | 120% |
| Myristic acid (up to 43 μM) | |||
| Normal HSA | 37% | 8% | 0.6%b |
| Glycated HSA | 15% | 4.5%c | 8%c |
| Oleic acid (up to 600 μM) | |||
| Normal HSA | 310% | −27%a | 53% |
| Glycated HSA | 7% | 36% | 27% |
| Palmitic acid (up to 400 μM) | |||
| Normal HSA | 64% | 68% | 69% |
| Glycated HSA | 23% | 21% | 85% |
| Stearic acid (up to 80 μM) | |||
| Normal HSA | 8% | 57% | 37% |
| Glycated HSA | 7%c | 20% | 36%c |
All of the indicated changes are relative to the free concentration measured for a drug in the absence of any fatty acid at pH 7.4 and 37°C. Each of the given values represents an increase in the free drug concentration, except for the case of gliclazide in the presence of oleic acid and normal HSA.
All changes in the free drug concentration, except those indicated by this footnote, were significantly different at the 95% confidence level when comparing the results measured at the upper end of the elevated fatty acid concentration range versus the results obtained when no fatty acid was present.
All changes in the free drug concentration, except those indicated by this footnote, were significantly different at the 95% confidence level when comparing the results measured at the upper end of the normal fatty acid concentration range for normal HSA versus glycated HSA.
For most of the combinations of fatty acids, drugs and types of HSA that were examined, the change in free drug concentration that was measured as the fatty acid concentration was increased gave a reasonably good fit to a linear model. Some examples of such fits are shown in Figure 3 for normal HSA and linoleic acid. In this figure, the correlation coefficients of the best-fit responses for acetohexamide, gliclazide and tolbutamide were 0.784 (n = 8), 0.918 (n = 9) and 0.931 (n = 9). Similar results were seen for most of the other combinations of fatty acids, drugs and types of HSA that were examined. The corresponding best-fit lines typically had a positive slope, with the only exception occurring for gliclazide in the presence of oleic acid and normal HSA, as discussed in the next paragraph. There were also a few situations in which a large initial change in free drug concentration at low fatty acid conditions required the use of a nonlinear fit or a multi-linear fit to describe the data. This type of behavior was observed when using normal HSA and tolbutamide in the presence of oleic acid or stearic acid and acetohexamide in the presence of palmitic acid, as well as for gliclazide and glycated HSA in the presence of oleic acid. However, in each of these cases a net positive slope was still obtained.
The results in Table 1 and 2 show that almost all of the tested combinations of drugs and fatty acids resulted in an increase in free drug concentration, or weaker drug binding, as the concentration of fatty acids was increased. This general observation agrees with the previous screening studies that used trace amounts of the same drugs along with immobilized preparations of normal HSA or glycated HSA and nanomolar concentrations of the given fatty acids [20]. This decrease in drug binding could be produced by direct competition of a fatty acid with a drug for binding sites or through negative allosteric interactions [34,35]. The presence for either type of interaction fits with the fact that some fatty acids may bind either at or near Sudlow sites I and II, as illustrated for palmitic acid in Figure 2. The only case in which different behavior was noted occurred for gliclazide in the presence of oleic acid and normal HSA, for which a decrease in free drug concentration and stronger drug binding was seen as the amount of added fatty acid was increased. This behavior can occur if a positive allosteric effect is present during the binding of a fatty acid and a drug with HSA [35].
It was noted that the same general changes in free drug concentrations in the presence of fatty acids tended to occur for each drug with normal HSA or glycated HSA. This observation was again in agreement with the previous screening studies using immobilized forms of these proteins [20]. Some differences did occur in the relative size of the effects for normal versus glycated HSA (e.g., as seen in some of the results for linoleic acid and palmitic acid); however, the most significant difference occurred in the results for gliclazide and acetohexamide in the presence of oleic acid. For gliclazide that was combined with normal HSA, a decrease in free drug concentration was seen as the amount of oleic acid was increased, but for the glycated HSA an increase in free drug concentration was noted under otherwise identical conditions. For acetohexamide, a large increase in free drug concentration was measured in the presence of oleic acid and normal HSA, while only a small increase was seen for the same drug in the presence of oleic acid and glycated HSA.
Myristic acid (i.e., a medium length saturated fatty acid) gave only a small or moderate change in binding for each of the tested drugs even at elevated fatty acid levels (i.e., less than 8% change for gliclazide and tolbutamide at 43 μM myristic acid, and a change of 37% or less for acetohexamide). Small or moderate changes in binding were also noted for each drug in the presence of medium-to-long chain saturated fatty acids such as lauric acid (<13% change) and stearic acid (<57% change). Larger changes in binding were observed for these drugs in the presence of linoleic acid (i.e., a long chain unsaturated fatty acid) and palmitic acid (a long chain saturated fatty acid). Both of these fatty acids have relatively high concentrations in serum and gave moderate-to-large changes in binding for the sulfonylurea drugs (i.e., a 36–120% change and a 21–85% change, respectively, at elevated fatty acid levels). All of these general trends agree with those noted for immobilized normal HSA and glycated HSA at lower fatty acid concentrations [20]. Oleic acid is an unsaturated long chain fatty acid that also occurs at a relatively high concentration in serum. The small-to-moderate changes in binding seen in the presence of oleic acid for tolbutamide with normal HSA and for all of the drugs in the presence of this fatty acid and glycated HSA further agreed with the results in Ref. [20].
Effects of low levels of fatty acids on the binding of sulfonylurea drugs with HSA
The next stage of this study used competition studies and HPAC to more closely examine the changes in binding that occurred for sulfonylurea drugs in the presence of fatty acids and normal HSA or glycated HSA. The analysis of these systems was simplified by using fatty acid concentrations that were up to 100-fold below the levels found in serum, as employed previously in a screening study to emphasize the initial, high affinity interactions for these fatty acids with HSA [20]. In this current report these conditions were used to examine the competition of these fatty acids with sulfonylurea drugs in a quantitative fashion and to compare the types of interactions that were occurring on normal HSA and glycated HSA between these fatty acids and drugs. During these experiments, the retention time (tR) for a small amount of an injected drug was measured and used to calculate the retention factor for the same drug (k) by using the relationship k = (tR − tM)/tM, where tM is the elution time for a non-retained solution (e.g., sodium nitrate for the columns used in this study). Examples of some typical chromatograms that are obtained through this approach for both normal HSA and glycated HSA columns can be found in Ref. [20].
In this study, the change in the retention factor for a drug that was injected onto a normal HSA column or glycated HSA column was examined as various concentrations of fatty acids were added to the mobile phase. As described previously for other systems, the presence of no significant changes in the value of k for a drug as the fatty acid concentration is increased would indicate that no competition or allosteric effects are occurring between the drug and fatty acid on the column [40]. A decrease in k with an increase in the concentration of a fatty acid may be caused by direct competition of these agents or through negative allosteric effects, while an increase in k could be produced through positive allosteric effects. A review of the equations and models that can be used to differentiate between these possible effects can be found in Refs. [40,41].
Tables 3–5 summarize the results for competition studies in which small amounts of acetohexamide, tolbutamide or gliclazide were injected onto columns that contained normal HSA or glycated HSA and various fatty acids in the mobile phase. The overall trends noted in this study were in agreement with Ref. [20] and the results in the previous section, in which most of the combinations of fatty acids and sulfonylurea drugs gave a decrease in binding for both normal HSA and glycated HSA as the concentration of fatty acids in the mobile phase was increased. This decrease in binding was indicated in the chromatographic studies by a decrease in the retention time of the injected drug and a decrease in its retention factor (k), or an increase in the value of 1/k, as the fatty acid concentration was raised.
Table 3.
Change in the reciprocal of the retention factor (1/k) for acetohexamide in the presence of increasing concentrations of fatty acids applied to normal HSA and glycated HSA columns
| Fatty Acid | Correlation Coefficienta | Type of Competitionb |
|---|---|---|
| Lauric acid (1.5–6 nM) | ||
| Normal HSA | r = 0.896 (n = 6) | Mixed mode |
| Glycated HSA | r = 0.390 (n = 6)c | Direct(?)c, KaI = 9.6 (± 11.4) × 106 M−1 |
| Linoleic acid (100–250 nM) | ||
| Normal HSA | r = 0.941 (n = 6) | Mixed mode |
| Glycated HSA | r = 0.977 (n = 7) | Direct, KaI = 1.2 (± 0.1) × 106 M−1 |
| Myristic acid (4–24 nM) | ||
| Normal HSA | r = 0.887 (n = 6) | Direct, KaI = 5.2 (± 1.4) × 106 M−1 |
| Glycated HSA | r = 0.987 (n = 6) | Direct, KaI = 1.6 (± 0.1) × 107 M−1 |
| Oleic acid (125–546 NM) | ||
| Normal HSA | r = 0.638 (n = 6)c | Direct(?)c, KaI = 2.6 (± 1.6) × 105 M−1 |
| Glycated HSA | r = 0.500 (n = 7)c | Direct(?)c, KaI = 3.2 (± 2.4) × 104 M−1 |
| Palmitic acid (100–400 nM) | ||
| Normal HSA | r = 0.928 (n = 6) | Direct, KaI = 3.8 (± 0.8) × 105 M−1 |
| Glycated HSA | r = 0.966 (n = 7) | Direct(?)c, KaI = 2.0 (± 11.5) × 108 M−1 |
| Stearic acid (10–80 nM) | ||
| Normal HSA | r = 0.854 (n = 7) | Direct, KaI = 1.0 (± 0.3) × 106 M−1 |
| Glycated HSA | r = 0.946 (n = 8) | Direct, KaI = 3.3 (± 0.5) × 106 M−1 |
The correlation coefficients are those obtained for a plot made according to Eqn. (1).
The values in parentheses represent a range of ± 1 S.D. A mixed mode interaction (e.g., direct competition plus a positive allosteric effect) was indicated in plots made according to Eqn. (1) by an initial decrease and then gradual increase in the retention factor for the injected drug as the concentration of fatty acids in the mobile phase was increased. A decrease in the retention factor for the drug when there was an increase in the concentration of the fatty acids could be produced by either a net negative allosteric effect or direct competition; in this study, the overall fit of the results to Eqn. (1) generally suggested that direct competition was present in most of these cases. In these latter cases, the apparent value of KaI for the fatty acid, as obtained by using Eqn. (1), is provided.
The correlation coefficient obtained in this case was not significant at the 95% confidence level or, in the case of palmitic acid and glycated HSA, the resulting value of KaI had a level of uncertainty that made it difficult to determine if direct competition was present in the given system. The symbol “(?)” indicates that the variation in the data was too large or the change in the retention factor was too small to obtain a reliable determination of exact type of interaction that was present or of the corresponding value of KaI in the case of direct competition.
Table 5.
Change in the reciprocal of the retention factor (1/k) for tolbutamide in the presence of increasing concentrations of fatty acids applied to normal HSA and glycated HSA columns
| Fatty Acid | Correlation Coefficienta | Type of Competitionb |
|---|---|---|
| Lauric acid (1.5–6 nM) | ||
| Normal HSA | r = 0.873 (n = 6) | Mixed mode |
| Glycated HSA | r = 0.448 (n = 6)c | Direct(?)c, KaI = 1.0 (± 1.0) × 107 M−1 |
| Linoleic acid (100–250 nM) | ||
| Normal HSA | r = 0.954 (n = 7) | Mixed mode |
| Glycated HSA | r = 0.979 (n = 7) | Direct, KaI = 1.1 (± 0.1) × 106 M−1 |
| Myristic acid (4–24 nM) | ||
| Normal HSA | r = 0.909 (n = 6) | Direct, KaI = 4.1 (± 0.9) × 106 M−1 |
| Glycated HSA | r = 0.982 (n = 6) | Direct, KaI = 1.4 (± 0.1) × 107 M−1 |
| Oleic acid (125–546 nM) | ||
| Normal HSA | r = 0.688 (n = 6)c | Direct(?)c, KaI = 3.8 (± 2.1) × 105 M−1 |
| Glycated HSA | r = 0.901 (n = 7) | Direct, KaI = 7.1 (± 1.5) × 104 M−1 |
| Palmitic acid (100–400 nM) | ||
| Normal HSA | r = 0.926 (n = 6) | Direct, KaI = 3.1 (± 0.6) × 105 M−1 |
| Glycated HSA | r = 0.936 (n = 6) | Direct(?)c, KaI = 2.6 (± 2.6) × 107 M−1 |
| Stearic acid (10–80 nM) | ||
| Normal HSA | r = 0.890 (n = 7) | Direct, KaI = 6.5 (± 1.5) × 105 M−1 |
| Glycated HSA | r = 0.973 (n = 8) | Direct, KaI = 3.0 (± 0.3) × 106 M−1 |
The correlation coefficients are those obtained for a plot made according to Eqn. (1).
The values in parentheses represent a range of ± 1 S.D. A mixed mode interaction (e.g., direct competition plus a positive allosteric effect) was indicated in plots made according to Eqn. (1) by an initial decrease and then gradual increase in the retention factor for the injected drug as the concentration of fatty acids in the mobile phase was increased. A decrease in the retention factor for the drug when there was an increase in the concentration of the fatty acids could be produced by either a net negative allosteric effect or direct competition; in this study, the overall fit of the results to Eqn. (1) generally suggested that direct competition was present in most of these cases. In these latter cases, the apparent value of KaI for the fatty acid, as obtained by using Eqn. (1), is provided.
The correlation coefficient obtained in this case was not significant at the 95% confidence level or, in the case of palmitic acid and glycated HSA, the resulting value of KaI had a level of uncertainty that made it difficult to determine if direct competition was present in the given system. The symbol “(?)” indicates that the variation in the data was too large or the change in the retention factor was too small to obtain a reliable determination of exact type of interaction that was present or of the corresponding value of KaI in the case of direct competition.
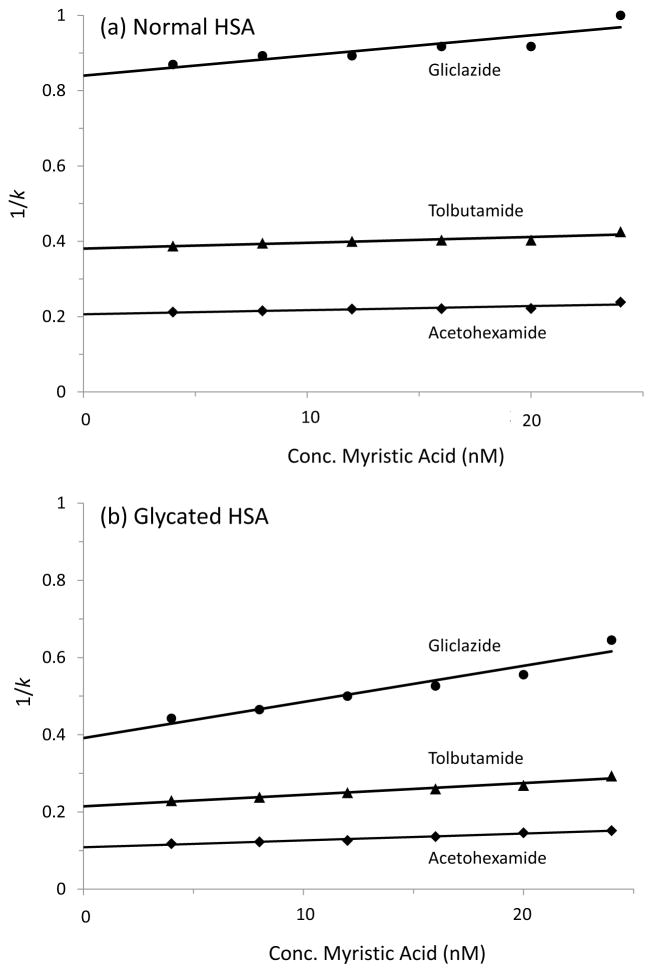
When plots were prepared of 1/k versus fatty acid concentration, many of these graphs gave a linear increase in 1/k under the conditions used in this study, as shown in Figure 4. As illustrated in this figure, and by the best-fit parameters provided in the Supportive/Supplementary Material, many of the combinations of drugs and fatty acids gave similar shifts in retention for normal HSA versus glycated HSA. This similarity in trends suggested that the same general regions and types of interactions between the drugs and fatty acids were present in both the normal HSA and glycated HSA. However, the glycated HSA also tended to have a larger change in binding, which confirmed that glycation could alter these interactions [14,20–25].
Figure 4.
Observed change in the retention factor (k) for gliclazide (●), tolbutamide (▲) and acetohexamide (◆) on HPLC columns containing immobilized (a) normal HSA or (b) glycated HSA and in the presence of mobile phases containing various concentrations of myristic acid. All values shown in this figure are the averages of three replicates and had relative standard deviations in the range of ±0.1–1.6%.
The linear plots of 1/k versus fatty acid concentration that were seen for many of the sulfonylurea drugs and fatty acids suggested that some type of direct competition was present between these solutes under these conditions, as predicted by Eqn. (1) [40,41].
| (1) |
According to Eqn. (1), a linear relationship would be expected between 1/k for an injected drug (A) and the concentration of a fatty acid (I) in the mobile phase for a system that has direct competition at a single site or a cluster of sites with similar binding affinities for A and I [40,46,47], as occurs for sulfonylurea drugs at Sudlow sites I and II [21–24]. The terms KaA and KaI in Eqn. (1) are the association equilibrium constants (i.e., where Ka is the reciprocal of the dissociation equilibrium constant, Kd) for the drug and fatty acid with an immobilized binding agent in the column (e.g., normal HSA or glycated HSA), VM is the column void volume, and mL is the moles of binding sites in the column.
Based on Eqn. (1) it was possible to classify each drug and fatty acid combination with regards to whether the system involved direct competition or a mixed-mode interaction (e.g., allosteric interactions plus direct competition). These classifications are provided in Tables 3–5. Although similar classifications were seen for normal HSA and glycated HSA with the tested drugs and many of the fatty acids, quite different behavior was seen in some cases. For instance, lauric acid and linoleic acid gave mixed-mode interactions on the normal HSA column for all of the drugs but gave confirmed or possible direct competition with these drugs on the glycated HSA column. These differences again indicated that glycation can affect the types of interactions that occur between fatty acids and sulfonylurea drugs, even at low fatty acid concentrations.
It was further possible to use Eqn. (1) and the best-fit parameters of the plots for systems found to have direct competition to estimate the overall value of KaI for each fatty acid at its site(s) of competition with an injected drug. These values were obtained by finding the ratio of the slopes to the intercepts for plots of 1/k versus [I]. The resulting values of KaI are provided in Tables 3–5 and were in the general range of 105–108 M−1. These results gave good agreement with association equilibrium constants that have been reported for the same fatty acids with normal HSA in solution [33,39].
A change in KaI was often seen for fatty acids when going from normal HSA to glycated HSA even in cases for which similar types of interactions were seen between the fatty acid and a sulfonylurea drug. For example, acetohexamide, tolbutamide and gliclazide all gave a decrease in retention in the presence of myristic acid and stearic acid on both the normal HSA and glycated HSA columns and results that were consistent with a direct competition model. The KaI values obtained for myristic acid at its sites of competition with these drugs on normal HSA were in the range of 4.1–6.4 × 106 M−1 and increased to 1.4–2.4 × 107 M−1 for glycated HSA. Stearic acid also showed an increase in affinity (KaI) that went from 0.65–1.0 × 106 M−1 for normal HSA to 3.0–3.6 × 106 M−1 for glycated HSA. These values represented a net 3.1- to 3.8-fold increase in affinity for myristic acid when using glycated HSA and a 3.3- to 4.9-fold increase in affinity for stearic acid, with all of these differences being significant at the 95% confidence level.
CONCLUSION
Binding studies conducted by ultrafiltration and in the presence of various fatty acids demonstrated that an increase free drug concentration, or weaker binding, generally occurred for both normal HSA and glycated HSA as the concentration of fatty acids was increased. Such an effect could be due to direct competition or negative allosteric interactions between the fatty acids and drugs. The only exception noted was for gliclazide in the presence of oleic acid and normal HSA, for which an increase in overall binding was noted, as could be produced through a positive allosteric effect. Small or moderate changes in binding were found to be produced by lauric acid, myristic acid and stearic acid, while linoleic acid and palmitic acid gave moderate-to-large changes in binding. Oleic acid gave small-to-moderate increases in free drug concentrations for all of the tested drugs in the presence of glycated HSA and for tolbutamide in the presence of normal HSA; however, a large increase in free drug concentration for acetohexamide was seen in the presence of oleic acid and normal HSA.
Chromatographic columns containing normal HSA or glycated HSA were next used to examine in more detail the effects of glycation on the interactions between common sulfonylurea drugs and fatty acids on this protein. Relatively low fatty acid concentrations were employed in this part of the study to emphasize the initial, high affinity interactions that occurred as fatty acids were added to the system. Although similar overall changes in retention were seen for most combinations of drugs and fatty acids, some of these systems followed a direct competition model while others involved a mixed-mode interaction between the drugs and fatty acids under the conditions used in this study. There were some cases in which differences occurred in the interaction mechanism between normal HSA and glycated HSA (e.g., as seen for all of the drugs with linoleic acid). Even for those systems in which the same general type of interaction was present, changes were observed in the affinity of the fatty acids at their sites of competition with the drugs. This latter effect was noted for myristic acid and stearic acid with all of the investigated drugs.
These results confirmed that glycation, as a result of the metabolic changes that occur during diabetes, can alter the binding of drugs and fatty acids with HSA. In addition, it was found that changes in the levels of fatty acids, as can also take place in diabetes, can further alter these interactions through various mechanisms. Together, this research demonstrates the importance of considering how changes in the concentrations and types of metabolites (e.g., in this case, glucose and fatty acids) can alter the function of a protein such as HSA and its ability to interact with drugs or other agents. These effects, in turn, may alter the non-bound fraction of a drug or solute, thus affecting the apparent activity and ability of this drug or solute to bind with other proteins or receptors and to cross cell membranes [11–14,19–25]. As a result, experiments like those described in this report are expected to gain in importance as greater emphasis is placed on personalized medicine, or on the role that a disease state and metabolic changes may play on the customization of treatment for patients [14,24,48,49].
Supplementary Material
Table 4.
Change in the reciprocal of the retention factor (1/k) for gliclazide in the presence of increasing concentrations of fatty acids applied to normal HSA and glycated HSA columns
| Fatty Acid | Correlation Coefficienta | Type of Competitionb |
|---|---|---|
| Lauric acid (1.5–6 nM) | ||
| Normal HSA | r = 0.902 (n = 6) | Mixed mode |
| Glycated HSA | r = 0.513 (n = 6)c | Direct(?)c, KaI = 1.8 (± 1.5) × 107 M−1 |
| Linoleic acid (100–250 nM) | ||
| Normal HSA | r = 0.961 (n = 7) | Mixed mode |
| Glycated HSA | r = 0.972 (n = 7) | Direct, KaI = 1.6 (± 0.2) × 106 M−1 |
| Myristic acid (4–24 nM) | ||
| Normal HSA | r = 0.885 (n = 6) | Direct, KaI = 6.4 (± 1.7) × 106 M−1 |
| Glycated HSA | r = 0.965 (n = 6) | Direct, KaI = 2.4 (± 0.3) × 107 M−1 |
| Oleic acid (125–546 nM) | ||
| Normal HSA | r = 0.894 (n = 6) | Mixed mode |
| Glycated HSA | r = 0.796 (n = 7) | Direct, KaI = 6.2 (± 2.1) × 104 M−1 |
| Palmitic acid (100–400 nM) | ||
| Normal HSA | r = 0.965 (n = 6) | Direct, KaI = 6.3 (± 0.9) × 105 M−1 |
| Glycated HSA | r = 0.905 (n = 7) | Direct(?)c, KaI = 4.5 (± 10.2) × 107 M−1 |
| Stearic acid (10–80 nM) | ||
| Normal HSA | r = 0.885 (n = 7) | Direct, KaI = 7.4 (± 1.8) × 105 M−1 |
| Glycated HSA | r = 0.982 (n = 8) | Direct, KaI = 3.6 (± 0.3) × 106 M−1 |
The correlation coefficients are those obtained for a plot made according to Eqn. (1).
The values in parentheses represent a range of ± 1 S.D. A mixed mode interaction (e.g., direct competition plus a positive allosteric effect) was indicated in plots made according to Eqn. (1) by an initial decrease and then gradual increase in the retention factor for the injected drug as the concentration of fatty acids in the mobile phase was increased. A decrease in the retention factor for the drug when there was an increase in the concentration of the fatty acids could be produced by either a net negative allosteric effect or direct competition; in this study, the overall fit of the results to Eqn. (1) generally suggested that direct competition was present in most of these cases. In these latter cases, the apparent value of KaI for the fatty acid, as obtained by using Eqn. (1), is provided.
The correlation coefficient obtained in this case was not significant at the 95% confidence level or, in the case of palmitic acid and glycated HSA, the resulting value of KaI had a level of uncertainty that made it difficult to determine if direct competition was present in the given system. The symbol “(?)” indicates that the variation in the data was too large or the change in the retention factor was too small to obtain a reliable determination of exact type of interaction that was present or of the corresponding value of KaI in the case of direct competition.
Acknowledgments
This research was supported by the National Institutes of Health under grant R01 DK069629 and was conducted in facilities that were renovated under NIH grant RR015468-01.
Footnotes
SUPPORTIVE/SUPPLEMENTARY MATERIAL
Additional material is provided for HPAC studies, including other examples of plots of 1/k versus fatty acid concentration and further details on the best-fit lines that were obtained for such plots.
References
- 1.Unwin N, Whiling D, Gan D, Jacqmain O, Ghyoot G, editors. Diabetes Atlas. International Diabetes Federation; Brussels: 2009. [Google Scholar]
- 2.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. U.S. Centers for Disease Control and Prevention; Atlanta, GA: 2008. [Google Scholar]
- 3.Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–1358. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- 4.Lapolla A, Fedele D, Seraglia R, Traldi P. The role of mass spectrometry in the study of non-enzymatic protein glycation in diabetes: an update. Mass Spectrom Rev. 2006;25:775–797. doi: 10.1002/mas.20090. [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Cerami A. Nonenzymatic glycosylation of proteins in diabetes mellitus. Rec Adv Diabetes. 1984;1:173–180. [Google Scholar]
- 6.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812–3817. [PubMed] [Google Scholar]
- 7.Rohovec J, Maschmeyer T, Aime S, Peters JA. The structure of the sugar residue in glycated human serum albumin and its molecular recognition by phenylboronate. Chem Eur J. 2003;9:2193–2199. doi: 10.1002/chem.200204632. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MWA, Rasheed Z, Khan WA, Ali R. Biochemical, biophysical and thermodynamic analysis of in vitro glycated human serum albumin. Biochemistry (Moscow) 2007;72:146–152. doi: 10.1134/s0006297907020034. [DOI] [PubMed] [Google Scholar]
- 10.Murtiashaw MH, Winterhalter KH. Non-enzymatic glycation of human albumin does not alter its palmitate binding. Diabetologia. 1986;29:366–370. doi: 10.1007/BF00903346. [DOI] [PubMed] [Google Scholar]
- 11.Seedher N, Kanojia M. Reversible binding of antidiabetic drugs, repaglinide and gliclazide, with human serum albumin. Chem Biol Drug Design. 2008;72:290–296. doi: 10.1111/j.1747-0285.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 12.Hervé F, Urien S, Albengres E, Duché JC, Tillement JP. Drug binding in plasma: a summary of recent trends in the study of drug and hormone binding. Clin Pharmacokin. 1994;26:44–58. doi: 10.2165/00003088-199426010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Barzegar A, Moosavi-Movahedi AA, Sattarahmady N, Hosseinpour-Faizi MA. Spectroscopic studies of the effects of glycation of human serum albumin on L-trp binding. Protein Pept Lett. 2007;14:13–18. doi: 10.2174/092986607779117191. [DOI] [PubMed] [Google Scholar]
- 14.Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M, Hage DS. Review: glycation of human serum albumin. Clin Chim Acta. 2013 doi: 10.1016/j.cca.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2:1114–11121. doi: 10.1177/193229680800200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SN. In: Goodman and Gilman’s the pharmacological basis of therapeutics. 11. Brunton LL, Lazo JS, Parker KL, editors. Chap. 60 McGraw-Hill; New York: 2006. [Google Scholar]
- 17.Crooks MJ, Brown KF. Binding of sulfonylureas to serum albumin. J Pharm Pharmacol. 1974;26:304–311. doi: 10.1111/j.2042-7158.1974.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 18.Imamura Y, Kojima Y, Ichibagase H. Effect of simultaneous administration of drugs on adsorption and excretion. XIX. Binding of acetohexamide and its major metabolite, (−)-hydroxyhexamide, to human serum albumin. Chem Pharm Bull. 1985;33:1281–1284. doi: 10.1248/cpb.33.1281. [DOI] [PubMed] [Google Scholar]
- 19.Joseph KS, Hage DS. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J Chromatogr B. 2010;878:1590–1598. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basiaga SB, Hage DS. Chromatographic studies of changes in binding of sulfonylurea drugs to human serum albumin due to glycation and fatty acids. J Chromatogr B. 2010;878:3193–3197. doi: 10.1016/j.jchromb.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph KS, Anguizola J, Jackson AJ, Hage DS. Chromatographic analysis of acetohexamide binding to glycated human serum albumin. J Chromatogr B. 2010;878:2775–2781. doi: 10.1016/j.jchromb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph KS, Anguizola J, Hage DS. Binding of tolbutamide to glycated human serum albumin. J Pharm Biomed Anal. 2011;54:426–432. doi: 10.1016/j.jpba.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda R, Anguizola J, Joseph KS, Hage DS. High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin. Anal Bioanal Chem. 2011;401:2811–2819. doi: 10.1007/s00216-011-5382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clarke W, Cerny RL, Hage DS. Development of affinity microcolumns for drug-protein binding studies in personalized medicine: interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal Chem. 2013;85:4453–4460. doi: 10.1021/ac303734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AJ, Anguizola J, Pfaunmiller EL, Hage DS. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal Bioanal Chem. 2013;405:5833–5841. doi: 10.1007/s00216-013-6981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagstrom-Toft E, Hellstrom L, Moberg E. Lipolytic response during spontaneous hypoglycemia in insulin-dependent diabetic subjects. Hormone Metabol Res. 1998;30:586–593. doi: 10.1055/s-2007-978938. [DOI] [PubMed] [Google Scholar]
- 27.Chase HP, Glasgow AM. Juvenile diabetes mellitus and serum lipids and lipoprotein levels. Am J Dis Children. 1976;130:1113–1117. doi: 10.1001/archpedi.1976.02120110075010. [DOI] [PubMed] [Google Scholar]
- 28.Bomba-Opon D, Wielgos M, Szymanska M, Bablok L. Effects of free fatty acids on the course of gestational diabetes mellitus. Neuroendocrin Lett. 2006;27:277–280. [PubMed] [Google Scholar]
- 29.Gosmanov AR, Smiley DD, Robalino G, Siquiera J, Khan B. Effects of oral and intravenous fat load on blood pressure endotelial function sympathetic activity, and oxidative stress in obese healthy subjects. Am J Physiol Endocrinol Metab. 2010;299:E953–E958. doi: 10.1152/ajpendo.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 31.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 32.Peters T., Jr . All About Albumin. Academic Press; San Diego: 1996. [Google Scholar]
- 33.Spector AA. Fatty acid binding to plasma albumin. J Lipids Res. 1976;16:165–179. [PubMed] [Google Scholar]
- 34.Noctor TAG, Wainer IW, Hage DS. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J Chromatogr. 1992;577:305–315. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 35.Simard JR, Zunszain PA, Hamilton JA, Curry S. Location of high and low affinity fatty acid binding sites on human serum albumin revealed by NMR drug-competition analysis. J Mol Biol. 2006;361:336–351. doi: 10.1016/j.jmb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharya AA, Grüne T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol. 2000;303:721–732. doi: 10.1006/jmbi.2000.4158. [DOI] [PubMed] [Google Scholar]
- 37.Petitpas I, Grune T, Bhattacharya AA, Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol. 2001;314:955–960. doi: 10.1006/jmbi.2000.5208. [DOI] [PubMed] [Google Scholar]
- 38.Curry S, Mandelkow H, Brick P, Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nature Struct Biol. 1998;5:827–835. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- 39.Richieri GVS, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–7580. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- 40.Hage DS, Anguizola JA, Jackson AJ, Matsuda R, Papastavros E, Pfaunmiller E, Tong Z, Vargas-Badilla J, Yoo MJ, Zheng X. Chromatographic analysis of drug interactions in the serum proteome. Anal Methods. 2011;3:1449–1460. doi: 10.1039/C1AY05068K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hage DS. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J Chromatogr B. 2002;768:3–30. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M, Matsunaga R, Hara S, Nakamura M, Ohkura Y. Highly sensitive determination of free fatty acids in human serum by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1986;375:27–35. doi: 10.1016/s0378-4347(00)83688-9. [DOI] [PubMed] [Google Scholar]
- 43.Shimomura Y, Sugiyama S, Takamura T, Kondo T, Ozawa T. Quantitative determination of the fatty acid composition of human serum lipids by high-performance liquid chromatography. J Chromatogr B. 1986;383:9–17. doi: 10.1016/s0378-4347(00)83435-0. [DOI] [PubMed] [Google Scholar]
- 44.Takayama M, Ikeda T, Kotoku S. Determination of the free fatty acid composition of human serum in normal subjects and diabetes and liver cirrhosis patients. Med Sci Res. 1992;20:665–666. [Google Scholar]
- 45.Schulz M, Iwersen-Bergmann S, Andressen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care. 2012;16:R136. doi: 10.1186/cc11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wa C, Cerny RL, Hage DS. Identification and quantitative studies of protein immobilization sites by stable isotope labeling and mass spectrometry. Anal Chem. 2006;78:7967–7977. doi: 10.1021/ac0609935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loun B, Hage DS. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. J Chromatogr. 1992;579:225–235. [PubMed] [Google Scholar]
- 48.Hood L, Heath J, Phelps M, Lin B. Systems biology and new technologies enable predictive and preventive medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 49.Lim D, Dickherber A, Compton C. Before you analyze a human specimen, think quality, variability and bias. Anal Chem. 2011;83:8–13. doi: 10.1021/ac1018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.