Abstract
Background
The cyclooxygenase-2 (COX-2) enzyme plays a major role in tumor progression and resistance to chemotherapy. A Phase-II study was undertaken to determine the activity of a dose attenuated schedule of irinotecan, capecitabine, and the COX-2 inhibitor celecoxib in patients with advanced colorectal cancer.
Methods
The eligibility criteria included a pathologically or cytologically confirmed diagnosis of adenocarcinoma of the colon or rectum that was metastatic. Patients received a combination of irinotecan 70 mg/m2 over 30 min I.V. on days 1 and 8, capecitabine 1,000 mg/m2 twice per day orally on days 1–14, and celecoxib at a daily dose of 800 mg continuously. Cycles were repeated every 21 days
Results
Fifty-one patients were enrolled (median age 58 years; M : F 31 : 20). The objective response rate was 21/51=41% [95% confidence intervals (CI), 0.28–0.55]. The median time to progression was 7.7 months (95% CI, 6.2–8.6 months). Median survival time and probability of survival at 1 year were 21.2 months (95% CI, 13.8–n/a), and 75% (95% CI, 0.63–0.88), respectively. The major toxicity was Grade 3 or 4 diarrhea, seen in 24 and 10% of patients, respectively. There were no treatment related deaths.
Conclusions
The lower dose intensity of irinotecan appeared to maintain activity and improve tolerability when combined with capecitabine. The addition of celecoxib to irinotecan and capecitabine did not appear to significantly increase the activity of this doublet based on the RECIST criteria for objective response.
Keywords: Colorectal cancer, Irinotecan, Celecoxib, Capecitabine
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer mortality in the United States resulting in 55,000 deaths per year [1]. The sequential introduction of the active agents irinotecan and oxaliplatin significantly increased the survival of patients with CRC [2]. However, <50% of patients with metastatic disease are alive at 2 years. The improvement in the outcome of patients with CRC is dependent on the development of more effective systemic therapies that incorporate, in addition to chemotherapy, agents with novel mechanisms of action.
The longer treatment times of modern therapies require the development of chemotherapy platforms that are simpler to administer with less acute and cumulative toxicities. Three randomized trials demonstrated improvements in response rate and survival in patients with CRC treated with irinotecan/5-flourouracil (5-FU) doublets over 5-FU in metastatic CRC [3–5]. The irinotecan and infusional 5-FU (FOLFIRI) regimen offered improved safety and efficacy over the bolus 5-FU (IFL) based combination [6]. However, disadvantages of an infusional 5-FU regimen are the complications and discomfort associated with the necessary indwelling central venous catheter. Capecitabine is an oral fluoropyrimidine with equivalent response rate and improved tolerability over bolus 5-FU [7, 8]. Several schedules of irinotecan and capecitabine have been reported. In phase-I trials, the maximal tolerated doses of weekly irinotecan and capecitabine were 70–150 and 2,000–2,500 mg/m2, respectively [9].
The cyclooxygenase (COX) enzymes catalyze the rate limiting step in the conversion of arachidonate to prostaglandin H2 (PGH2), the immediate substrate for prostaglandin, and thromboxane synthesis [10]. Prostaglandins play a critical role in carcinogenesis [11], angioneogenesis, and chemoresistance [12–14]. Cyclooxygenase-2 (COX-2) therefore represents a very attractive target in the therapy of CRC. It is preferentially expressed in most CRCs as compared to adjacent normal epithelium [11, 15]. COX-2 expression is associated with variables such as angiogenesis [13], inhibition of apoptosis [16, 17], and invasion, which portend to a poor prognosis [15, 18]. In preclinical systems, its blockage has sensitized colon cancer cells to cytotoxic therapy [19, 20], preliminary in vivo data exist for a biological activity of celecoxib in the interference of the natural history of CRC development [21]. Clinical trials suggested an improved safety profile of celecoxib in comparison to non-selective COX inhibitors with respect to gastrointestinal and renal toxicity [22, 23].
We therefore hypothesized that COX-2 inhibition by celecoxib would improve the outcome of patients with metastatic CRC treated with combination chemotherapy. We also hypothesized that a dose-attenuated schedule of weekly irinotecan and capecitabine would have improved tolerability and comparable efficacy to conventional irinotecan/fluoropyrimidine combination. In order to test these hypotheses, we identified two aims: (1) to evaluate the potential benefit (response rate) of the addition of celecoxib to chemotherapy and (2) to evaluate the toxicity of the weekly low-dose irinotecan schedule with capecitabine.
Materials and methods
Patient eligibility
Patients with a histologic or cytologic diagnosis of colorectal adenocarcinoma and radiological evidence of metastatic disease were eligible. The eligibility criteria also included no previous chemotherapy for CRC except for patients relapsing more than 6 months after completion of adjuvant chemotherapy. Patients were required to have a Southwest Oncology Group (SWOG) performance status of 0–2, and adequate hematologic, renal and hepatic function defined by the following parameters: neutrophil count ≥1,500 per mm3, platelet count ≥100,000 per mm3, creatinine ≤1.5 mg/dl, total serum bilirubin ≤1.5 mg/dl, and aspartate transaminase (AST) <3 times the upper limit of the institutional normal range (ULN). Patients were excluded if they had other active malignancy within the preceding year except for adequately treated basal cell, squamous cell skin cancer, or in situ cervical cancer. Additional exclusion criteria included active peptic ulcer disease within the preceding year, sulfa allergy, Gilbert's syndrome. All patients provided a signed informed consent in accordance with the Institutional Human Investigation Committee guidelines prior to enrolment on the study.
Study design and treatment plan
Irinotecan (Camptosar, Pfizer Crop., New York, NY) was administered at a dose of 70 mg/m2 intravenously over 30 min on days 1 and 8 of each treatment cycle. Capecitabine (Xeloda, Hoffman-La Roche, Nutely, NJ) was given at a dose of 1,000 mg/m2 orally twice per day on days 1– 14. Celecoxib (Celebrex, Pfizer Crop.) was administered orally at a dose of 400 mg twice per day. In order to achieve steady-state levels prior to initiation of chemotherapy, celecoxib was started on day 7. Celecoxib was continued until the end of study participation. Treatment cycles were repeated every 21 days. Compliance with celecoxib and capecitabine was monitored by pill counts and drug diaries.
Dose reductions were undertaken for irinotecan on day 8 and capecitabine at any time during the cycle based on the toxicity encountered within that cycle. Irinotecan was held for a neutrophil count of <1,000 per mm3 or platelets <50,000 per mm3. No dose adjustments for capecitabine or celecoxib were performed for hematologic toxicity. The irinotecan dose was reduced by 25% for Grade 2 diarrhea on day 8. Capecitabine was held for Grade 2 diarrhea and/or Grade 2 or higher hand foot syndrome and restarted when toxicity was down to Grade 1. Irinotecan and capecitabine were held for Grade 3 or 4 non-hematologic toxicity until recovery to Grade 1 or lower.
A new cycle of therapy could only begin if the neutrophil count was ≥1,500 per mm3, platelet count was ≥100,000 per mm3 and all relevant non-hematological toxicities were Grade 1 or lower. Dose adjustments on day 1 of a new cycle were based on the worst grade of toxicity in the preceding cycle as follows: patients who sustained either febrile neutropenia or Grade 3 or 4 myelosuppression received irinotecan at a dose of 75% of the commencing dose of the previous cycle. For Grade 2 or 3 non-hematologic toxicities excluding alopecia, hand foot syndrome, nausea, or vomiting the doses of irinotecan and capecitabine were reduced by 25%. A 50% dose reduction of irinotecan and capecitabine was performed for Grade-4 non-hematologic toxicity. Only the capecitabine dose was adjusted for hand foot syndrome. A 25% dose reduction of capecitabine was performed for Grade 2 or 3 hand foot syndrome. Celecoxib was withheld for patients who developed peptic ulcer disease or a rise in serum creatinine level until recovery. The celecoxib dose was not adjusted for other toxicities. Once the dose of any drug was reduced during a treatment cycle, re-escalation was not permitted in subsequent cycles.
Patients requiring a delay in therapy of longer than 2 weeks or who required more than two dose reductions because of toxicities were removed from the study. In addition, patients were removed from the study for either disease progression or withdrawal of the consent.
On-study evaluation
Imaging studies were performed at baseline and repeated after every two cycles of therapy or whenever there was clinical or biochemical suspicion of disease progression. Objective tumor responses (primary endpoint) were determined by the RECIST criteria and categorized as complete response, partial response, disease progression, or stable disease [24]. Objective responses required one additional confirmatory follow-up scan at least 3 weeks after the documentation of response. Response duration (RD) was measured from the start date of the best response until the date of progression. Overall survival (OS) was measured from study registration to date of death or last follow up. Time to progression (TTP) was measured from study registration to the date of first documented progressive disease, or death. Time to treatment failure (TTF) was measured from study registration to the date of first documented progressive disease, or date off treatment due to toxicity, patient refusal of further treatment, or death, whichever occurred first. Toxicities were evaluated at a minimum on day 1 of each cycle and graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 2.0 [25].
Statistical methods
This two-institution phase-II trial was planned with a Simon two-stage design [26]. The particular design chosen had near-optimal Simon-like properties, and resulted from the Simon algorithm modifications of Hintze [27]. The primary endpoint was complete or partial response (CR + PR). Therefore, the null hypothesis was that the true, unknown response rate was 35% or less, and the alternative hypothesis (which would indicate a successful study) was that the true, unknown response rate was 55% or more. We wished to distinguish these regions of the true, unknown response rate: at most 0.35 versus at least 0.55. The 2-stage design called for a maximum of 44 response evaluable (r-e) patients, 21 in Stage 1 and 23 in Stage 2. At least eight responses among the first 21 r-e patients were needed to justify beginning Stage 2 of accrual. Patients were considered r-e if they completed atleast two cycles of therapy. The design had a Type-I error of 0.092 and power of 0.905.
Exact, minimum-width 90% confidence intervals (CI) for response and toxicity rates were calculated using the Casella method [28] as implemented in StatXact software [29]. Standard Kaplan-Meier estimates of the censored RD, TTF, TTP, and OS distributions were computed. Due to the small sample sizes, survival statistics (e.g., median 1-year rate, etc.) were estimated more conservatively using linear interpolation [30] among successive event times on the Kaplan-Meier curves.
Results
Patient characteristics
Patients' characteristics are presented in Table 1. Fifty-one patients from two institutions (WSU and UM) were enrolled onto the study between June 2002 and November 2005. The median age at study entry was 58 years (range 32–78 years) with proportionally more males (61%). Seventy-six percent of the patients had a performance status of 1 or 2. Eighty-eight percent of patients had liver metastases and 65% had metastasis involving more than one site.
Table 1. Characteristics of the 51 patients with advanced colorectal cancer treated with irinotecan, capecitabine, and celecoxib.
| Characteristics Median age (range) | Number 58 (32–78) | Percentage |
|---|---|---|
| Sex | ||
| Male | 31 | 61 |
| Female | 20 | 39 |
| Race | ||
| African-American | 16 | 31 |
| Caucasian | 35 | 69 |
| Performance status | ||
| 0 | 12 | 24 |
| 1 | 38 | 75 |
| 2 | 1 | 2 |
| Metastatic site | ||
| Liver | 45 | 88 |
| Lung | 21 | 41 |
| Peritoneum | 6 | 12 |
| Other | 29 | 57 |
| Number of metastatic sites | ||
| 1 | 18 | 35 |
| 2 | 20 | 39 |
| 3 | 9 | 18 |
| 4 | 4 | 8 |
| Site of primary disease | ||
| Colon | 40 | 78 |
| Rectum | 11 | 22 |
| Prior adjuvant therapy | ||
| Chemotherapy | 8 | 16 |
| Chemoradiotherapy | 6 | 12 |
Treatment administration
A total of 354 cycles were administered with a median of eight cycles per patient (range 0–18). Forty-five patients received two or more cycles of therapy. Two patients received less than one cycle of therapy for the following reasons: one patient developed uncontrolled hiccups and the other had a very rapid decline in performance status. Four patients received only one cycle of therapy for the following reasons: three patients developed severe diarrhea, and one patient was unable to take oral medication. Twenty-three (45%) patients required dose reduction of capecitabine and 21 (41%) patients required a dose reduction of irinotecan. The most common causes for dose reductions were diarrhea and hand foot syndrome. There were no treatment-related deaths.
Subsequent treatment
Three patients were lost to follow up with no information available regarding their subsequent treatment. One patient continues on the current treatment. Six patients had no subsequent treatment after being off the study. Subsequent treatments included; oxaliplatin (36), bevacizumab (23), and cetuximab (11). Twelve patients received three or more lines of therapy and four patients underwent surgery for resection and/or ablation of liver metastases.
Objective response
Two patients (4%) had complete response. Nineteen (37%) patients had a partial response and 19 patients had stable disease. The overall response rate (CR + PR) based on an intent to treat analysis was 41% (95% CI, 0.28–0.55). The median RD was 6.1 months (95% CI, 5.1–7.5 months).
Time to progression, time to treatment failure, and survival
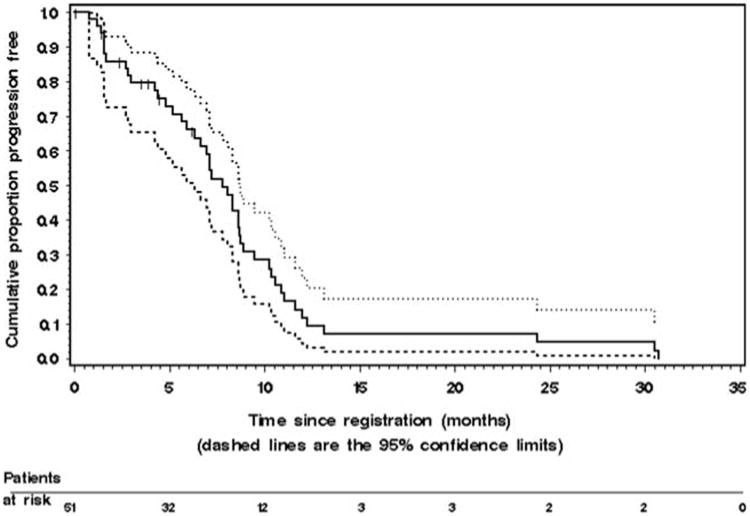
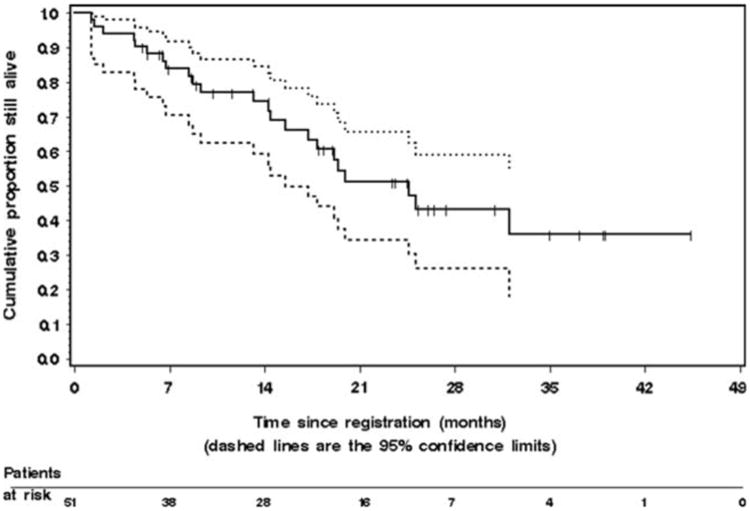
Analyses were performed on an intention to treat basis. The Kaplan–Meier estimate of TTP for the 51 patients is given in Fig. 1. The median TTP was 7.7 months (95% CI, 6.2– 8.6 months). The median time to treatment failure (which includes patients removed from study due to toxicity or patient request) was 5.9 months (95% CI, 4.4–7.0 months). Median survival time and probability of survival at 1 year were 21.2 months [95% CI, 13.8-not available months], and 75% (95% CI, 0.63–0.88), respectively. The Kaplan–Meier estimate of OS is given in Fig. 2. Twenty-eight patients are still alive for a censoring rate of 55%.
Fig. 1.
The Kaplan–Meier estimate of time to progression (TTP) in the 51 patients with metastatic colorectal cancer who were treated with irinotecan, capecitabine, and celecoxib. The dashed lines represent the 95% confidence interval (CI) about each successive estimate of the progression-free rate. The median TTP was 7.7 months (95% CI, 6.2–8.6 months). The 6-month TTP rate was 65% (95% CI, 0.52–0.79). The 12-month TTP rate was 12% (95% CI, 0.02–0.21)
Fig. 2.
The Kaplan–Meier estimate of overall survival in the 51 patients with metastatic colorectal cancer enrolled on the study. The dashed lines represent the 95% confidence interval (CI) about each successive estimate of the survival rate. The median survival was 21.2 months [95% CI, 13.8-not available months]. The 1-year survival rate was 75% (95% CI, 0.63–0.88). The 2-year survival rate was 48% (95% CI, 0.31–0.64)
Toxicity
All 51 patients began treatment and were evaluable for toxicity. The treatment was generally well tolerated in the outpatient setting. No treatment-related deaths were reported. Table 2 summarizes Grades 3 and 4 toxicities observed on the study. The most common toxicity was diarrhea. Grades 1 and 2 diarrhea were observed in 12 (24%) and 9 (18%) of patients, respectively. Grade 3 or 4 diarrhea was documented in 24 and 10% of patients, respectively. Grades 1 and 2 hand foot syndrome was observed in 11 (22%) and 7 (14%) of patients, respectively. Grade 3 or 4 hand foot syndrome was seen in 14 and 0% of patients, respectively. No Grade 3 or 4 thrombocytopenia or anemia was observed. Grade 3 neutropenia was present in 10% of the patients and no Grade-4 neutropenia was observed. Eleven (22%) patients were hospitalized for treatment related toxicity including: diarrhea (5), vomiting and dehydration (3), and infection (3). There were no cardiovascular complications potentially attributed to celecoxib, irinotecan, or capecitabine.
Table 2. The frequency of Grade 3 or 4 treatment related toxicities in 51 patients with metastatic colorectal cancer treated with celecoxib, capecitabine, and irinotecan.
| Type of toxicity | Grade 3 | Grade 4 | ||
|---|---|---|---|---|
|
|
|
|||
| Number | Percentage | Number | Percentage | |
| Neutropenia | 5 | 10 | 0 | 0 |
| Diarrhea | 12 | 24 | 5 | 10 |
| Fatigue | 3 | 6 | 1 | 2 |
| Nausea | 3 | 6 | 1 | 2 |
| Vomiting | 1 | 2 | 2 | 4 |
| Infection | 1 | 2 | 0 | 0 |
| Stomatitis | 1 | 2 | 0 | 0 |
| Hand foot syndrome | 7 | 14 | 0 | 0 |
The data are expressed as the worst toxicity per patient. Toxicity was assessed using the NCI-CTC Version 2.0 scale for toxicity grading [25]
Discussion
Irinotecan-based combination therapy for advanced CRC offers the following advantages: lack of major cumulative toxicities such as the neuropathy associated with prolonged oxaliplatin usage and the limitations of using oxaliplatin in an increasing number of patients with metastatic disease who have been exposed to the drug in the adjuvant setting. Randomized trials have demonstrated no significant difference between infusional 5-FU combined with either irinotecan or oxaliplatin in advanced CRC [31, 32]. With the established activity of capecitabine in advanced disease its incorporation in combination therapies has also been studied. Two schedules of irinotecan and capecitabine have been reported. In the first schedule irinotecan was administered every 3 weeks at a dose of 250–350 mg/m2[33]. Two randomized trials comparing this schedule with FOLFIRI have demonstrated inferior activity and increased toxicity of a capecitabine-based regimen [6, 34]. In the second schedule, irinotecan was administered weekly at a dose of 70–150 mg/m2 with comparable activity to FOLFIRI [6, 9]. The major advantage of the weekly irinotecan regimen is the low incidence of hematological toxicity, with the absence of Grade-4 myelotoxicity. Therefore, weekly irinotecan and capecitabine should be especially considered in patients with CRC and an increased risk of myelotoxicity. This group includes patients who may be deficient in uridine glucournosyl transferase (UGT), patients who had received prior radiation to the pelvic area, and patients who experienced prolonged myelosuppression with FOLFIRI regimen.
Although no treatment-related morality was observed in this study, the incidence of Grade 3 (24%) and Grade 4 (10%) diarrhea was significant. Fuchs et al. reported a randomized trial of capecitabine combined with weekly irinotecan (120 mg/m2) or every 3 weeks irinotecan (300 or 350 mg/m2) in previously treated metastatic CRC [6]. The incidence of Grade 3 or 4 diarrhea was significantly higher in the weekly irinotecan arm (36% compared to 19%, P = 0.002). The incidence of Grades 3 and 4 myelotoxicity was similar in both arms of the study (29% in the weekly versus 34% in the every three-week schedule). Therefore, the current dose-attenuated schedule of irinotecan appears to offer an improvement in myelotoxicity but no significant impact on the incidence of Grade 3 or 4 diarrhea. The lack of treatment related major sepsis and/or mortality is probably explained in the disassociation between severe gastrointestinal toxicities from severe neutropenia. It is also likely that that a reduction in the dose of capecitabine to 1,500– 1,750 mg/m2 per day will improve the frequency of Grade 3 or 4 diarrhea.
In the present study, the triple combination therapy of irinotecan, capecitabine, and celecoxib resulted in a response rate of 41% and a median time of progression of 7.7 months in patients with metastatic CRC. These results are comparable to those previously reported with oxaliplatin or irinotecan-based combination chemotherapy regimens in advanced CRC [31, 32, 35, 36]. Moreover, the duration of the responses and of disease stabilization were within the range seen in most combination chemotherapy regimens. It is therefore unlikely that celecoxib contributed significantly to the anti-tumor activity of this chemotherapy regimen based on response rate (the primary endpoint). The median 1- and 2-year survival rates of 21 months, 75 and 48%, respectively, indicate significant activity of the chemotherapy regimen with <50% of patients with bevacizumab in the second-line setting.
Two recently reported randomized trials evaluated the role of celecoxib in patients with advanced CRC. In the first trial, the addition of celecoxib to irinotecan-based chemotherapy resulted in an inferior response rate (26% vs. 46%) and median progression-free survival (6.9 months vs. 7.8 months). In the second trial, the addition of celecoxib to FOLFIRI/CAPIRI regimens had no apparent impact on response rate and progression-free survival.
This apparent lack of benefit of a COX-2 inhibitor may not necessarily rule out COX-2 enzyme as a target for drug development in CRC for the following reasons. First, patients, who went on this study and the other randomized trials, were not selected on the basis of tumoral expression/ activity of COX-2 or any other related biomarker. Second, even in the face of a high COX-2 expression, other collateral survival pathways will require additional agents in combination with celecoxib for effective blockade of survival pathways. Third, it is not known whether there is any pharmacokinetic interaction between celecoxib and either capecitabine or irinotecan. Finally, it is not known whether continued exposure to celecoxib influenced the expression of COX-2 in tumor cells and/or negatively altered the cell kinetics of tumor cells. For example, celecoxib can arrest cell cycle progression [37, 38] resulting in an antagonistic effect to S-phase-specific chemotherapeutic agents such as capecitabine. An intermittent schedule of administration of celecoxib following chemotherapy, possibly at higher doses, could result in chemosensitization without cell cycle arrest. Such schedules could be evaluated in preclinical models of CRC.
In conclusion, the anti-tumor activity of irinotecan and capecitabine did not appear to be significantly improved with concurrent administration of the COX-2 inhibitor celecoxib. Further, preclinical investigations are also needed to identify characteristics of CRC cell lines that could benefit from inhibition of the COX-2 pathway. Development of predictive markers of COX-2 blockade would facilitate the design of trials in CRC for patients who would potentially benefit from the addition of celecoxib to chemotherapy. The dose-attenuated schedule of weekly irinotecan (70 mg/ m2) and capecitabine (2,000 mg/m2 day) is active and has an improved safety profile with respect to myelosuppression and lack of major sepsis or treatment related deaths.
Acknowledgments
This study was supported in part by Cancer Center Support Grant CA-22453 from the National Cancer Institute and by Pfizer Pharmaceuticals and Roche.
Contributor Information
Bassel F. El-Rayes, Email: elrayesb@karmanos.org, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA.
Mark M. Zalupski, University of Michigan, Ann Arbor, MI, USA
Stephanie G. Manza, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA
Barbara Rusin, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA.
Ann Marie Ferris, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA.
Ulka Vaishampayan, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA.
Lance K. Heilbrun, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA
Raghu Venkatramanamoorthy, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA.
Anthony F. Shields, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA
Philip A. Philip, Karmanos Cancer Institute, Wayne State University, 4100 John R Street, Detroit, MI 48201, USA
References
- 1.Jemal A, et al. Cancer statistics. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23(36):9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 5.Kohne CH, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European organisation for research and treatment of cancer gastrointestinal group study 40986. J Clin Oncol. 2005;23(22):4856–4865. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Marshall J, Mitchell E, et al. A randomized trial of first-line irinotecan, fluoropyrimidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C) Presented at ASCO; Atlanta, GA: 2006. [Google Scholar]
- 7.Hoff PM, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19(8):2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Hoff PM, Harper P, et al. Oral capecitabine vs intravenours 5-fluorouracil and leucovorin: intregrated efficacy data and novel analyses from two lagre, randomized, phase III trials. Br J Cancer. 2004;90(6):1190–1197. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewes M, et al. Capecitabine and irinotecan as first-line chemotherapy in patients with metastatic colorectal cancer: results of an extended phase I study {dagger} Ann Oncol. 2003;14(9):1442–1448. doi: 10.1093/annonc/mdg376. [DOI] [PubMed] [Google Scholar]
- 10.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncolgene. 1999;18(55):7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 11.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 12.Tsujii M, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 13.Masunaga R, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6(10):4064–4068. [PubMed] [Google Scholar]
- 14.Bamba H, et al. Prostaglandins up-regulate vascular endothelial growth factor production through distinct pathways in differentiated U937 cells. Biochem Biophys Res Commun. 2000;273(2):485–491. doi: 10.1006/bbrc.2000.2969. [DOI] [PubMed] [Google Scholar]
- 15.Sano H, Kawahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55(17):3785–3789. [PubMed] [Google Scholar]
- 16.Ohd JF, Wikstro K, Sjolander A. Leukotrienes induce cell-survival signaling in intestinal epithelial cells. Gastroenterology. 2000;119(4):1007–1018. doi: 10.1053/gast.2000.18141. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, et al. Intracellular unesterified arachidonic acid signals apoptosis. PNAS. 2000;97(21):11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. PNAS. 1997;94(7):3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, et al. Combination of cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem Pharmacol. 2005;70(5):658–667. doi: 10.1016/j.bcp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Matsunga N, Yamanda M, Ohira M, et al. Combined treatment with selective cyclooxygenase-2 inhibitor and fluorinated pyrimidines for liver metastasis of colon cancer. Biochem Pharmacol. 2004;70(5):658–667. [PubMed] [Google Scholar]
- 21.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 22.Whelton A, Fort JG, Puma JA, et al. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and refecoxib in older hypertensive osteoarthritis patients. AM J Ther. 2001;8(2):89–95. doi: 10.1097/00045391-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Silverstein FE, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000;284(10):1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.CTEP. Common terminology criteria for adverse events, Version 2.0. 1999 Apr 30; ( http://www.ctep.cancer.gov), DCTD, NCI, NIH, DHHS.
- 26.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.Hintze J. Power and sample size (PASS) user's guide. NC-SS; Kaysville, UT: 2004. [Google Scholar]
- 28.Casella G. Refining binomial confidence intervals. CA J Stat. 1987;14:113–129. [Google Scholar]
- 29.Mehta C, Patel N. StatXact 6; statistical software for exact nonparametric inference, user manual. Cytel Software Corporation; Cambridge, MA: 2004. pp. 1–29. [Google Scholar]
- 30.Lee ET, Wang J. Statistical methods for survival data analysis. 3rd. Wiley; New York: 2003. pp. 76–91. [Google Scholar]
- 31.Tournigand C, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 32.Colucci G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the gruppo oncologico dell' italia meridionale. J Clin Oncol. 2005;23(22):4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs CS, et al. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21(5):807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 34.De Greve J, Koehne C, Hartmann J, et al. Capecitabine plus irinotecan versus 5-FU/FA/irinotecan ± celecoxib in first line treatment of metastatic colorectal cancer (CRC) Long-term results of the prospective multicenter EORTC phase III study 40015. Presented at ASCO; Atlanta, GA: 2006. [Google Scholar]
- 35.Goldberg RM, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Grothey A, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22(7):1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Grosch S, et al. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15(14):2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 38.Dvory-Sobol H, et al. Celecoxib leads to G2/M arrest by induction of p21 and down-regulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer. 2006;42(3):422–426. doi: 10.1016/j.ejca.2005.11.009. [DOI] [PubMed] [Google Scholar]