Abstract
Epidemiologic studies have consistently shown that sleeping < 7 hr and ≥ 8 hr is associated with increased mortality and morbidity. The risks of short sleep may be consistent with results from experimental sleep deprivation studies. However, there has been little study of chronic moderate sleep restriction and no evaluation of older adults who might be more vulnerable to negative effects of sleep restriction, given their age-related morbidities. Moreover, the risks of long sleep have scarcely been examined experimentally. Moderate sleep restriction might benefit older long sleepers who often spend excessive time in bed (TIB), in contrast to older adults with average sleep patterns. Our aims are: (1) to examine the ability of older long sleepers and older average sleepers to adhere to 60 min TIB restriction; and (2) to contrast effects of chronic TIB restriction in older long vs. average sleepers. Older adults (n=100) (60–80 yr) who sleep 8–9 hr per night and 100 older adults who sleep 6–7.25 hr per night will be examined at 4 sites over 5 years. Following a 2-week baseline, participants will be randomized to one of two 12-week treatments: (1) a sleep restriction involving a fixed sleep-wake schedule, in which TIB is reduced 60 min below each participant’s baseline TIB; (2) a control treatment involving no sleep restriction, but a fixed sleep schedule. Sleep will be assessed with actigraphy and a diary. Measures will include glucose tolerance, sleepiness, depressive symptoms, quality of life, cognitive performance, incidence of illness or accident, and inflammation.
Keywords: Long Sleep, Inflammation, Sleep Restriction, Cumulative Sleep Debt, Sleepiness, Hypersomnia
1. Introduction
Epidemiologic studies tend to show that self-reported sleep durations of < 7 hr and ≥ 8 hr are associated with increased mortality [14] and morbidity, including cardiovascular disease [5], diabetes [6], and stroke [7]. These associations have been observed following control for over 30 covariates, follow-up durations of 10–20 years, and across all adult age strata.
The risks associated with short sleep are consistent with a vast literature indicating detrimental effects of experimental sleep restriction [8–11]. However, experimental studies have concentrated on short-term (≤ 5 days) manipulations involving profound sleep restriction (loss of ≥ 4 hr per night) in young adults. There has been little experimental study of chronic moderate sleep restriction, which is far more pervasive, and therefore more relevant to public health.
The risks of long sleep have scarcely been examined experimentally [12]. However, given epidemiologic data showing adverse effects of long sleep, long sleepers possibly could benefit from moderate sleep restriction. There are particularly compelling rationales for examining the risks/benefits of chronic moderate sleep restriction in older adults, as conflicting hypotheses regarding effects of sleep restriction can be experimentally evaluated.
Older adults might be more vulnerable than young adults to negative effects of sleep restriction, as short sleep duration is associated with inflammation [13]. Even among long sleepers, negative effects of sleep restriction might be accentuated if long sleep truly reflects an extra need for sleep, or underlying morbidity, or advanced aging, as many have posited.
On the other hand, older adults might tolerate (or benefit) from moderate sleep restriction. Older adults tend to spend more time in bed (TIB), despite decreases in total sleep time with age [14], and this “extra” TIB could contribute to age-associated sleep fragmentation, depression, and low energy, which might be ameliorated by TIB restriction. Even among older adults who sleep an average duration, moderate TIB restriction might have benefits without much sleep curtailment [15]. The longer a person’s habitual TIB, the greater might be the tolerance or positive benefits of chronic sleep restriction. Indeed, our recent study of older long sleepers (≥ 8.5 hr) found no adverse effects of 8 weeks of TIB restriction (90 min) [16].
The study will investigate the effects of chronic, moderate TIB restriction on multiple outcomes in older adults with average vs. long sleep duration, with examination of inflammatory biology dynamics as a potential mechanism. Cellular and genetic markers of inflammation are activated by sleep loss [17, 18], and are associated with morbidity risk [19–21]. Further, the study will provide an extensive assessment of whether long sleepers can adhere to sleep restriction with benefits, or at least without adverse consequences. These trials might begin exploration of sleep restriction as a potential large-scale risk-prevention intervention.
The aims of this randomized controlled trial are: (1) to examine the ability of older (ages 60–80) long (8–9 hr) and average sleepers (6–7.25 hr) to adhere to a 60 min time-in-bed restriction for 12 weeks; (2) to contrast effects of TIB restriction on health-related measures and on markers of inflammation, in long vs. average sleepers.
2. Methods
2.1. Study Overview
This 5-year home-based randomized controlled trial [22] will examine health, mood, quality of life, and performance effects of moderate restriction of time in bed (TIB) for 12 weeks, and will also evaluate inflammatory markers that are related to these outcomes. Across four experimental sites, we will contrast n=100 adults (25 per site) ages 60–80 yr who report sleeping ≥ 8 hr but ≤ 9 hr per night (hereafter: long sleepers) and n=100 (25 per site) age-matched adults who report sleeping 6–7.25 hr per night (hereafter: average sleepers). For both the long sleepers and the average sleepers, we will compare the effects of sleep restriction vs. control treatment, and these effects will be contrasted between long sleepers vs. average sleepers. Following a 2-week baseline period, participants will be randomly assigned to one of two 12-week treatments. (1) In a sleep restriction treatment, participants will be assigned to a fixed sleep-wake schedule, in which TIB will be reduced 60 min below each participant’s median baseline TIB. (2) In a control treatment, participants will undergo no sleep restriction, but will follow a fixed sleep schedule consistent with baseline TIB. Throughout the experiment, sleep will be monitored continuously by wrist actigraphy, supplemented with daily diaries. Experimental questionnaires will be entered via a study web site. Foci will include body weight, glucose tolerance, sleepiness, depression, quality of life, neurobehavioral performance, cellular and genomic markers of inflammation, incidence of illness, incidence of automobile accidents, and physical activity. Follow-up assessments for each participant will be conducted for 12 months after completion of the intervention. The study is registered in clinicaltrials.gov (NCT01642719).
2.2. Participant Recruitment and Screening
Participants will be 100 adults ages 60–80 years who report sleeping an average of 8–9 hr per night, and n=100 in the same age range who report sleeping an average of 6.0–7.25 hr per night.
Recruitment will involve a multi-stage process of initial questionnaire screening, informing prospective participants about the study, medical screening, and further screening based upon baseline recording. Approximately equal numbers of participants will be recruited from each of 4 experimental sites: Columbia, SC, Los Angeles, CA, Tucson, AZ, and New York, NY. Recruitment will be structured to reflect the racial/ethnic distribution of the populations at each location.
2.3. Initial Questionnaire Screening
After initial contact and a brief phone screen, potential participants will be given detailed information about the study, and complete questionnaires. Exclusion criteria will include: (1) reported average sleep duration of < 8.0 hr or > 9.0 hr for the long sleepers, or < 6 hr or > 7.25 hr for the average sleepers; (2) spending > 30 min time in bed in the morning and/or night outside of the major sleep period (e.g., watching tv), which could make it hard to define TIB; (3) expected change in usual sleep duration in the near future (e.g., change in work schedule); (4) reported average napping of > 2 naps/day or total nap duration of > 90 min/day; (5) recent shift-work (previous 2 months) or travel across multiple time zones (previous 4 weeks), or plans for performing shift-work or transmeridian travel during the intervention; (6) risk of severe sleep apnea (STOP questionnaire) [23]; (7) obesity (body mass index ≥35), a strong predictor of apnea; (8) high daytime sleepiness (Epworth Sleepiness Scale ≥ 10) [24]; (9) moderately severe depression (Patient Health Questionnaire-9 ≥15 [25]; (10) use of hypnotics or other drugs prescribed to promote sleep; (11) alcohol or drug use disorder; (12) any medical, neurologic, or psychiatric illness causing long sleep; factors associated with significant abnormalities of inflammation (a key outcome variable), including (13) several medical disorders (e.g., rheumatoid arthritis), (14) medications (e.g., steroids) and (14) current smoking (see 6.2.a); and (15) any health or mental condition that would contraindicate participation in the rigors of the study (e.g., MI in previous 3 years). Note that some quantity of sleep apnea and period limb movements in sleep is so common in people over 60 yr that exclusion for mild apnea or PLMS would mean that only a highly biased and rare selection of persons ≥60 years could be studied. Based upon our previous data (supported by NIH HL71560) showing tolerance to sleep restriction in older adults [16, 26], as well as our goal of obtaining a more representative sample, the present study will not exclude for moderate depression (PHQ-9 <15), moderate sleep apnea (AHI <15), Type 2 diabetes, or use of antidepressant drugs, so long as there is a period of stable use (≥ 8 weeks) prior to the experiment. Besides race and ethnicity, other demographics assessed will include employment, socioeconomic and marital status, the Dysfunctional Beliefs and Attitudes about Sleep Scale [27], and the Munich Chrontype Questionnaire.
2.4. Laboratory Screening Day 1
Potential participants who appear suitable based upon questionnaire responses will meet with the experimenters for further study orientation and screening. Each participant will then be invited to sign a written informed consent to participate in the remainder of the study, under the direction of the IRB of the respective institution. Immediately after signing consent, participants will practice using the study website, which will be used for the study questionnaires. Participants who are unwilling or unable to use this technology (n~20) will be enrolled, so long as they are willing to call a dedicated laboratory answering machine for their daily sleep diary, and to complete the paper and pencil questionnaires as scheduled. The participants’ personal physicians will be informed about the study and will have the opportunity to advise against participation.
2.5. Sleep Apnea Screening (1 night)
Absence of severe sleep apnea will be determined with the WatchPat device [28], an FDA-approved home apnea screening device. An AHI of ≥ 15 events per hr of sleep will result in exclusion.
2.6. Physical Examination and Medical/Sleep History
The next steps will be physical examination, screening laboratory tests (e.g., complete blood count, metabolic panel) and interview with a physician, which will determine the participant’s sleep history; help rule out obvious sleep disorders or medical causes of long sleep; and establish adequate health for study participation.
2.7. Scheduling
Following screening, the participants will be assessed for a 2-week baseline and a 12-week treatment. The 14-week assessment will be scheduled to avoid circumstances that might greatly alter sleep (e.g., travel). Staff will have interview contact with participants after baseline and after treatment weeks 2, 4, 8, and 12.
2.8. Home Baseline Recording
Besides pre-treatment data, the baseline period will also provide a final screen for participants’ ability and willingness to adhere to the protocol. During the 14-day baseline, participants will be asked to maintain usual habits of sleep, napping, exercise, and caffeine and alcohol intake, and they will wear a wrist actigraphic monitor for continuous assessment of sleep-wake activity and illumination. Each participant will be provided with a notebook with detailed instructions for using the study web site, a set of hard copies of the questionnaires to which they can refer when using the web site, and a customized schedule for completing each questionnaire. Staff will monitor the web site daily to verify successful completion of the questionnaires and adherence to the protocol. For the participants who record their responses on a laboratory answering machine, staff will enter the responses into the web site. Staff will contact participants up to 3 times during baseline to offer help, if needed. Participants providing less than 11 days of satisfactory data (e.g., completing the questionnaires and wearing actigraphs) during baseline (80% of the days) will be excluded from the study.
2.9. Sleep Habits Interview
Participants will undergo a semi-structured interview with a study anthropologist to assess social, environmental, cognitive and behavioral factors that might explain why the participants are long or average sleepers. For example, participants will be asked questions that probe their life and family history of sleep; whether they are night owls or morning larks; how much sleep they think they need for health, well-being, etc; whether they feel bad when they cannot sleep their usual sleep duration; whether they worry about not getting enough sleep; the extent to which they sleep as long as they do because they have time to do so, they are bored, they are avoiding stressors, whether they have heard that they should sleep ≥ 8 hr; whether their sleep duration can be partly attributed to habit; and whether their sleep habits were altered following a health or life event.
2.10. End of Baseline Visit
At the end of baseline, research staff will download actigraphic data. Reported long sleepers demonstrating a median baseline actigraphic TIB of < 8 hr or > 9 hr will be excluded, and reported average sleepers demonstrating a median baseline TIB of <6 or > 7.25 hr will be excluded from further participation in the study. Moreover, any participant demonstrating a median nap frequency of > 2/day, or median nap duration of > 90 min/day will be excluded from further participation in the study. Participants who have passed this final screen will then receive experimental randomization and instructions.
2.11. Experimental Treatment Randomization
At 4 study sites, a total of N=200 will pass through baseline screening. Blocked randomization (in groups of 5 subjects per site) will be used to assign participants by a ratio of 3:2 to a sleep restriction treatment or control treatment, stratified based upon sex and baseline TIB duration (< 8.5 hr vs ≥ 8.5 hr for the long sleepers; < 6.5 hr vs ≥ 6.5 hr for the average sleepers). A 3:2 assignment provides more assessment of the effects of sleep restriction with almost the same statistical power as a 3:3 assignment.
Following treatment assignment, participants will be given standardized statements designed to minimize potential expectancy biases. For example, they will be warned about potential negative effects of TIB restriction, but they will also be told that the interventions may have no effect or even positive effects. After these statements, they will complete 5-point Likert questions about their expected ability to complete the study, and of expected changes in sleep, sleepiness, health, mood, which will be used to assess how expectancy might influence outcomes. Participants will be assigned a treatment without concealment of the treatment. The principal investigators will be blinded to the treatment assignment, except following the occurrence of an adverse event or drop out from the study.
2.11.1. Sleep Restriction
Participants randomized to sleep restriction treatments (n=60 long and n=60 average sleepers) will be asked to reduce their TIB by 60 min below their median baseline TIB, and to maintain this sleep restriction every night for 12 weeks. For example, if they spend 9.0 hr TIB during baseline, they will reduce their TIB to 8.0 hr. In previous research by one of the authors, participants reporting sleep durations of ≥ 8.5 hr tolerated reducing their TIB by a mean of 83 min without negative consequences [16].
The effects of 60 min TIB restriction for the average sleepers are more difficult to predict. Experimental studies have reported mixed findings regarding whether older average sleepers can tolerate chronic modest TIB restriction without negative effects [15, 29, 30]. The present study will provide a unique exploration of competing hypotheses that 60 min TIB restriction might result either in similar tolerance in long and average sleepers (perhaps via sleep consolidation) or rather symptoms of sleep debt.
Participants will be asked to keep a precise sleep schedule throughout the 12 weeks, turning the lights off for sleep and arising at the same times, and using an alarm clock, if needed. The strategy for reducing TIB will be negotiated on an individual basis with each subject, and can be changed slightly during the experiment. An attempt will be made to tailor the TIB restriction to each individual’s sleep pattern. For example, if a participant has difficulty falling asleep, his/her bedtime will be delayed; if there are problems with early morning awakening, the wake-up time will be advanced, etc. Retrospectively, it will be possible to explore the extent to which both objective TIB curtailment and TST curtailment are correlated with beneficial or adverse effects. The relative effects of delaying bedtime and advancing wake-time will also be assessed. The participants will be asked to maintain their usual napping habits. A moderate increase in caffeine intake (≤ 200 mg) will be permitted. Caffeine intake will be monitored daily on the web site.
2.11.2. Fixed TIB Control
Participants randomized to control treatment (n=40 long and n=40 average sleepers) will also maintain fixed bedtimes, wake-times, times in bed, and napping, consistent with each person’s average baseline. Increased caffeine intake (≤ 200 mg) will also be permitted. Thus, nonspecific effects, which might include contact with research staff, participation in the procedures, regularization of the sleep schedule, and expectation of benefit will be balanced.
2.12. Time Schedule
The 5-year project will examine 10–12 participants per year who enter randomization at each of 4 experimental sites. During 14-week intervals, 2–5 participants will be studied at each site.
2.13. Measurements Taken Before and After the 12-Week Intervention (timeline in Table 2)
Table 2.
Experimental Measures during the 2-Week Baseline and 12-Week Intervention.
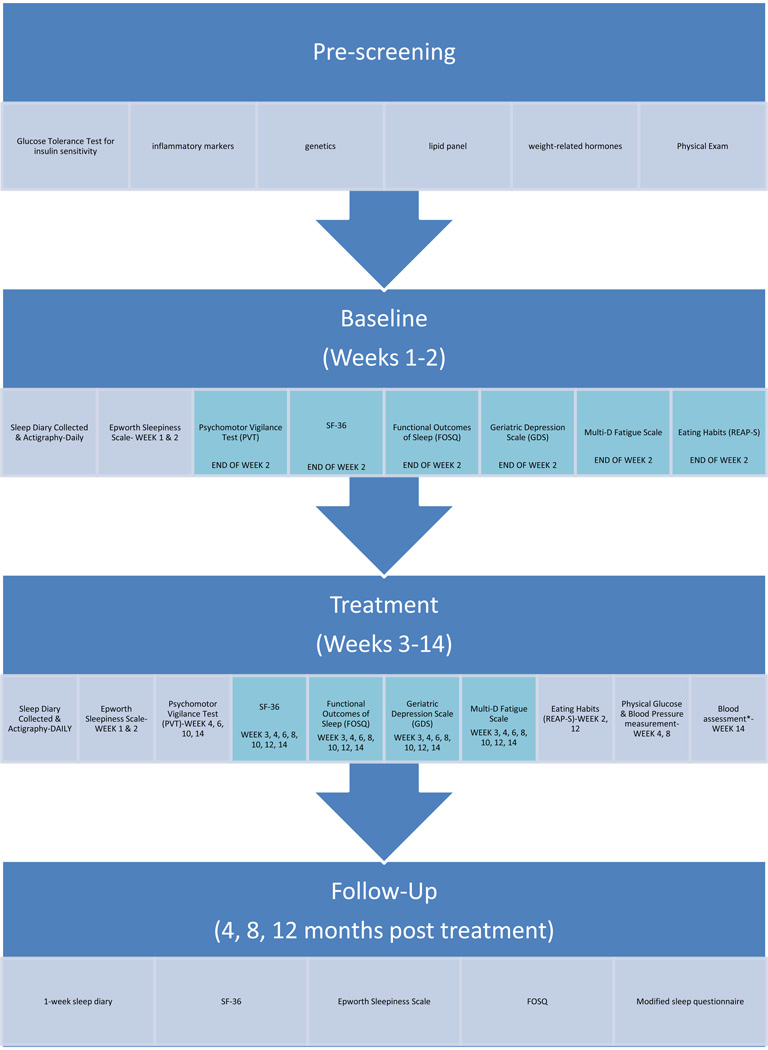 |
2.13.1. Metabolic Monitoring
About 1 week before the 2-week baseline, and during the last week of the study, participants will be given a morning oral glucose tolerance test (OGTT). Following a 12-hr fast and 24-hr abstention from exercise, participants will arrive at the laboratory. Staff will record body weight with a calibrated scale and height. Baseline BMI ≥ 35 will require study exclusion. Participants will remain in a seated position throughout the OGTT. A pre-ingestion blood sample (28 ml) will be drawn 5 min before glucose ingestion for the OGTT to allow for assessment of the lipid panel, genetic assessment, and markers of inflammation. Blood for plasma (10 ml) will be drawn into EDTA vacutainers, placed on ice, centrifuged at 4°C within 30 minutes, and plasma aliquots stored at −80°C for batch assays.
Blood for peripheral blood mononuclear cells (PBMC) (10 ml) will be drawn into heparinized vacutainers at room temperature. PBMC will be isolated using a Becton Dickinson (BD) Vacutainer™ CPT™ tube at each of the 4 sites. Aliquots of PBMCs (2 × 106 cells per/ml) will be unstimulated or stimulated with IL-6 (10 ng/ml) or with TNF-α (10 ng/ml)for 15 minutes at 37°C and the immediately fixed in 2% para-formaldehyde and stored at −80°C until transferred to UCLA’s IBC (see Resources) for subsequent intranuclear staining of the trascription factors signal transducer and activator of transcription (STAT 3) (IL-6 stimulant) or NFkB (TNF-α stimulant). The participant will then consume a 1.75 g/kg bolus of glucose (maximum dose: 75 g). Blood samples will be drawn at 30, 60, 90, and 120 min following glucose ingestion (2 ml per sample). Thus, the total amount of blood drawn will be 36 ml (~0.5 oz), We will assess fasting and post-ingestion levels of glucose and insulin and subsequent area under the curve .
2.13.2. Lipid Panel
Fasting samples will be obtained at commencement of the OGTT and mailed to the UCLA site (where these and OGTT assays are routine) for assay for total cholesterol, HDL, LDL, VLDL, and triglycerides. An association of long sleep with cholesterol has been attributed to long TIB and sleep fragmentation [31].
2.13.3. Markers of Inflammation
will be assessed at the UCLA site. Circulating markers of inflammation will include plasma levels of IL-6, TNF-a, and CRP. Molecular markers of inflammatory signaling will include the pro-inflammatory NF-KBK/Rel 235 family, and the signal transducer and activators of transcription (STAT). Recent infections, including URTI, will result in rescheduling of these measures. Also carefully assessed and statistically controlled will be information about biobehavioral confounds of inflammation including alcohol consumption, smoking history, BMI, physical activity; and use of medications (antidepressants, statins, and NSAID) [32].
2.13.4. Weight Related Hormones
will be assessed at the USC site. The hormones will include leptin, ghrelin, and obestatin. Previous research has indicated decreased in leptin (a satiety hormone) and increases in ghrelin (an appetite-promoting hormone) following rather profound sleep deprivation [33]. It will be interesting to observe whether there are changes in these hormones after chronic moderate sleep restriction.
2.13.5. DNA Assessment
DNA will be extracted from the PBMC samples. Sleep is influenced by many genes and polymorphisms [34, 35]. It is prudent now to collect DNA from a sample of long sleepers who will be extensively characterized. Later, using independent funds, we hope to genotype key polymorphisms in these samples for comparisons between groups and with population samples.
2.14. Measurements Taken During Baseline and During the 12-Week Intervention (Table 2)
Staff will contact the participants weekly (5–10 min) to assess their status and to troubleshoot obstacles to the protocol. Moreover, staff will visit the participants following baseline and weeks 2, 4, 8 and 12 of the experimental treatments to download actigraphic data and further check participants. To monitor tolerance of sleep restriction, many of the measures will be assessed more frequently during the initial 4 weeks of the treatments. Since adherence to the schedule is an important dependent variable, minimal efforts will be exerted to have the participants follow the schedule, and adherence will be a covariate in the statistical analyses. Staff will insure that the participants complete the study questionnaires and wear the actigraphs.
2.14.1. Actigraphic and Diary Sleep Assessment
Throughout the 14-week period, participants will wear wrist actigraphic monitors for continuous assessment of sleep-wake. Participants failing to wear the actigraph for ≥ 80% of any 2-week period during the experiment will be dropped from the study. All sites will use the Actiwatch Spectrum device (Philips Respironics). The large number of nights that can be recorded with actigraphy improves the stability of estimates of sleep in the presence of night-to-night variability, and actigraphy also provides monitoring of out-of-bed sleep, which may have greater face validity as a measure of consequences of sleep loss than subjective sleepiness measures or laboratory tests. Although actigraphy has some inaccuracies, in a design in which each subject's baseline serves as the control, relative change in sleep during the intervention should be highly reliable for contrasting treatment groups. The following variables will be assessed for each night: sleep latency, total sleep time, TIB, and sleep efficiency. In addition, daily measures of the number of naps and total duration of naps will be calculated. In a daily morning sleep diary, participants will input: (1) times and duration of recalled naps of the previous day; (2) medications used to promote nighttime sleep or daytime alertness; (3) time of getting into bed; (4) time of lights off trying to go to sleep, (5) final time of awakening; (6) final time of arising from bed; and self-estimations of (8) SOL, (9) TST, (10) WASO, and (11) overall sleep quality. The following illumination metrics will also be assessed: acrophase, mesor, first 4-hr and last 4-hr of wakefulness, time spent in darkness (<5 lux).
2.14.2. Subjective Sleepiness
At the end of each week, subjects will complete the Epworth Sleepiness Scale (ESS) [24]. Participants reporting ESS of ≥ 10 will be dropped from the experimental intervention as a safety precaution.
2.14.3. Physical Examinations
During screening and during treatment weeks 2, 6, and 12 or within 3 days following the experiment, participants will receive a physical exam to identify potential adverse effects of the experiment. Medical burden will be evaluated using the Charlson Index [36]. This final exam will also include 12-hr fasting glucose and blood pressure assessment.
2.14.4. Depression, Health-Related Quality-of-Life, Outcomes of Sleepiness, Fatigue
After baseline, and after treatment weeks 1, 2, and every 2 weeks thereafter, participants will complete web-based questionnaires for depression (Geriatric Depression Scale) [37], health-related quality of life (SF-36) [38], Functional Outcomes of Sleepiness (FOSQ) [39], and fatigue (Multi-Dimensional Fatigue Scale) [40]. With the emergence of pathological depression (GDS >16) or large decrements in SF-36 (10–15, depending on scale) or in FOSQ (decrease of 5 in global measure), the participant will be dropped from the experimental intervention and referred to appropriate care as needed.
2.14.5. Neurobehavioral Performance Battery
The following battery of tests will be administered following baseline, and following treatment weeks 2, 4, 8, and 12. Participants will be asked to refrain from napping, caffeine intake and exercise prior to testing.
Psychomotor Vigilance Test (PVT)
A 10-min PVT will measured sustained attention. The PVT is a widely used test, with established reliability, validity and high sensitivity for performance declines following sleep loss [41]. Metrics that will be assessed will include median reaction time (RT), the fastest 10% of responses, the slowest 10% of responses, the number of response “lapses” (RT > 500 ms), and standard deviation of responses.
The Stroop Color-Word Test [42]
is a test of cognitive interference. This test requires participants to read aloud words or colors while timed during three different tasks: randomly ordered words (task A); randomly ordered blocks of colors (task B); and randomly ordered words with conflicting colors (e.g., the word red in green ink). An interference score, calculated as the ratio of time on task C to the time on task B, will be the outcome measure for this test. Performance on the SCWT is impaired in individuals with OSA [43].
The Trail-making Test (TMT)[44]
is a task that assesses frontalcortex function In Part A of the TMT, participants connect 25 consecutively numbered circles in ascending numerical order. In Part B, participants connect 25 circles, alternating between ascending sequences of numbers and letters (i.e., 1, A, 2, B, etc). The difference between the time to complete Part B and Part A was used as the main outcome measure for this test. Performance on the TMT has been shown to be impaired in individuals with sleep apnea [45].
2.14.6. Daily Opportunity to Report Negative Effects, Accidents, or Emergencies
Every time participants access the website, they will be prompted to report whether they have had any negative or positive effecs of being in the study, and to provide open-ended comments. Participants will also be prompted to report accidents that may have occurred. Each time a participant has an accident while driving, while at home, or while working, the participant will complete a brief accident report on the web site, detailing the nature, severity, and presumed cause of the accident. Should the participants attribute any accident to sleepiness, fatigue, or to unknown causes, they will be excluded from further experimental regulation of their sleep, though we will continue to attempt to obtain the dependent measures. Likewise, participants will report visits to the emergency room or hospitalizations, which could result in study exclusion if attributed to one of these causes. Using informed consent, accident reports will be obtained from police or departments of motor vehicles records.
2.14.7. Physical Activity
During baseline week 2 and during treatment weeks 4, 8, and 12, an actigraph will also be worn on the waist (MTI, Inc.) for continuous estimation of physical activity. Such measurement has been validated against oxygen consumption [46]. At the conclusion of these weeks, physical activity will be further assessed with the Godin Physical Activity Recall Interview [47]. The proposed measures should detect moderate increases in physical activity that might result from sleep restriction. Since fatigue and sleepiness are predictors of inactivity, these data will also provide some indication of deleterious responses.
2.14.8. Upper Respiratory Tract Infections (URTI)
Perhaps the most ecologically valid method of determining the effects of sleep restriction on immune functioning is to assess the incidence of illness during the experiment. Incidence/severity of URTI will be assessed with the 21-item Wisconsin Upper Respiratory Symptom Survey [48], completed daily while the subject is experiencing symptoms. Quantified will be the number of days the participant notes illness and severity as determined by the sum of the scores from the individual items.
2.14.9. Eating Habits
will be assessed at baseline and after treatment weeks 2 and 12 with the Rapid Eating Assessment for Patients (REAP-Short Form) questionnaire [49]. On this 13-item scale, participants will be asked to indicate how frequently they skip breakfast, eat at restaurants, and eat various categories of food (e.g., processed meats, fried foods) using three response options (1=rarely/never, 2=sometimes, 3=usually/often). The total score will be derived by summing the scores of the 13 items.
2.14.10. Drop-Out Criteria
Detailed records of the date and reported reasons for drop-out will be recorded. The ombudsman will interview each drop-out, particularly focusing on whether the participant dropped out because of inability/unwillingness to restrict sleep, difficulty adhering to a rigid experimental schedule in general, health problems, or for unexpected personal or professional reasons. When drop-outs occur, we will attempt to continue collecting experimental data (besides actigraphy). Participants may also be excluded for failure to adhere at least 80% to the protocol of TIB stability and/or restriction and completion of study questionnaires, etc.
2.15. Post-Treatment Assessments
2.15.1. Ombudsman Reports
After the 12-week intervention, the study ombudsperson will contact each participant (20 min) and will attempt to elicit detailed (and blinded) feedback regarding difficulties or complaints about the experiment. The study ombudsperson will be otherwise independent of the study so that participants might feel more comfortable discussing these issues. Verifying that participants are treated fairly and courteously is an aim of the reports, which might prompt the ombudsperson to request modification of the approach to assessing participants, or possibly termination of the study if a volunteer reports severe negative reactions that are not otherwise detected.
2.15.2. Follow-up Assessment
Using the web site, the SF-36, ESS, FOSQ, and a 1-week sleep diary will be assessed at 4, 8 and 12 months after completing the study. In addition, participants will be asked to complete a questionnaire assessing whether they have modified their sleep after participating in the study. At the 5-y mark, final one-year follow-ups will not have occurred for a few participants, but will be obtained subsequently when possible.
2.15.3. Drop-Out Assessment
Detailed records of the date and reported reasons for drop-out will be recorded. The ombudsperson will interview each drop-out, particularly focusing on whether the participant dropped out because of inability or unwillingness to restrict or regulate sleep, difficulty adhering to a rigid experimental schedule in general, health problems, or for unexpected personal or professional reasons. When drop-outs occur, we will attempt to continue collecting experimental data (besides actigraphy) and will offer a small incentive. A multiple imputation approach will be used to handle missing data.
2.16. Data Safety Monitoring Board (DSMB)
A DSMB has been developed to safeguarding the interests of study participants, assess the safety and efficacy of study procedures, and to monitoring the overall conduct of the study. The DSMB will convene at least twice during the first year of the study, at least once in remaining years, and more often if necessary, for example, to discuss adverse events that might arise. The DSMB consists of two sleep scientists, a sleep physician, a biostatistician, and an ethicist. The DSMB is an independent group advisory first to the IRBs at the associated institutions, and second to the NHLBI. The DSMBis required to provide recommendations about starting, continuing, and stopping the study. In addition, the DSMB is asked to make recommendations, as appropriate, to the IRBs and NHLBI about: efficacy of the study intervention; benefit/risk ratio of procedures and participant burden; selection, recruitment, and retention of participants; adherence to protocol requirements; completeness, quality, and analysis of measurements; amendments to the study protocol and consent forms; performance of individual centers and core labs; participant safety; notification of and referral for abnormal findings.
2.17. Statistical Analyses and Power Calculations
Note: for each aim, there are rationales for examining treatment by group (long vs. average sleepers) effects, as well as conducting separate analyses of treatment effects for each group.
2.17.1. Aim 1
To examine whether older long and average sleepers…are able to adhere a 60-min TIB restriction for 12 week. For each participant, the average actigraphic nocturnal TIB will be computed for the 2-week baseline and for the 12-week treatment interval.
ANCOVA: ΔTIBi = β0 + β1LStrti + β2AStrti + β3LSconi + εi where we model the average change in the mean time in bed for each participant as a function of whether the the ith participant is a long sleeper in the treatment group LStrti, an average sleeper in the treatment group AStrti, or a long sleeper in the control group LSconi,. We will assess the hypotheses (1) H0: β1=60, β2=60, β3=0 [Was the sleep restriction as designed?], (2) β1 − β2=0 [Were the long and average sleepers both able to restrict sleep by the prescribed amount?] for which we will have 80% power to detect an effect size departure of 0.5, for example, a change of 50±15 min (assuming SD of 30 min).
ANCOVA: Ndaysi = β0 + β1LStrti + β2AStrti + β3LSconi + β4Agei + β5Genderi + εi where we model the number of days remaining in the study as a function of whether the the ith participant is a long sleeper in the treatment group LStrti, an average sleeper in the treatment group AStrti, a long sleeper in the control group LSconi, their age, and their gender. We will assess the hypotheses (1) H0: β1=0, β2=0, β3=0 [Did subjects complete the study regardless of group, treatment?], (2) β1 − β2=0 [Did the average vs. long sleepers stay in the study for the same amount of days?] for which we will have 80% power to detect an effect size of 0 5, e.g., duration in study of 60±10 days (SD= 20 days) This analysis accounts for dropouts.
2.17.2 Aim 2
To contrast effects of TIB sleep restriction on health-related function. Incidence and duration of upper respiratory tract infections and incidence of accidents will be reported as they occur and will be compared between treatments with Cox Proportional Hazards. All the other outcomes will be modeled using ANCOVA controlling for baseline values and degree of TIB restriction. Glucose tolerance/insulin sensitivity, body weight, and multiple measures of inflammation will be assessed at baseline and intervention week 12. Ghrelin, leptin, obestatin, and dietary recall will be assessed at baseline and the end of treatment weeks 2 and 12. Confounds such as BMI and alcohol use will be covariates in the analyses of inflammatory measures.
Most of the remaining variables will be assessed bi-weekly or more frequently. Variables measured daily will be summarized as bi-weekly mean values and models will be fit to the summary measures. Models for outcomes measured at least bi-weekly will include terms for assessing trends. Mean scores for baseline and for each of six 2-week treatment periods will be computed. We correct for multiple testing by setting α=0.01. Each outcome will be assessed using the generalized linear model specification: g(Yit) = β0 + β1LStrti+ β2AStrti+ β3LSconi + β4Agei + β 5Genderi + β6Racei +β7BaseYi + εit where g(Yit) is the link function transformation of the outcome for the ith participant at the tth treatment time as a function of whether the the ith participant is along sleeper in the treatment group LStrti, an average sleeper in the treatment group AStrti, a long sleeper in the control group LSconi, age, gender, race/ethnicity, and the average baseline levels. We are interested in the following three tests: (1) H0: β2=0 [For average sleepers, is there a treatment effect?], (2) β1 − β3=0 [For long sleepers is there a treatment effect?], (3) β1+ β2 − 2β3=0 [Is there a treatment effect across both long and average sleepers?], (4) β1− 2β2 + β3=0 [Is the treatment effect for long sleepers the same as the treatment effect for average sleepers?]. We will have 80% power for (1) to detect an effect size of 0.780 for one measure, 0.546 for two repeated measures and of 0.313 for six repeated measures (α =0.01). We will have 80% power for (2) to detect an effect size of 0.633 for one measure, 0.444 for two repeated measures and of 0.255 for six repeated measures (α =0.01). We will have 80% power for (3) and (4) to detect an effect size of 0.273 for a single measure, 0.192 for two repeated measures and of 0.110 for six repeated measures (α =0.01). Table 1 displays modest changes which would be significant when comparing variables at a specific time period (we will have higher power when there are repeated measures).
Table 1.
Differences in selected outcome variables which would be significantly different with the proposed sample size.
|
Variable |
SD | H1: Difference Yielding Effect of TIB Restriction in Average Sleepers |
H2: Difference Yielding Effect of TIB Restriction in Long Sleepers |
H3 H4:Difference Yielding Main Effect or Interaction |
Clinical Relevance |
|---|---|---|---|---|---|
| Fast Glucose | 15 | 100 vs. 112 mg/dl | 100 vs. 110 mg/dl | 100 vs. 105 mg/dl | 110–125 mg/dl is “high” |
| Gluc Tol. | 25 | 100 vs. 120 mg/dl | 100 vs. 116 mg/dl | 100 vs. 109 mg/dl | 140–199: impaired levels |
| Cholesterol | 20 | 180 vs. 196 | 180 vs. 193 | 180 vs. 187 | < 200 is desirable |
| IL-6 | 1 | 1.88 vs. 2.66 | 1.88 vs. 2.51 | 1.88 vs. 2.15 | Seen in partial sleep depriv |
| NFKb | 0.05 | 0.18 vs. 0.22 | 0.18 vs. 0.21 | 0.18 vs. 0.20 | Seen in partial sleep depriv |
| Epworth | 3.5 | 4.5 vs. 7.3 | 4.5 vs. 6.8 | 4.5 vs. 5.8 | 10 is high sleepiness |
| GDS | 5 | 6 vs. 10 | 6 vs. 10 | 6 vs. 8 | 10–19 is mild depression |
| SF-36 | 20 | 90 vs. 74 | 90 vs. 77 | 90 vs. 83 | ↓ of ≥15 is poor response |
| FOSQ | 10 | 15 vs. 7 | 15 vs. 8 | 15 vs. 11 | ↓ of >10 is poor response |
Further analyses will explore the extent to which adherence and tolerance of TIB restriction varies depending on (1) history of morbidities, (2) medication use; and (3) the extent to which subjects’ habitual sleep duration can be attributed to voluntary habits vs. biologicalneed ;and (4) whether the restriction was accomplished via delaying bedtime, advancing wake time. Post-hoc analysis will also assess whether responses to the intervention are moderated by changes in light exposure.
3. Discussion
3.1. Is Long Sleep Harmful?
Few concepts challenge prevailing assumptions in the sleep field more than the notion that 8 hr of sleep might be harmful. Much current research on sleep and health has focused on negative consequences of insomnia or inadequate sleep, but the present study will address an important empirical void in addressing long sleep.
Notwithstanding prevailing assumptions, epidemiologic evidence indicates that the risks of long sleep are at least as great as the risks of short sleep [2,12]. Moreover, long sleep or long TIB might be a greater societal risk because some studies have found that a greater proportion of adults report sleeping ≥ 8 hr than those reporting ≤ 6 hr [1]. Nonetheless, the notion that long sleep could be harmful remains a topic of skepticism, centered around the following arguments addressed below: (1) “long sleep” (i.e., 8 hr sleep), should be considered normal and healthy were it not for curtailment of sleep in our society; (2) self-reported sleep duration is more reflective of long TIB, not long TST; (3) associations of long sleep with mortality and morbidity must be explained by some confounding factor; and (4) there are no plausible mechanisms to explain how long sleep could be hazardous [50].
3.1.1. Societal Sleep Deprivation?
The notion that there is a societal epidemic of sleep deprivation has evolved partly in light of epidemiologic data cited above, as well several other lines of evidence. First, average sleep duration has apparently decreased over the last century [51], and from about 8 to 7 hr over the past few decades [52], a trend that has coincided with an increased prevalence of obesity [53], diabetes [54], and reported fatigue [55]. However, these parallel population trends might reflect other factors, such as declines in energy expenditure, and are not necessarily causally linked. Moreover, there has not been consistent evidence for an increase over the past 35 yr in the percentage of people reporting < 6 hr sleep [56–58], the amount most closely linked with morbidity.
Second, although people often expand their sleep on weekends or in laboratory ad libitum sleep conditions [59], this behavior might not reflect a “sleep debt” any more than banquet overeating is indicative of malnutrition. Surveys indicate that people are more likely to sleep extra amounts for pleasure rather than to replace lost sleep [60].
3.1.2. Long Total Sleep Time or Long TIB?
Research suggests that self-reported long sleepers [61], as well as average sleepers [62], tend to overestimate their TST by about 60 minutes compared with objective data. Thus, reported sleep durations correspond largely to TIB [62]. Mortality/morbidity risks associated with self-reported long sleep might be partly attributable to long TIB. However, given the high correlations of TIB with TST, reported long sleep is likely to be indicative of long physiologic sleep, as confirmed recently [63]. Research has also confirmed risks associated with objectively long sleep [64]. Moreover, studies that have discriminated between TIB and TST have found similar mortality associations with both measures [65].
3.1.3. Alternative Explanations for Risks Associated with Long Sleep
As with risks of short sleep, risks of long sleep might be explained by multiple factors associated both with long sleep and health problems, including sleep apnea [66], depression [67], low socioeconomic status [68], unemployment [69], race/ethnicity [70], and low physical activity [12, 71]. Moreover, long sleep might simply reflect a moribund state. Although epidemiologic studies have controlled for these factors, perhaps the control has not been comprehensive, nor have potential interactions between factors been fully assessed. On the other hand, statistical control for these factors might result in an underestimation of the risks. For example, if long sleep suppresses exercise [71], then controlling for exercise could obscure associations of long sleep with mortality. Randomized trials can experimentally control for confounding factors.
As much as 50% of reported sleep duration may be heritable [72], and the extent to which this genetic variation produces increased mortality via sleep duration per se or via independent causal pathways is unclear. Controlled experimental trials are needed to determine if excess mortality related to long sleep of genetic origin can be modified by sleep restriction or if long sleep due to other factors causes mortality and morbidity. Mendelian randomization studies might prove useful in assessing causality if genes responsible for long sleep are identified.
3.1.4. How Might Long Sleep be Hazardous?
Perhaps all healthy behaviors may be hazardous in excessive amounts, and a similar hazard associated with long sleep/TIB is plausible via several potential mechanisms [12]. First, just 2–5 days of bed-rest can elicit significant impairments in insulin sensitivity and cardiovascular function [73]. Second, long sleep is associated with increased sleep fragmentation, which has been associated with poor health outcomes in epidemiologic research [74], and in studies of experimentally-induced fragmentation [75]. Third, long sleep duration could elicit metabolic changes that could lead to dyslipidemia [76]. Fourth, lethargy and malaise, noted after acute sleep extension [77] and in long sleepers [78], could be indicative of a cytokine imbalance as activation of inflammatory signaling is associated with fatigue [79].
Moderate sleep restriction could counteract such hazardous effects. It is noteworthy that sleep restriction therapy is one of the most effective treatments for primary insomnia [80], which is often associated with excessive time in bed in an attempt to compensate for insomnia. So profound are the improvements that despite decreases in TIB by 1–3 hr, TST is often preserved [81]. Moreover, sleep restriction has had significant antidepressant effects [82].
Sleep restriction might be similarly beneficial for long sleepers. Evidence suggests that long sleepers might also be spending too much TIB, and this behavior can be partly explained by a conscious effort to go to sleep early, by misperceptions about what is adequate sleep, or by passive avoidance defenses [83].
3.2. Test of Cumulative Sleep Debt
Another widely held assumption that will be challenged in the present study is that chronic sleep restriction will result in cumulative sleep debt and many negative health consequences [84]. Experimental sleep restriction studies have been limited mostly to short-term manipulations involving profound sleep restriction. Research involving chronic sleep restriction has revealed results that are not consistent with the results from short-term manipulations. Indeed, several earlier studies found that TIB in young 8-hr sleepers could be reduced to 4.5–5.5 hr over several months with no impairments in performance or mood [85, 86]. However, these studies lacked sufficient verification of adherence or sensitivity of measures, and the studies failed to assess biological consequences of sleep loss. The present study will provide the most comprehensive examination of the influence of chronic moderate sleep restriction.
3.3. Aging and Sleep
Another limitation of experimental sleep deprivation studies is that they have focused mostly on young adults. Older adults might respond differently to sleep loss. The voluntary decision to spend long TIB seems apparent in older adults, who tend to spend more TIB than young adults despite a steady decline in objective sleep duration with age. Analogous to insomniacs, many older adults spend more TIB in an attempt to compensate for age-related disturbances in sleep [87]. Others seem to spend more TIB out of habit or mistaken beliefs [16]. However, there are vastly different viewpoints regarding the potential effects of TIB restriction in older adults.
There is some support for the hypothesis that reductions in sleep quantity and quality could contribute to morbidity and mortality associated with aging [88]. For example, research by Irwin et al. has shown an association of sleep disturbance with elevations in pro-inflammatory cytokines in older adults [13], which might be a mechanism by which poor sleep accelerates morbidity with aging.
In contrast, because aging is associated with increased wakefulness, and increased TIB [87], others have argued that older adults could benefit from moderate sleep restriction [89]. A study by Hoch et al. [15] found that 1-year of mild restriction of TIB (30 min) improved sleep in older adults who had no sleep complaints, and average baseline levels of TIB (7.8 hr) and average TST (5.8 hr) for their age. While sleep efficiency and delta power increased, TST also actually increased, despite the TIB restriction. Riedel et al. [90] found similar sleep benefits of moderate sleep restriction (~30 min) for 16 weeks in non-complaining older adults.
These studies did not assess biological variables. As reviewed above, increases in inflammation might occur with sleep restriction. Alternatively, modest sleep restriction might result in decreased inflammation by preserving delta sleep and eliciting decreases in sleep fragmentation [91]. This study will address these competing hypotheses by examining the effects of chronic 60-min TIB restriction in older adults who report average sleep (i.e., 6–7.25 hr), as well as those who report long sleep (8–9 hr).
Because long sleepers have relatively greater sleep fragmentation, older long sleepers might tolerate or benefit the most from moderate restriction of TIB. Our study will reduce an 8–9 hr reported sleep period to 7–8 hr in bed, which appears to be optimal in epidemiologic studies, and contrast this to reducing the sleep period below 6–7.25 hr. On the other hand, if initial longer TST or TIB reflects a greater sleep need, then long sleepers might experience the often-demonstrated consequences of inadequate sleep.
Acknowledgments
Supported by R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to MRI; R25HL105444 and R01MD004113 and UCLA CTSI UL1TR000124, and the Cousins Center for Psychoneuroimmunology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiat. 2002;2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Kurina LM, McClintock MK, Chen JH, et al. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013;23:361–370. doi: 10.1016/j.annepidem.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality; result from the JACC Study. Sleep. 2004;2004;27:51–54. [PubMed] [Google Scholar]
- 4.Hublin C, Partinen M, Koskenvuo M, et al. Sleep and mortality: a populationbased 22-year follow-up study. Sleep. 2007;2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diab Care. 2003;2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AI, Giles WH, Croft JB, et al. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurol. 1997;1997;48:904–911. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 8.Rechtschaffen A, Bergmann BM, Everson CA, et al. Sleep deprivation in the rat: X. Integration, discussion of the findings 1989. Sleep. 2002;25:68–87. [PubMed] [Google Scholar]
- 9.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Doyle WJ, Alper CM, et al. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 12.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;2004;8:159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MR, Wang M, Campomayor C, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Int Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 14.Carskadon MA, Brown ED, Dement WC. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 15.Hoch CC, Reynolds CFI, Buysse DJ, et al. Protecting sleep quality in later life: a pilot study of bed restriction and sleep hygiene. J Gerontol. 2001;56B:P52–P59. doi: 10.1093/geronb/56.1.p52. [DOI] [PubMed] [Google Scholar]
- 16.Youngstedt SD, Kline CE, Zielinski MR, et al. Tolerance of chronic 90-minute time-in-bedrestriction in older long sleepers. Sleep. 2009;2009;32:1467–1479. doi: 10.1093/sleep/32.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. Sep 18;2006166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 18.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Biol Psychiat. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 20.Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodondi N, Marques-Vidal P, Butler J, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 2010;171:540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicaTrials.gov NCT01642719. Chronic Moderate Sleep Restriction in Older Adults [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire. A tool to screen for obstructive sleep apnea. Anesthesiol. 2008;2008;108:812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness sscale. Sleep. 1991;1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 Validity of a brief depression severity measure. J Gen Intern Med. 2001;2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielinski MR, Kline CE, Kripke DF, et al. No effect of 8-week time-in-bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;2008;17:412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin CM, Stone J, Trinkle D, et al. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;1993;8:463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 28.Yuceege M, Firat H, Demir A, Ardic S. Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. J Clin Sleep Med. 2013;2013;9:339–344. doi: 10.5664/jcsm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds CF, III, Serody L, Okun ML, et al. Protecting sleep, promoting health in later life: a randomized clinical trial. Psychosom Med. 2010;2010;72:178–186. doi: 10.1097/PSY.0b013e3181c870a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg JF, Miedema HM, Tulen JH, et al. Long sleep duration is associated with serum cholesterol in the elderly: the Rotterdam Study. Psychosom Med. 2008;2008;70:1005–1011. doi: 10.1097/PSY.0b013e318186e656. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel K, Tasali E, Penev P, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004 2004 Dec 7;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 34.Retey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci. 2005;2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (−308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Jr., Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2004. [Google Scholar]
- 39.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 2007;2007;20:835–843. [PubMed] [Google Scholar]
- 40.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychsom Res. 1995;1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 41.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;1997;20:267–277. [PubMed] [Google Scholar]
- 42.Golden C, Freshwater S. A Manual for the Adult Stroop Color and Word Test. Chicago: Stoelting; 2002. [Google Scholar]
- 43.Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive function. Sleep. 2004;2004;27:685–693. [PubMed] [Google Scholar]
- 44.Reitan RM, Davison LA. Clinical Neuropsychology: Current Status and Applications. New York: John Wiley; 1974. [Google Scholar]
- 45.Bedard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treament with CPAP. J Clin Exp Neuropsychol. 1993;1993;15:330–341. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 46.Slootmaker SM, Chin A, Paw MJ, et al. Concurrent validity of the PAM accelerometer relative to the MTI Actigraph using oxygen consumption as a reference. Scand J Med Sci Sports. 2009;2009;19:36–43. doi: 10.1111/j.1600-0838.2007.00740.x. [DOI] [PubMed] [Google Scholar]
- 47.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Spor Sci. 1985;1985;10:141–146. [PubMed] [Google Scholar]
- 48.Barrett B, Locken K, Schwamman J, et al. The Wisconsin Upper Respiratory Symptom Survey: Development of an instrument to measure the common cold. J Fam Pract. 2008;2008;51:265. [PubMed] [Google Scholar]
- 49.Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav. 2006;2006;38:286–92. doi: 10.1016/j.jneb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;2006;29:878–879. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 51.Ekirch R. Sleep we have lost: Pre-industrial slumber in the British Isles. Amer Histor Rev. 2001;2001;106:343–386. [PubMed] [Google Scholar]
- 52.Bixler E. Sleep and society: an epidemiological perspective. Sleep Med. 2009;2009;10:S3–S6. doi: 10.1016/j.sleep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 53.James WP. The epidemiology of obesity: the size of the problem. J Int Med. 2010;2010;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 54.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 55.Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;1996;19:462–464. doi: 10.1093/sleep/19.6.462. [DOI] [PubMed] [Google Scholar]
- 56.Knutson KL, Van Cauter E, Rathouz PJ, et al. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rowshan Ravin A, Bengtsson C, Lissner L, et al. Thirty-six year secular trends in sleep duration and sleep satisfaction, and associations with mental stress and socioeconomic factors - results of the Population Study of Women in Gothenburg, Sweden. J Sleep Res. 2010;2010;19:496–503. doi: 10.1111/j.1365-2869.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 58.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev. 2012;2012;16:223–230. doi: 10.1016/j.smrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Webb WB, Agnew HW., Jr. Are we chronically sleep deprived? Bull Psychonom Soc. 1975;6:47–48. [Google Scholar]
- 60.Anderson C, Horne JA. Do we really want more sleep? A population-based study evaluating the strength of desire for more sleep. Sleep Med. 2008;2008;9:184–187. doi: 10.1016/j.sleep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Kline CE, Zielinski MR, Devlin TM, et al. Self-reported long sleep in older adults is closely related to objective time in bed. Sleep Biol Rhythms. 2010;2010;8:42–51. doi: 10.1111/j.1479-8425.2009.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauderdale DS, Knutson KL, Yan LL, et al. Self-reported and measured sleep duration: how similar are they? Epidemiol. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel SR, Blackwell T, Ancoli-Israel S, Stone KL. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35:641–8. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011 2011 Jan;12(1):28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 66.Bliwise DL, King AC, Harris RB. Habitual sleep durations and health in a 50–65 year old population. J Clin Epidemiol. 1994;1994;47:35–41. doi: 10.1016/0895-4356(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 67.Patel SR, Malhotra A, Gottlieb DJ, et al. Correlates of long sleep duration. Sleep. 2006;2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hale L. Who has time to sleep? J Public Health. 2005;27:205–211. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- 69.Kronholm E, Harma M, Hublin C, et al. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;2006;15:276–290. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 70.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morgan K. Long sleep duration and all-cause mortality in later life: a possible consequence of sedentary death syndrome. Sleep. 2007;30:A115. [Google Scholar]
- 72.de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;2002;76:479–486. doi: 10.1016/s0031-9384(02)00699-6. [DOI] [PubMed] [Google Scholar]
- 73.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;2007;27:2750–2756. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett LS, Barbour C, Langford B, et al. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 2002;2002;159:1884–1890. doi: 10.1164/ajrccm.159.6.9808107. [DOI] [PubMed] [Google Scholar]
- 75.Horner RL. Autonomic consequences of arousal from sleep: mechanisms and implications. Sleep. 1996;1996;19:S193–S195. doi: 10.1093/sleep/19.suppl_10.s193. [DOI] [PubMed] [Google Scholar]
- 76.Kaneita Y, Uchiyama M, Yoshiike M, et al. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;2008;31:645–625. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taub JM, Berger RJ. Extended sleep and performance: the Rip Van Winkle effect. Psychon Sci. 1969;1969;16:204–205. [Google Scholar]
- 78.Baekeland F, Hartmann E. Reported sleep characteristics: effects of age, sleep length and psychiatric impairment. Compr Psychiat. 1971;12:141–147. doi: 10.1016/0010-440x(71)90005-8. [DOI] [PubMed] [Google Scholar]
- 79.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiat. 1994;1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 81.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;1987;10:45–56. [PubMed] [Google Scholar]
- 82.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for Insomnia in Patients with High and Low Depressive Symptom Severity: Adherence and Clinical Outcomes. J Clin.Sleep Med. 2011;2011;7:645–652. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hartmann E, Baekeland F, Zwilling GR. Psychological differences between long and short sleepers. Arch Gen Psychiat. 1972;1972;26:463–468. doi: 10.1001/archpsyc.1972.01750230073014. [DOI] [PubMed] [Google Scholar]
- 84.Van Dongen HPA, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 85.Mullaney DJ, Johnson LC, Naitoh P, et al. Sleep during and after gradual sleep reduction. Psychophysiol. 1977;14:237–244. doi: 10.1111/j.1469-8986.1977.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 86.Webb WB, Agnew HW., Jr. The effects of a chronic limitation of sleep length. Psychophysiol. 1974;1974;11:265–274. doi: 10.1111/j.1469-8986.1974.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 87.Prinz PN, Vitiello MV, Raskind MA, et al. Geriatrics: sleep disorders and aging. N Engl J Med. 1990;1990;323:520–526. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 88.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of followup. Psychosom Med. 2003;2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 89.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiat Neuro. 2000;2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 90.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 91.Irwin MR, Valladares EM, Motivala S, et al. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosom Med. 2006;2006;68:159–166. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]


