Abstract
Given the limited information on Clostridium difficile infection (CDI) during hematopoietic stem cell transplantation (HSCT), we examined the recent epidemiology of CDI in HSCT recipients at our institution. During the two-yr retrospective study period (2005–2006), 361 transplants were performed: 60% allogeneic and 40% autologous. Among all hospitalized patients in a non-outbreak setting, CDI rates in HSCT recipients were ninefold higher than those in general patients and 1.4-fold higher than those in patients with cancer (24.0 vs. 2.6 vs. 16.8/10 000 patient-days respectively). Sixty-two episodes of CDI occurred in 51 (14%) HSCT recipients: 39 (18%) allogeneic vs. 12 (8%) autologous (p = 0.01). Almost half of CDI episodes occurred within 30 d post-HSCT and 22% before HSCT. Clostridium difficile toxin assay was initially positive in 28% of the first, 31% of the second and 27% of the third stool samples tested. All but one patient responded to therapy with metronidazole or vancomycin. Severe CDI occurred in one patient and recurrent CDI in two patients. CDI is common during HSCT especially in allogeneic transplants during the peri-HSCT period. Prospective studies to better define the epidemiology and identify unique risk factors for CDI and more accurate tests to confirm the diagnosis in this population are needed.
Keywords: Clostridium difficile infection, epidemiology, hematopoietic transplantation
Diarrhea occurs in almost 90% of patients undergoing hematopoietic stem cell transplantation (HSCT) (1-4). Of the multiple causes including chemotherapy and graft-versus-host disease (GVHD) of the gastrointestinal tract, Clostridium difficile infection (CDI) accounts for 1.3–20.4% of all diarrheal illnesses in HSCT recipients (1, 3, 4).
The epidemiology of CDI has dramatically changed in recent years (5). In the United States, the number of hospitalized patients with a discharge diagnosis of CDI has doubled since the year 2000, with an estimated 178 000 CDI cases in 2003 (6). Moreover, a new virulent strain (NAP1) that produces excessive amounts of toxins A and B has caused outbreaks of severe CDI (5, 7, 8). Yet, there are limited reports on the recent epidemiology of CDI in HSCT recipients (1, 9-11). Until recently, CDI has been considered a “nuisance disease with minor morbidity” during HSCT and has not received the same attention as invasive fungal or viral infections.
We studied the epidemiology of CDI in HSCT recipients at our institution from 2005 to 2006 and determined the rates of CDI among HSCT recipients compared to hospitalized oncology and general patient groups. The characteristics of CDI in autologous and allogeneic HSCT recipients were evaluated. Finally, the utility of enzyme immunoassay (EIA) testing for C. difficile toxin was examined in the HSCT population.
Methods
The study was conducted at the Karmanos Cancer Center and Harper University Hospital. This tertiary care center has approximately 450 acute care beds, with oncology and HSCT recipients being housed in a 100-bed wing within the same building. The study was conducted in accordance with the guidelines of the institutional review board of Wayne State University.
Retrospective review was performed of the medical records of patients who underwent HSCT between January 1, 2005, and December 31, 2006, and met the definition for CDI. A case of CDI was defined as a patient with diarrhea and a positive result of a laboratory assay for C. difficile toxin in the stool, and/or endoscopic or histopathologic evidence of pseudomembranous colitis. Recurrent CDI was defined as repeated episodes of CDI within eight wk. Severe CDI was defined as CDI necessitating admission to an intensive care unit, resulting in colectomy or death within 30 d after disease onset (12). Response to therapy was defined as resolution of diarrhea and abdominal symptoms.
Patient data were reviewed for the 30 d preceding CDI diagnosis and for 60 d after to identify risk factors, CDI recurrences, and to determine mortality. Patient characteristics, clinical features, laboratory data, therapy and outcomes were evaluated. In patients with recurrent CDI only, the initial episode was considered in the analysis. The Charlson’s Comorbidity Index was used to grade the severity of comorbid illnesses (13). Rates of CDI in HSCT, oncology and general patient groups were determined from surveillance data that were obtained by the Hospital’s Epidemiology Department in accordance with national guidelines (14). Detection of C. difficile toxin in the stool was performed by EIA (C. difficile tox A/B II™ assay, laboratory assay; Wampole, Princeton, NJ, USA) that detects both toxin A and toxin B. CDI was classified by exposure setting using the Centers for Disease Control and Prevention (CDC) surveillance definitions (12). Routine peri-transplant antimicrobial prophylaxis at our institution included norfloxacin, acyclovir and fluconazole begun on day 7 (day 0 being the day of HSCT) of transplantation and continued until engraftment.
Statistical methods
Descriptive statistics were used to summarize the categorical and continuous variables. Dichotomous categorical variables were compared by HSCT type (autologous vs. allogeneic) using Fisher’s exact test (two-sided). Continuous categorical variables were compared by HSCT type using the independent sample t-test (two-sided). No adjustments were made for multiple comparisons.
Results
During the two-yr study period, the CDI rate/10 000 patient-days among HSCT recipients was 24.0 compared to 2.6 in general patients and 16.8 in oncology patients. Epidemiological investigation identified no clusters or outbreaks of CDI in the HSCT or other patient groups.
Of the 361 HSCTs performed, 216 (60%) were allogeneic and 145 (40%) were autologous transplants. Sixty-two episodes of CDI were identified in 51 (14%) of HSCT recipients. CDI occurred in 39 (18%) allogeneic compared to 12 (8%) autologous recipients (p = 0.01). The characteristics of all patients with CDI are summarized in Table 1. Most (82%) of the allogeneic HSCT recipients received a preparative regimen consisting of busulphan, cytosine arabinoside and cyclophosphamide.
Table 1.
Characteristics of 51 hematopoietic stem cell transplantations recipients with Clostridium difficile infection
| Characteristic(s) | Mean ± SD or N (%) |
|---|---|
| Demographics | |
| Age (yr) | 42.9 ± 18.1 |
| Men | 29 (57) |
| Caucasians | 44 (86) |
| Transplant characteristics | |
| Type of transplant | |
| Autologous | 12 (8) |
| Allogeneic | 39 (18) |
| Underlying malignancy | |
| Acute myelogenous leukemia | 15 (29) |
| Non-Hodgkin’s lymphoma | 12 (24) |
| Acute lymphocytic leukemia | 7 (14) |
| Hodgkin’s lymphoma | 4 (8) |
| Neuroblastoma | 3 (6) |
| Chronic myelogenous leukemia | 2 (4) |
| Multiple myeloma | 2 (4) |
| Othersa | 6 |
SD, standard deviation.
One patient each with chronic lymphocytic leukemia, myelodysplastic syndrome, paroxysmal nocturnal hemoglobinuria, T-cell lymphoma, mantel-cell lymphoma and medulloblastoma.
Using the CDC surveillance criteria, 92% of the CDI episodes were health-care-facility (HCF)-associated. The mean duration from hospital admission to diagnosis of CDI was 19 d, with 18% identified within two d and 42% within 2–14 d of admission. The mean duration from HSCT to CDI diagnosis was 13 d. The temporal distribution of CDI with regard to HSCT is shown in Fig. 1. Most cases of CDI (71%) occurred in the peri-transplant period, within 30 d before or after HSCT.
Fig. 1.
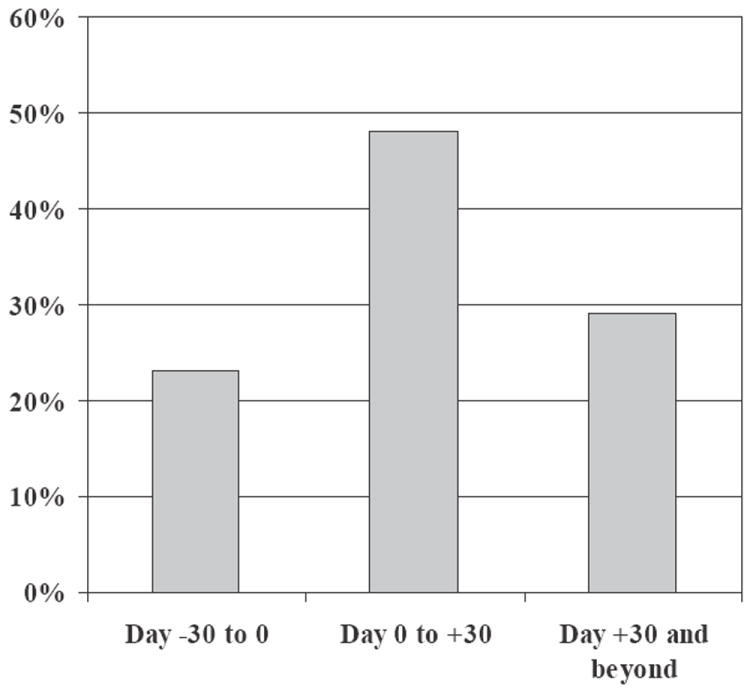
Temporal relationship of Clostridium difficile infection to hematopoietic stem cell transplantation.
The characteristics of the CDI among allogeneic and autologous HSCT recipients are compared in Table 2. There were no significant differences in the demographics of the allogeneic and autologous HSCT recipients. The mean duration from HSCT to CDI diagnosis was similar in both groups. Conventional risk factors for CDI including exposure to antibiotics, use of proton pump inhibitors, total parenteral nutrition, prior gastrointestinal procedures and low albumin were comparable in the allogeneic and autologous groups. All patients had received more than one antibiotic within 30 d of CDI diagnosis. Exposure to the quinolones, especially norfloxacin that was used as prophylaxis during neutropenia, occurred in 64% of cases. Exposure to cefepime, used for empiric therapy of febrile neutropenia, was noted in 74% of cases. Allogeneic recipients had a slightly higher mean Charlson’s Comorbidity Index (2.5 vs. 2). Eighteen of the allogeneic HSCT recipients with CDI had GVHD, of whom 13 had GVHD involving the gastrointestinal tract.
Table 2.
Characteristics of 51 hematopoietic stem cell transplantation (HSCT) recipients with Clostridium difficile infection (CDI) by type of transplant
| Characteristic(s) | Allogeneic (N = 39) | Autologous (N = 12) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 44.6 ± 15.9 | 37.4 ± 23.9 | 0.34 |
| Men (%) | 22 (56) | 7 (58) | 1.0 |
| Caucasian ethnicity (%) | 33 (85) | 11 (92) | 1.0 |
| Onset of CDI | |||
| Time from HSCT to CDI (d) | 13.8 ± 16.4 | 11.1 ± 17.1 | 0.65 |
| Time from admission to CDI (d) | 19.6 ± 20.15 | 13.5 ± 13.1 | 0.15 |
| Conventional risk factors for CDI | |||
| General risk factors | |||
| Antibiotica (%) | 39 (100) | 12 (100) | NA |
| Total number of prior antibiotics | 2.6 ± 1.1 | 2.3 ± 1.1 | 0.5 |
| Quinolones (%) | 24 (62) | 8 (67) | 1.0 |
| Cefepime (%) | 29 (74) | 8 (67) | 0.7 |
| Imipenem (%) | 6 (15) | 2 (17) | 1.0 |
| Vancomycin (%) | 20 (51) | 5 (42) | 0.74 |
| Proton pump inhibitors (%) | 23 (59) | 5 (42) | 0.34 |
| Prior gastrointestinal surgery or procedure (%) | 7 (18) | 1 (8) | 0.7 |
| Diabetes (%) | 8 (21) | 0 | 0.1 |
| Nasogastric tube (%) | 1 (3) | 0 | 1.0 |
| Total parenteral nutrition (%) | 10 (26) | 3 (25) | 1.0 |
| Low albumin (<2.5 g/dL) (%) | 30 (77) | 9 (75) | 1.0 |
| Charlson’s Comorbidity Index | 2.5 ± 1.04 | 2.0 ± 0.29 | 0.02 |
| Risk factors related to HSCT | |||
| Number of chemotherapy agents in preparative regimen | 2.7 ± 1.97 | 2.5 ± 1.62 | 0.64 |
| Radiation as part of preparative regimen (%) | 8 (21) | 1 (8) | 0.7 |
| Neutropenia at onset of CDI (%) | 22 (56) | 8 (67) | 0.73 |
| Clinical features of CDI | |||
| Abdominal pain (%) | 8 (21) | 3 (25) | 0.7 |
| Fever (%) | 8 (21) | 3 (25) | 0.7 |
| Treatment of CDI | |||
| Metronidazole (oral/IV) (%) | 33 (85) | 10 (83) | 1.0 |
| Vancomycin alone (oral) | 0 | 0 | N/A |
| Metronidazole (oral/IV) plus vancomycin (oral)b (%) | 6 (15) | 2 (17) | 1.0 |
| Duration of treatment (d)c | 11.4 ± 3.9 | 10.7 ± 1.6 | 0.5 |
| Complications of CDI | |||
| Severe CDI | 0 | 1 (8%) | 0.24 |
| Recurrent CDI | 2 (5%) | 0 | 1.0 |
| Death | 0 | 0 | N/A |
Table entries are either mean ± SD or N (%).
N/A, not applicable.
All patients received >1 antibiotic during the prior 30-d period.
All patients received metronidazole as initial therapy that was switched to oral vancomycin in 15% of allogeneic and 17% of autologous HSCT recipients owing to drug intolerance.
The exact duration of therapy was available in 28 allogeneic and six autologous transplant recipients.
The clinical features of CDI were comparable in the allogeneic and autologous groups. Metronidazole was used in all cases as initial therapy for a mean duration of 11 d. Metronidazole was given by oral route in 37 patients and parenteral route in 14 patients as a result of oral intolerance. Therapy in eight patients was switched to oral vancomycin as a result of intolerance to metronidazole. All but one of the patients responded to therapy with resolution of diarrhea and abdominal symptoms. One autologous recipient developed severe CDI resulting in ICU admission. Two allogeneic HSCT recipients (4%) developed recurrent CDI. The initial episode of CDI in both patients occurred just prior to the HSCT, and they subsequently developed a total of 10 episodes of CDI after HSCT.
Clostridium difficile toxin assay was positive for the first time in 28% of the first, 31% of the second and 27% of the third stool sample tested. A total of 27.4% (n = 14) patients had the first test negative and second positive and 16% (n = 8) had the first and second tests negative and third positive. In patients with an initial negative test and a subsequent positive test, the mean interval between the two tests was 5.6 d. None of the patients had tests performed on consecutive days.
Discussion
Our report of 62 episodes of CDI in 51 of 361 HSCT recipients is the largest study of CDI in HSCT and is the first to report the comparative rates of CDI in HSCT and other hospitalized patient populations. In our study of patients hospitalized within the same building in a non-outbreak setting, the rate of CDI was ninefold higher in HSCT recipients compared to the general hospitalized population and 1.4-fold higher than that of other oncology patients. Substantial antibiotic usage, exposure to chemotherapy agents and prolonged hospital stay may be some of the risk factors for the high rate noted in HSCT recipients. CDI accounted for up to 11% of diarrheal episodes in HSCT recipients in the years before 2000 (7, 15), in contrast to rates as high as 20% reported in more recent studies in HSCT patients (1, 9-11). This recent increase in rates of CDI among HSCT recipients appears to parallel the increase in CDI in the general population (16).
CDI occurred significantly more often in our allogeneic recipients than in the autologous recipients (18% vs. 8%, p = 0.01). This is in contrast to the two prior studies of CDI in both autologous and allogeneic HSCT recipients that reported CDI primarily among the autologous patients (1, 4). Both allogeneic and autologous recipients in our study had one or more of the previously described risk factors for CDI including the use of antibiotics, H2 blockers, proton pump inhibitors, gastrointestinal procedures, enteral tubes and feedings, chemotherapy, total body irradiation, and low albumin (2, 14). Receipt of cephalosporins has been reported to be a risk factor for CDI in allogeneic transplants (17). Cephalosporins and quinolones have minimal activity against C. difficile and have been postulated to increase the risk of CDI, compared to antibiotics such as the penicillins that possess better activity against C. difficile (17, 18). In the setting of endemic CDI in HSCT population, the routine use of quinolone prophylaxis during neutropenia and the use of cefepime for empiric therapy of febrile neutropenia may need re-evaluation.
Like prior studies of CDI in HSCT, we were unable to discern any unique risk factors for CDI attributable to allogeneic or autologous HSCT (2). In allogeneic recipients with CDI, pre-existing GVHD was present in approximately half (18 of 39) of the cases and most of these patients (13 of 18) had GVHD of the gastrointestinal tract. The development of new-onset GVHD as well as worsening of pre-existing GVHD after CDI has been reported (9). However, none of our patients developed new-onset GVHD or worsening GVHD after CDI. The exact relationship between CDI and GVHD needs further elucidation.
The mean time to onset of CDI from HSCT in our study was approximately two wk, which is comparable to mean time to onset of 4–38 d reported in prior studies (9, 11). Importantly, 22% of CDI occurred just before HSCT. Prior studies have only focused on CDI post-HSCT and hence may have underestimated the true burden of CDI during HSCT. Nearly 70% of CDI occurred within 30 d before to 30 d after HSCT. Thus, all HSCT recipients with diarrheal illness that occurs early during the peri-HSCT period should be carefully evaluated for CDI. The predominance of CDI in the peri-HSCT period suggests that conventional risk factors such as exposure to antibiotics and conditioning regimens likely play a significant role in the pathogenesis of CDI in this population.
The most commonly used confirmatory tests for the diagnosis of CDI are EIAs that detect C. difficile toxins A and B. EIAs for CDI have a sensitivity of 32–73% (19); however, these assays have not been validated in the setting of HSCT. Repeated testing with EIA for diagnosis of CDI is not recommended in the general population (19). However, in our study among HSCT recipients, the first EIA test was positive in only 28% of cases, second test in 31% and the third test in 27% of cases. Given the diverse causes of diarrhea in HSCT recipients, it is possible that CDI was absent during the initial testing and occurred later in the diarrheal illness. An initial negative EIA test in this population could also imply that the test lacked sufficient sensitivity. The “gold standard” anaerobic toxigenic C. difficile culture and cytotoxin cell assay have long turnaround times and are labor-intensive (19). Newer more sensitive and specific tests such as two-stage glutamate dehydrogenase antigen assay followed by cytotoxin neutralization or rapid C. difficile PCR assays may be useful and should be validated in this population (19, 20).
All patients with diarrhea and a positive test for CD toxin responded to specific therapy for CDI with resolution of diarrhea and abdominal symptoms. All received metronidazole as initial therapy; oral vancomycin was restricted by formulary guidelines for use only in patients with severe CDI or intolerance to metronidazole (21). All but one patient responded to treatment; serious complications or recurrences were infrequent, and there were no deaths. The good response to therapy with metronidazole was expected as almost all patients had non-severe CDI. The relative lack of severe CDI in our HSCT recipients is similar to that reported in most prior studies (3, 9, 15). However, it is noteworthy that in a recent report by Dubberke, 57% of 37 allogeneic HSCT recipients with CDI were classified as having severe disease with an increase in mortality during the 180-d period of follow-up (22). The high rate of severe CDI noted may be in part attributable to the less stringent definition of severe disease or to factors such as differences in the HSCT population or to the presence of more virulent strains of C. difficile such as the NAP1 strain. The rate of recurrent CDI in our study is lower than the 15–30% rate reported in the general population (23). The risk factors associated with recurrent CDI include severity of initial episode and advanced age (24). The low recurrence rate in our study may be attributable to lack of severe cases and the younger age of our patients.
As was expected, almost all cases of CDI (92%) in our study were classified as HCF-associated (prior hospitalization within eight wk of CDI) (14). However, it is noteworthy that 22% of the HCF-associated cases had onset of symptoms in the community. This is consistent with the increasing rates of community-onset CDI recently reported in the general population (25, 26). CDI should no longer be considered strictly as a nosocomial disease with symptom onset only after hospital admission.
Our study has the inherent limitations of a retrospective design and was performed at a single center. However, some key observations have been made. Patients undergoing HSCT were at the highest risk of CDI among the various patient populations hospitalized at our tertiary care institution. The majority of CDI occurred during the peri-transplant period, and allogeneic HSCT recipients were more likely than autologous HSCT recipients to develop CDI. Sensitivity of the diagnostic EIA test for CDI appears to be low. Prospective studies are needed to better understand the epidemiology of CDI and to evaluate the utility of newer molecular diagnostic tests for CDI in the HSCT population.
Acknowledgments
None.
Dr. Heilbrun and Mr. Smith are supported in part by National Institutes of Health Cancer Center Support Grant CA-22453.
Footnotes
Preliminary data were presented in part at the 45th Annual Meeting of the Infectious Diseases Society of America, San Diego, CA, USA (October 4–7, 2007).
Conflict of interest: None of the authors have any conflict of interest related to this study.
References
- 1.van Kraaij MG, Dekker AW, Verdonck LF, et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant. 2000;26:299. doi: 10.1038/sj.bmt.1702484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobak D, Arfons LM, Creger RJ, Lazarus HM. Clostridium difficile-associated disease in human stem cell transplant recipients: coming epidemic or false alarm? Bone Marrow Transplant. 2008;42:705. doi: 10.1038/bmt.2008.317. [DOI] [PubMed] [Google Scholar]
- 3.Avery R, Pohlman B, Adal K, et al. High prevalence of diarrhea but infrequency of documented Clostridium difficile in autologous peripheral blood progenitor cell transplant recipients. Bone Marrow Transplant. 2000;25:67. doi: 10.1038/sj.bmt.1702086. [DOI] [PubMed] [Google Scholar]
- 4.Tomblyn M, Gordon L, Singhal S, et al. Rarity of toxigenic Clostridium difficile infections after hematopoietic stem cell transplantation: implications for symptomatic management of diarrhea. Bone Marrow Transplant. 2002;30:517. doi: 10.1038/sj.bmt.1703703. [DOI] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 6.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavan P, Sochor M, Nyc O, et al. Pseudomembraneous clostridium after autologous bone marrow transplantation. Bone Marrow Transplant. 1998;21:521. doi: 10.1038/sj.bmt.1701117. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti S, Lees A, Jones SG, Milligan DW. Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transplant. 2000;26:871. doi: 10.1038/sj.bmt.1702627. [DOI] [PubMed] [Google Scholar]
- 10.Gorschluter M, Glasmacher A, Hahn C, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2001;33:786. doi: 10.1086/322616. [DOI] [PubMed] [Google Scholar]
- 11.Arango JI, Restrepo A, Schneider DL, et al. Incidence of Clostridium difficile-associated diarrhea before and after autologous peripheral blood stem cell transplantation for lymphoma and multiple myeloma. Bone Marrow Transplant. 2006;37:517. doi: 10.1038/sj.bmt.1705269. [DOI] [PubMed] [Google Scholar]
- 12.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 13.Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 15.Bilgrami S, Feingold JM, Dorsky D, et al. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1039. doi: 10.1038/sj.bmt.1701773. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263. doi: 10.1016/j.ajic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 18.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 19.Peterson LR, Robicsek A. Does my patient have Clostridium difficile infection? Ann Intern Med. 2009;151:176. doi: 10.7326/0003-4819-151-3-200908040-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kvach EJ, Ferguson D, Riska PF, Landry ML. Comparison of BD GeneOhm Cdiff real-time PCR assay with a two-step algorithm and a toxin A/B enzyme-linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection. J Clin Microbiol. 2010;48:109. doi: 10.1128/JCM.01630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 22.Dubberke ER, Reske KA, Srivastava A, et al. Clostridium difficile-associated disease in allogeneic hematopoietic stem-cell transplant recipients: risk associations, protective associations, and outcomes. Clin Transplant. 2010;24:192. doi: 10.1111/j.1399-0012.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Palmore TN, Sohn S, Malak SF, Eagan J, Sepkowitz KA. Risk factors for acquisition of Clostridium difficile-associated diarrhea among outpatients at a cancer hospital. Infect Control Hosp Epidemiol. 2005;26:680. doi: 10.1086/502602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney JA, Dial S, Barkun A, Suissa S. Antimicrobial drugs and community-acquired Clostridium difficile-associated disease, UK. Emerg Infect Dis. 2007;13:761. doi: 10.3201/eid1305.061124. [DOI] [PMC free article] [PubMed] [Google Scholar]


