Summary
In photoreceptors, the assembly of signaling molecules into macromolecular complexes is important for phototransduction and maintaining the structural integrity of rod outer segments (ROSs). However, the molecular composition and formation of these complexes are poorly understood. Using immunoprecipitation and mass spectrometry, 4.1G was identified as a new interacting partner for the cyclic-nucleotide gated (CNG) channels in ROSs. 4.1G is a widely expressed multifunctional protein that plays a role in the assembly and stability of membrane protein complexes. Multiple splice variants of 4.1G were cloned from bovine retina. A smaller splice variant of 4.1G selectively interacted with CNG channels not associated with peripherin-2–CNG channel complex. A combination of truncation studies and domain-binding assays demonstrated that CNG channels selectively interacted with 4.1G through their FERM and CTD domains. Using immunofluorescence, labeling of 4.1G was seen to be punctate and partially colocalized with CNG channels in the ROS. Our studies indicate that 4.1G interacts with a subset of CNG channels in the ROS and implicate this protein–protein interaction in organizing the spatial arrangement of CNG channels in the plasma membrane of outer segments.
Key words: Retina, Photoreceptors, Outer segments, CNG channels, 4.1 proteins, 4.1G, Mass spectrometry
Introduction
Assembly of proteins into macromolecular complexes within cellular compartments facilitates signal transduction by providing speed and specificity. In rod photoreceptor cells, the special arrangement of protein complexes is partly achieved through the highly ordered structure of the outer segment, consisting of a stack of discs enclosed by a plasma membrane. Each disc is made of double membranes embedded with the light-sensitive visual pigment rhodopsin and circumscribed by a specialized hairpin margin referred to as the disc rim. The disc rim and the plasma membrane of the outer segment each have a distinct protein composition (Molday and Molday, 1987), and are connected through dynamic complexes formed by proteins within these structures. Immunoprecipitation and cross-linking studies have shown that the rod photoreceptor cyclic-nucleotide-gated (CNG) channel forms a complex with the Na+/K+-Ca2+ exchanger in the plasma membrane (Molday and Molday, 1998; Schwarzer et al., 2000) and the peripherin-2–rom-1 complex on the disc rim (Poetsch et al., 2001). The latter interaction, involving the glutamic-acid-rich protein (GARP) domain of the channel, is supported by a recent in situ fluorescence complementation study in Xenopus laevis rod photoreceptors (Ritter et al., 2011). These protein interactions not only facilitate phototransduction, but also help to stabilize the highly ordered structure of the outer segment (Zhang et al., 2009). To date, little is known about additional proteins that may constitute these complexes.
Members of the protein 4.1 family play a crucial role in the assembly and stability of protein complexes in the plasma membrane. The first member to be discovered, 4.1R, was shown to be essential for maintaining normal cell shape by connecting plasma membrane proteins to the spectrin-actin cytoskeleton in erythrocytes (Tyler et al., 1979; Ungewickell et al., 1979). To date, four additional 4.1 family members have been described: 4.1G [general (Parra et al., 1998; Walensky et al., 1998)], 4.1N [neural (Walensky et al., 1999)], 4.1B [brain (Parra et al., 2000)] and 4.1O [ovary (Ni et al., 2003)]. All members of the 4.1 family share the membrane-binding (FERM) domain, the spectrin-actin-binding (SAB) domain, the C-terminal domain (CTD), and non-conserved regions at the N-terminus (U1) and between domains (U2, U3).
The most widely expressed homolog, 4.1G, is abundantly expressed in many tissues including the nervous system (brain, spinal cord, Schwann cells, microglia and retina) (Ohno et al., 2005; Ohno et al., 2006; Rose et al., 2008), heart (Pinder et al., 2012), testis (Ohno et al., 2005; Terada et al., 2010) and adrenal gland (Wang et al., 2010). The gene encoding 4.1G has one translation initiation site but undergoes extensive, tissue-specific alternative splicing, giving rise to many isoforms, some of which lack the SAB domain (Wang et al., 2010; Yang et al., 2011).
Various physiological functions have been assigned to 4.1G because of its ubiquitous expression and its increasing number of interacting partners, the majority of which are ion channels and receptors, including SERC2 (Pinder et al., 2012), GluR1 and GluR4 (Coleman et al., 2003), parathyroid hormone receptor (Saito et al., 2005), metabotropic glutamate receptor (Tateyama and Kubo, 2007) and adenosine receptor (Lu et al., 2004a; Lu et al., 2004b). 4.1G has been implicated in increasing the surface membrane localization of these channels and receptors, and more specifically, directing proteins to lipid rafts where specific networks of signaling proteins congregate in the plasma membrane (Gibson et al., 2012). In the nervous system, 4.1G is required for the precise localization of glial adhesion molecules and axonal proteins in the internodes (Ivanovic et al., 2012). 4.1G is also essential for the assembly of tight junction protein complexes in neuroglia (Xia and Liang, 2012) and the assembly of extracellular matrix adhesion sites in astrocytes (Jung and McCarty, 2012).
In the retina, 4.1G has been found in the neuronal synaptic layers, as well as in the photoreceptor layer (Rose et al., 2008), using immunofluorescence microscopy. An earlier proteomics study also detected the presence of 4.1G in bovine rod photoreceptor outer segment (ROS) preparations (Kwok et al., 2008). However, the role of 4.1G in photoreceptors remains elusive. In this study, we have identified 4.1G as a binding partner of the CNG channel in photoreceptor rod outer segments and examined its mode of interaction.
Results
4.1G interacts with the rod cyclic nucleotide-gated channel
To identify interacting partners of the rod cyclic nucleotide-gated (CNG) channel, an antibody against the beta (CNGB1) subunit was used to immunoprecipitate the channel and associated proteins from bovine ROS for analysis by mass spectrometry. Beside the two CNG channel subunits (CNGA1 and CNGB1), 4.1G (also known as 112 kDa protein) was detected with a high level of confidence (Table 1A). A total of 14 peptides of 4.1G were found of which 11 were unique. In addition, peripherin-2 (peripherin/Rds) and sodium/potassium/calcium exchanger (SLC24A1) were also among the top proteins identified, confirming the previously established interaction of these proteins as a complex (Molday and Molday, 1998; Poetsch et al., 2001; Schwarzer et al., 2000).
Table 1. Proteins identified by mass spectrometry analysis from co-immunoprecipitation experiments.
Significant results (P<0.05) with a score >70 are shown.
Databases used were IPI_Bovine BOVINE_v_315 for A and SwissProt 57.1 for B and C.
emPAI, exponentially modified protein abundance index, is an approximate relative quantification of the proteins in a mixture and is derived from the ratio of the number of experimentally observed peptides over the number of expected peptides for a protein.
Common contaminants such as keratin and trypsin have been omitted. Scoring is probability-based and is reported as −10*Log10(P) where P is the absolute probability.
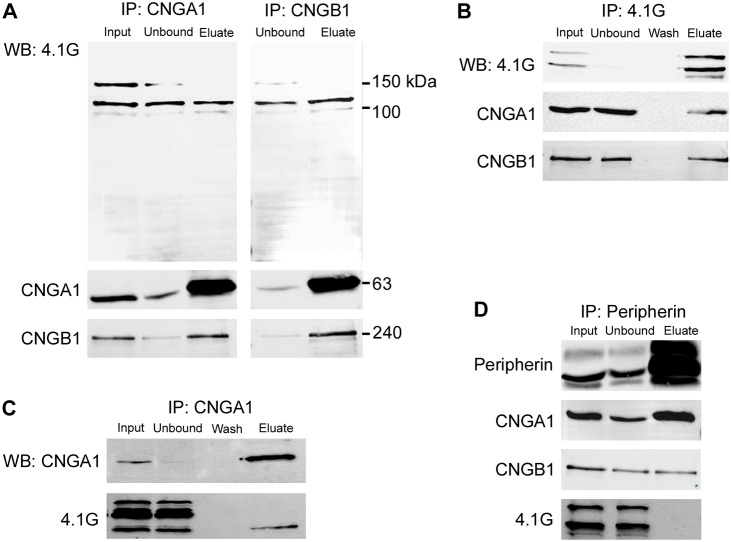
Western blotting of the immunoprecipitates using the polyclonal antibody against the C-terminus of 4.1G confirmed the interaction and further revealed that the 4.1G polyclonal antibody recognized two dominant variants in ROSs (Fig. 1A, input). Only the smaller 4.1G variant co-immunoprecipitated with CNGB1 (Fig. 1A, eluate). Other weaker bands were detected by the 4.1G antibody, but whether these bands are additional variants of 4.1G or degraded products remains to be determined. Immunoprecipitation with the anti-CNGA1 antibodies also pulled down 4.1G from bovine ROSs supporting their interaction as a complex (Fig. 1A).
Fig. 1.
Association of 4.1G with CNG channels. (A) Co-immunoprecipitation of 4.1G with CNG channels. Bovine rod outer segment (ROS) extracts were immunoprecipitated with antibodies against CNGA1 (PMc-1D1; left panel) and CNGB1 (Garp-8G8; right panel). The fractions (input, unbound and eluate) were separated by SDS-gel electrophoresis and the immunoblots labeled with anti-4.1G antibody (top), anti-CNGA1 antibody (middle) or anti-CNGB1 antibody (bottom). Note that only the smaller 4.1G variants were pulled down with the CNG channels. The larger 4.1G variant that was absent from the bound fraction was present in diminished amount in the unbound fraction compared to the input fraction, possibly as a result of nonspecific adsorption to the immunoaffinity matrix or proteolytic degradation. (B) Reverse co-immunoprecipitation of CNG channels with 4.1G. Bovine ROS extracts were immunoprecipitated with antibody against 4.1G. Western blots were labeled with anti-4.1G antibody (top), anti-CNGA1 antibody (middle) or anti-CNGB1 antibody (bottom). (C) Co-immunoprecipitation of 4.1G with CNGA1. Mouse retinal membranes were immunoprecipitated with antibody against CNGA1. Western blots were labeled with anti-CNGA1 (top) or anti-4.1G (bottom) antibodies. Note that similar to the observation in bovine ROS, only the smaller 4.1G variant was pulled down with the CNGA1. (D) Co-immunoprecipitation of CNG channels with peripherin-2. Bovine ROS extracts were immunoprecipitated by anti-peripherin antibody (Per-2B6). Western blots were labeled with anti-peripherin-2, anti-CNGA1, anti-CNGB1 or anti-4.1G antibody. Note that 4.1G was not pulled down with peripherin-2 or this population of CNG channels.
To further establish their interaction, the 4.1G polyclonal antibody was used to immunoprecipitate 4.1G and associated proteins from bovine ROSs. The top proteins identified with a high level of confidence were 4.1G (Epb41l2) and the CNGA1 and CNGB1 channel subunits (Table 1B). At the time of the analysis, the complete sequence of bovine 4.1G had not been published. Hence, only peptides of the mouse and human 4.1G were identified. However, these peptides are completely conserved with those from bovine 4.1G as revealed by later sequence analysis. Western blotting of the 4.1G immunoprecipitation complex using anti-CNGA1 and anti-CNGB1 antibodies supported their interaction (Fig. 1B). 4.1G was also detected by mass spectrometry as one of the main proteins in immunoprecipitates of CNGA1 from mouse retinal membrane extracts prepared by centrifugation (Fig. 1C; Table 1C). The interaction of 4.1G with CNGA1 and CNGB1 observed in both the bovine and mouse retina supports the significance of this binding. Intriguingly, as observed in bovine ROSs, western blotting with the 4.1G antibody revealed that only the shorter 4.1G variant of ∼100 kDa (Fig. 1C) co-precipitated with CNGA1 and CNGB1 from mouse retinal membranes. These observations suggest that each variant may have specific roles even within the same cellular environment conforming to the known complexity and functional diversity of splice variants in the 4.1 protein family.
The CNG channel located on the plasma membrane of the photoreceptor ROS has been shown to interact with the peripherin-2–rom-1 complex on the rim region of the disc membrane (Poetsch et al., 2001), thereby forming a physical linkage that is important for the structural organization and stability of the outer segment. However, 4.1G does not appear to participate in this peripherin-2 channel complex as immunoprecipitation with an anti-peripherin-2 antibody failed to pull down any 4.1G (Fig. 1D). This indicates that 4.1G selectively binds to a population of the channels that is free of peripherin-2.
Splice variants of 4.1G in the retina
Because the complete mRNA sequence of the bovine 4.1G was not available at the initiation of this project, the novel observation of a 4.1G splice variant interacting with the CNG channel motivated the cloning of the retinal 4.1G variants and the generation of a highly specific 4.1G antibody in order to gain further understanding of the mechanism of this interaction.
The 4.1G protein in bovine retina was originally detected in one of the first proteomics studies of photoreceptors (Kwok et al., 2008). Twelve peptides from a protein called 112 kDa protein with the accession number IPI00697691 were among the peptides detected in this proteomic study. Bioinformatics search of IPI00697691 resulted in a match in the EMBL database to bovine protein sequence UPI0000F3237F. To find the corresponding nucleotide sequence, Blast search (tBlastn) of UPI0000F3237F against the National Center for Biotechnology Information (NCBI) database yielded the predicted bovine miscellaneous RNA, LOC538959, which comprises 4387 nucleotides and two tandem open reading frames (ORFs), interrupted by a stop codon after the first 159 amino acids. Pairwise alignment of the ORFs of LOC538959 and UPI0000F3237F showed almost perfect identity, other than the premature stop codon (Fig. 2). The EMBL sequence encodes a protein of 1001 amino acid (aa) residues with a predicted molecular mass of 112 kDa. It was also predicted to have conserved domains across the 4.1 protein family including the FERM domain, the SAB domain and the CTD. The RNA sequence of LOC538959 surrounding the start codon and the final stop codon were used to design primers to clone the full-length 4.1G from bovine retinal tissue.
Fig. 2.
Schematic representation of bovine 4.1G predicted RNA sequence and protein sequence. The predicted bovine 4.1G RNA sequence (top; LOC538959) has two translation-initiation sites (ATG) as indicated at basepair (bp) positions 91 and 649 and two stop codons (TGA) at positions 570 and 3114 bp. Its corresponding open reading frames (ORFs) are identical to the protein sequence (1–159 aa; 181–1001 aa) from the EMBL database (bottom).
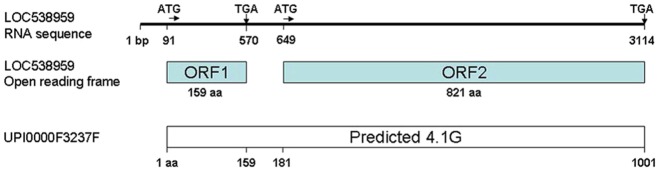
Amplification of full-length 4.1G coding region in the bovine retina resulted in three PCR products with sizes of 2205 basepairs (bp; short), 2775 bp (middle) and 2922 bp (long). Sequence analysis revealed that the short transcript lacks exons 12, 13 and 14; the middle transcript lacks exons 12 and 13, and the long transcript lacks only exon 12 (Fig. 3A). In the EMBL database, there is supporting evidence for 4.1G cDNAs without exons 12–14, such cDNAs have been sequenced from human (Q68DV2.1), mouse (Q80UE5.1) and orangutan (Q5RC68.1). The cloned bovine 4.1G cDNAs have deduced ORFs encoding proteins of 734, 924 and 973 aa, respectively. Pairwise alignment revealed the absence of aa residues 607 to 866, 607 to 676, and 607 to 627 of the predicted UPI0000F3237F in the short, middle and long cDNA, respectively, corresponding to the SAB domain and the U3 region of the predicted 4.1G. The adrenal gland 4.1G is another isoform that does not contain the SAB domain (Wang et al., 2010), although this domain is observed in most of the 4.1G proteins characterized to date (Ramez et al., 2003; Taylor-Harris et al., 2005).
Fig. 3.
Characterization of retinal 4.1G. (A) Schematic representation of domain and exon structures of the predicted 4.1G protein in comparison with the cloned retinal 4.1G variants. The epitope of the monoclonal antibody against bovine 4.1G was mapped to amino acid residues 62–112 (#). Anti-4.1G polyclonal antibody was generated against a peptide containing amino acid residues 965–984 (*). (B) Western blot of HEK-293 cell lysates labeled with Rho-1D4 antibody. HEK-293 cells were transfected with bovine 4.1G short (lane 2), middle (lane 3) and long (lane 4) variants each having a 1D4 tag at the C-terminus. Non-transfected HEK-293 cell lysate was loaded as control (lane 5). Molecular markers were loaded as reference (lane 1).
Expression of cloned 4.1G variants
To investigate the relative expression levels of the various variants, the 4.1G cDNAs encoding a 1D4-tag at the C-terminus were cloned into pcDNA3 and transfected into HEK-293 cells. The expressed 4.1G proteins were analyzed by immunoblotting with the tag-specific Rho-1D4 antibody. The apparent sizes of the bovine 4.1G variants (125, 180 and 200 kDa) in HEK-293 cells (Fig. 3B) were larger than their predicted molecular mass. Similar observations were noted in 4.1G proteins expressed in other tissues (Parra et al., 1998; Peters et al., 1998), as well as other members of the 4.1 family: 4.1R (Conboy et al., 1991; Tang et al., 1990), 4.1N (Walensky et al., 1999) and 4.1B (Parra et al., 2000). In the negative control, no protein was detected with the Rho 1D4 antibody in non-transfected cells.
Specificity of 4.1G antibodies
The specificity of 4.1G monoclonal (Epb41l2-13B2) and polyclonal antibodies was confirmed by immunoblotting the endogenous 4.1G from bovine and mouse retina (Fig. 4A). The monoclonal antibody recognized a single band migrating with an apparent molecular mass of 125 kDa in the bovine ROS and a band of ∼140 kDa in the mouse retinal membrane lysate. In contrast, the polyclonal antibody recognized two dominant bands (125 and 150 kDa) in the bovine ROS and multiple bands in the mouse retina with a prominent band of ∼140 kDa. The presence of multiple 4.1G variants detected in the retina is in general agreement with that observed in the mouse brain (Parra et al., 2000; Pinder et al., 2012), testis (Yang et al., 2011) and muscle (Okumura et al., 2010). The difference in labeling pattern observed with the two antibodies may be attributed to their immunological strength with the polyclonal antibody being stronger, allowing the detection of weakly expressed variants. Furthermore, the polyclonal antibody was generated against a 20 aa region in the C-terminal domain that is conserved between human, mouse and bovine. This region shares an overall 60% similarity with other 4.1 homologs, specifically 90% with 4.1B, 80% with 4.1R and 70% with 4.1N. The sequence similarity suggests the possibility of cross-reactivity with other 4.1 members as observed for antibodies generated against similar region (Scott et al., 2001). Owing to the nature of the anti-4.1G polyclonal antibody, it was used only for immunoprecipitation studies in which the identities of the proteins could be confirmed by mass spectrometry.
Fig. 4.
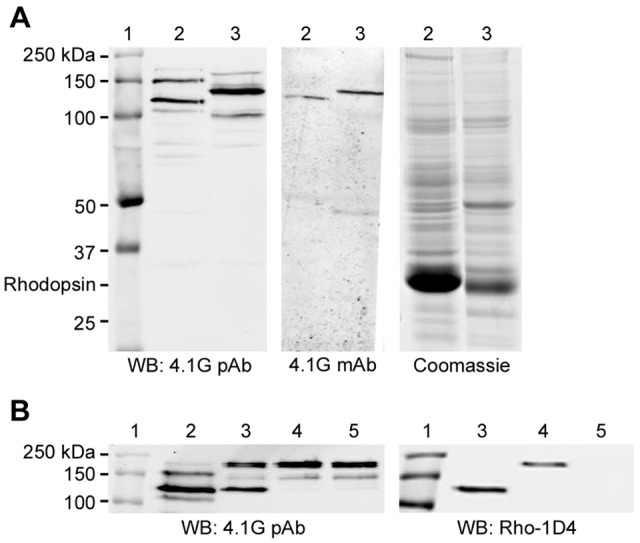
Specificity of 4.1G polyclonal and monoclonal antibodies. (A) Western blots of bovine ROS extracts and mouse retinal membranes. The left panel was labeled with the 4.1G polyclonal antibody (pAb) which detected two dominant variants (125 and 150 kDa) in the bovine ROS (lanes 2) and one dominant (140 kDa) in the mouse retina (lanes 3). The middle panel shows a western blot labeled with the 4.1G monoclonal antibody (mAb) which detected only the dominant variant in the bovine (125 kDa) and mouse (140 kDa) retina. The Coomassie-Blue-stained gel (right panel) is shown for the bovine and mouse samples. Molecular markers were loaded as reference (lane 1). (B) Western blots labeled with the 4.1G polyclonal antibody comparing the endogenous 4.1G variants in the bovine ROS with the cloned 4.1G exogenously expressed in HEK-293 cells. Lane 1, molecular markers; lane 2, lysate of bovine ROS; lane 3, cell lysate transfected with short bovine 4.1G; lane 4, cell lysate transfected with middle bovine 4.1G; lane 5, mock transfection. Note that the short bovine 4.1G (734 aa, lane 3) was of similar molecular mass to the most dominant variant in the bovine retina (lane 2), which were both ∼125 kDa. The 4.1G antibody also detected the endogenous 4.1G in all HEK-293 cell samples, including the mock-transfected cell lysate (∼200 and 150 kDa, lane 5). An immunoblot labeled with Rho-1D4 antibody showed the expression of the 4.1G variants.
To determine the epitope of the Epb41l2-13B2 monoclonal antibody, truncation constructs of 4.1G were generated. Subsequently, smaller constructs of the U1 region were produced with an increment of 50 aa residues to refine the antibody-binding site. The epitope of the antibody was mapped to the region spanning 62–112 aa in the U1 region of 4.1G. According to pairwise alignment, this region shares high similarity (74%) in the bovine and the mouse protein sequences. In terms of homolog specificity, it has been widely noted that there is limited homology within the U1 domain in the 4.1 family members. Moreover, the region of the antibody epitope (62–112 aa) in 4.1G shares only 28% similarity with 4.1R and is absent in 4.1B and 4.1N, attesting to the homolog specificity of the monoclonal antibody.
The physiological importance of the various 4.1G clones was assessed by a visual comparison of 4.1G in the retina and the heterologously expressed protein using gel electrophoresis. Fig. 4B showed that the dominant variant found in the bovine ROS (125 kDa) is of similar molecular mass to the short bovine 4.1G (734 aa). The middle variant of bovine 4.1G (924 aa) is of similar size to a faint band migrating at ∼180 kDa in the bovine ROS and is of the same apparent size as one of the endogenous 4.1G variants found in lysates from mock-transfected HEK-293 cells. Because the major short bovine 4.1G clone appears to be the most physiologically relevant variant in the retina, this clone was selected for domain interaction studies.
FERM domain of 4.1G is required for interaction with the CNG channel
The bovine 4.1G clone was used to further confirm the association between 4.1G and the rod CNG channel. Transient double-transfections of HEK-293 cells with cDNAs encoding 3F4-tagged 4.1G and CNGA1 or CNGB1 were performed. The 4.1G–3F4 co-immunoprecipitated with CNGA1 and CNGB1 subunits (Fig. 5A). This amount, however, was less than that observed in immunoprecipitation studies of bovine and mouse retinal tissues suggesting that additional photoreceptor proteins or photoreceptor-specific post-translational modifications may be needed to enhance the interaction of 4.1G and the channel subunits.
Fig. 5.
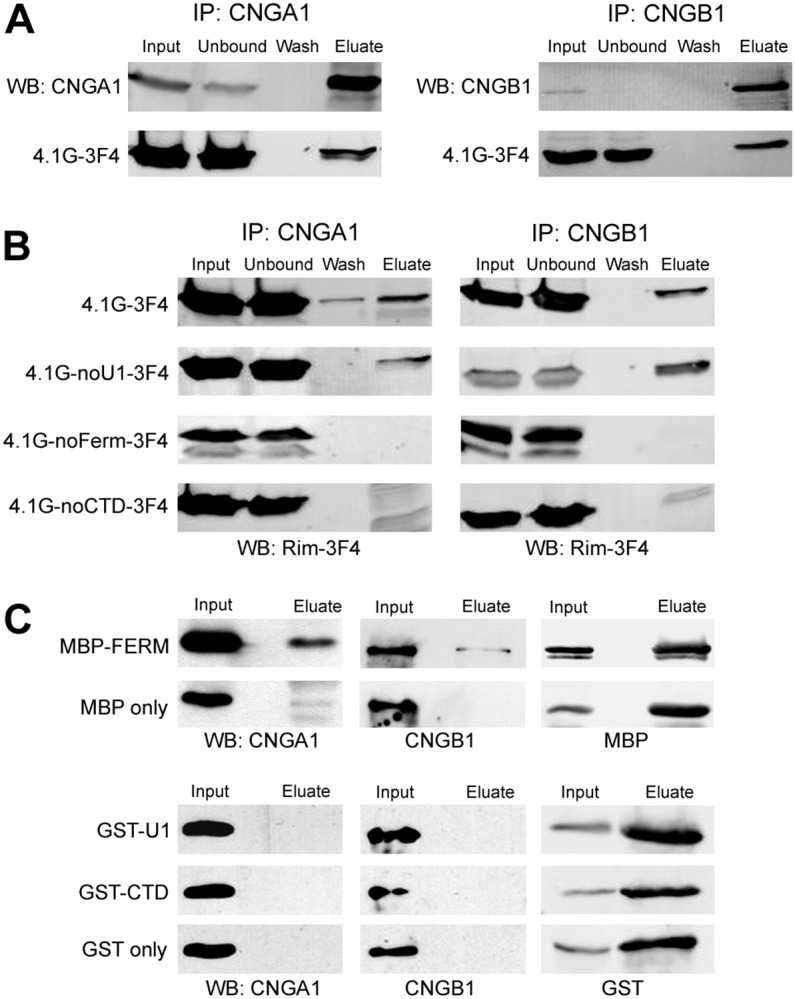
Domains of 4.1G required for binding to CNG channels. (A) Co-immunoprecipitation of 4.1G with CNG channels. Lysates of HEK-293 cells co-transfected with 4.1G-3F4 and CNGA1 (left panel) or CNGB1 (right panel) were incubated with anti-CNGA1 (PMc-1D1) or anti-CNGB1 (Garp-8G8) antibody column, respectively. Western blots of various fractions (input, unbound, wash and eluate) were labeled with anti-CNGA1 (top left) or anti-CNGB1 (top right) antibody and Rim-3F4 antibody (bottom). (B) Co-immunoprecipitation of 4.1G deletion mutants with CNG channels. Lysates of HEK-293 cells co-transfected with deletion construct of 4.1G and CNGA1 (left panel) or CNGB1 (right panel) were incubated with anti-CNGA1 or anti-CNGB1 antibody column, respectively. Western blots were labeled with Rim-3F4 to detect 4.1G full length (top) or deletion mutants (bottom 3) all of which had a 3F4 tag at the C-terminus. Note that 4.1G lacking the FERM domain or the CTD domain failed to co-immunoprecipitate with CNGA1 and CNGB1. (C) Co-immunoprecipitation of CNG channels with MBP–FERM. The domains of 4.1G (U1, FERM, CTD) were fused to either MBP or GST and incubated with bovine ROS and immunoprecipitated with either amylose resin or glutathione resin. Western blots of the input and eluate fractions were labeled with anti-CNGA1 (left panel), anti-CNGB1 antibody (middle panel) or an antibody against MBP or GST (right panel). Note that CNGA1 and CNGB1 were co-immunoprecipitated with MBP–FERM only. MBP and GST alone were used as control.
To determine the domain of 4.1G required for the binding of CNGA1 and CNGB1, deletion constructs of 4.1G were co-transfected with either CNGA1 or CNGB1 into HEK-293 cells and co-immunoprecipitation experiments were performed (Fig. 5B). 4.1G lacking the U1 domain co-immunoprecipitated with CNGA1 and CNGB1. However, further deletion of the FERM domain abolished the ability of 4.1G to interact with CNGA1 and CNGB1. Deletion of the CTD also prevented this interaction. These studies suggest that both the FERM domain and the CTD are required for optimal interaction of 4.1G with the CNG channel subunits.
To further delineate the 4.1G-binding site for the channels, individual domains fused with either GST or MBP were incubated with bovine ROSs. CNGA1 and CNGB1 co-precipitated with MBP–FERM, but failed to co-precipitate with the GST–U1 or GST–CTD (Fig. 5C). Control experiment using MBP alone did not pull-down any CNG channel subunits. These results indicate that the 4.1G FERM domain is crucial for the binding of CNGA1 and CNGB1. The 4.1G CTD may play a role in modulating the interaction.
Comparison of 4.1G and CNGs distribution in photoreceptors
To examine the localization of 4.1G in photoreceptors, mouse retinal sections were labeled with the 4.1G isoform-specific monoclonal antibody (Epb41l2-13B2). Punctate staining was detected throughout the length of the photoreceptor outer segments with additional labeling observed in the outer nuclear layer (Fig. 6). Double labeling with Epb41l2-13B2 and anti-CNGB1 polyclonal antibody showed partial colocalization of 4.1G and CNGB1 in the outer segment of photoreceptors (Fig. 6). The presence of 4.1G in the specialized photoreceptor outer segment compartment was further established by western blotting of highly pure ROS membranes prepared by hypotonic lysis of ROSs followed by flotation on 5% Ficoll solution. The amount of 4.1G detected in these ROS membranes was dependent on the addition of 2 mM MgCl2 (Fig. 7 left panel). In ROS membranes treated with EDTA used to chelate magnesium ions, the majority of 4.1G was found in the soluble fraction (Fig. 7 right panel).
Fig. 6.
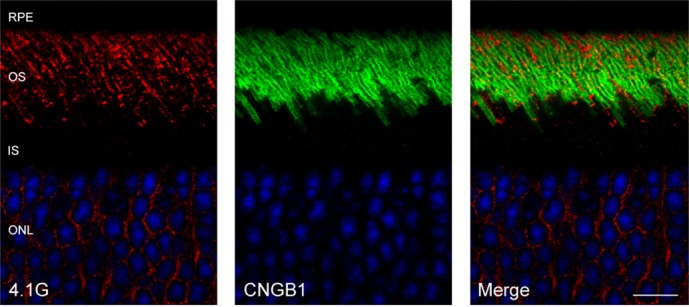
Localization of 4.1G in photoreceptors in the mouse retina. Immunofluorescence labeling of mouse retinal cryosection with monoclonal antibody against 4.1G and anti-CNGB1 antibody. Punctate staining of 4.1G in the photoreceptor outer segments partially co-localized with CNGB1 labeling.
Fig. 7.
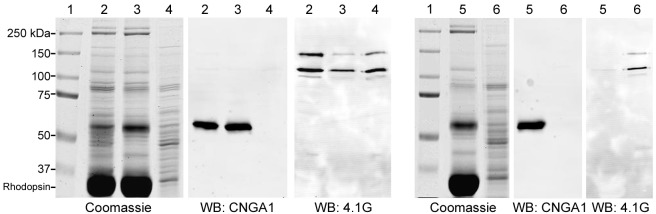
Distribution of 4.1G in bovine photoreceptors. SDS-polyacrylamide gels and immunoblots of bovine ROS membrane preparations. Lanes 1, molecular mass makers; lanes 2, crude ROSs prepared by sucrose gradient; lanes 3, ROS membrane vesicles prepared by hypotonic lysis of ROSs followed by flotation on Ficoll with the addition of 2 mM MgCl2; lanes 4, respective ROS soluble fraction (MgCl2); lanes 5, Ficoll ROS membrane vesicles prepared in the presence of 1 mM EDTA; lanes 6, respectively ROS soluble fraction (EDTA). The SDS-polyacrylamide gel was either stained with Coomassie Blue or transferred to Immobilon membranes and labeled with anti-CNGA1 or anti-4.1G antibody. The soluble fraction was not contaminated with the membrane fraction as CNGA1 was not detected in this fraction (lanes 4 and 6). 4.1G was detected in soluble fractions (lanes 4 and 6) and it was only present in the ROS membrane fraction when MgCl2 is added (lane 3).
Discussion
4.1 proteins exhibit diverse and complicated expression patterns attributed to alternative splicing with multiple splice variants often found within a certain tissue. Multiple splice variants of 4.1G were detected in the retina of both bovine and mouse with our anti-4.1G polyclonal antibody. An impressive molecular heterogeneity of 4.1G has been reported in various tissues with the apparent molecular mass of splice variants ranging from 70 to 200 kDa. Most studies have focused on human and rodent tissues, but alternative splicing of 4.1G is also common in the bovine retina as evident by the multiple cDNAs cloned in this study. Interestingly, only one of the cDNAs obtained from bovine retinal tissue encoded a protein with a similar molecular mass to one of the endogenous 4.1G variants in the retina. Furthermore, an even longer variant in bovine retina is predicted by the peptide sequences obtained from the mass spectrometry. However, this hypothetical longer 4.1G is likely to be expressed at a low level because the peptide specific to this variant was only detected once out of the three mass spectrometry experiments performed on bovine retina.
It is conceivable that each 4.1G splice variant could play a unique role within the same cell as they differ in their structural motifs. Alternatively, the different splice variants may be expressed in distinct cells or cellular compartments. The smaller variant in the bovine retina identified in this study (125 kDa) lacks the spectrin-actin-binding (SAB) domain suggesting that it has acquired a function outside the conventional role of 4.1 as an adaptor protein linking membrane proteins to the spectrin-actin cytoskeleton. As shown by immunoprecipitation and western blotting, this specific variant interacts with the CNG channel in the plasma membrane of rod photoreceptor outer segments. The fact that only peptides located within the short variant were found by mass spectrometry supports the selective preference of this variant in outer segments. Binding to the CNG channel requires the FERM domain and to some extent the CTD of 4.1G. Both these domains have been shown to be responsible for protein–protein interaction of 4.1 proteins. In particular the CTD domain appears to be associated with the binding of membrane receptors more frequently (Gibson et al., 2012; Lu et al., 2004a; Lu et al., 2004b; Rose et al., 2008). In contrast to the selective interaction of the SAB-less 4.1G with CNG channels observed in the retina, all of the three 4.1G variants in the mouse testis were co-immunoprecipitated with nectin-like 4 (NECL4) through binding of the FERM domain (Yang et al., 2011). The mechanism regulating the specific variant interaction is perplexing as the domain of interaction is present in all variants. Additional 4.1G domains, binding proteins or even ions that mediate this selection remain to be determined. There is recent evidence suggesting that calcium may play a role in regulating the activity of 4.1G. A conformational change is induced in the 4.1G protein when a calcium-binding protein associates with the U1 domain of 4.1G (Nunomura et al., 2013). Moreover, our studies have found that 4.1G is detected in the Ficoll-prepared ROSs only in the presence of MgCl2, further supporting a degree of ion-dependence on the interaction of 4.1G with the CNG channel.
It is interesting to note that whereas the CNG channels are specific in binding to the SAB-less 4.1G variant, 4.1G itself also discriminates against CNG channels that are complexed with peripherin-2. There may be a functional purpose for having distinct populations of CNG channels. The 4.1G-bound population may serve a functional role in targeting and accumulating CNG channels to the plasma membrane in order to achieve the speed required for phototransduction. The peripherin-2-bound population of CNG channels has been implicated in playing a structural role in maintaining a proper spatial relationship between the plasma membrane and the disc membrane within the outer segments (Poetsch et al., 2001; Zhang et al., 2009). However, whether CNG channels and peripherin-2 serve as the sole factors in providing the structure integrity of photoreceptor outer segments remains debatable as relatively limited photoreceptor structural defects were observed in mice lacking CNGB1 (Hüttl et al., 2005; Zhang et al., 2009).
In this study, mass spectrometry analysis failed to detect further confirmable interacting partners of 4.1G. Ankyrin G has been reported to co-immunoprecipitate with CNG channels in the mouse retina. These studies have implicated ankyrin G in the transport of CNG channels to rod photoreceptor cilia (Kizhatil et al., 2009). Ankryin G is similar to 4.1 proteins in that it acts as an adaptor linking membrane proteins to the cytoskeleton. The mass spectrometry analysis performed on samples immunoprecipitated with the CNGA1 and CNGB1 antibodies failed to detect ankyrin G peptides. In fact, ankyrin G has not been found in mass-spectrometric-based proteomic studies of photoreceptor outer segments (Kiel et al., 2011; Kwok et al., 2008; Reidel et al., 2011; Skiba et al., 2013). Considering the size of this protein it is surprising that these proteomics studies have failed to detect peptides from ankyrin G in outer segment preparations. This discrepancy remains to be reconciled. Interestingly, another adaptor protein known as the cytoskeleton-associated protein 4 (Ckap4) co-immunoprecipitated with CNGA1 (Table 1C). Ckap4 is a transmembrane protein anchoring the endoplasmic reticulum (ER) to microtubules (Klopfenstein et al., 1998), through which the proper spatial distribution of ER with respect to the nucleus is maintained (Vedrenne et al., 2005). It remains to be determined whether Ckap4 interacts with the CNGA1 within the ROS.
The Epb41l2-13B2 monoclonal antibody revealed a punctate pattern of 4.1G throughout the length of the photoreceptor outer segments where it partially co-localized with the CNG channels. This punctate labeling pattern has also been documented for 4.1R in the heart (Baines et al., 2009; Pinder et al., 2012), 4.1B at the region of cell–cell contact in the brain (Parra et al., 2000) and 4.1G at tight junctions of OLN-93 cells (Xia and Liang, 2012). This high local density of 4.1 proteins has been implicated in facilitating signal transduction by promoting cell surface expression and stable retention of transmembrane proteins, and accumulating signaling molecules at precise cellular compartments. Whether 4.1G has any effect on the activity and the surface expression level of CNG channels or on the structural integrity of photoreceptor outer segments remains to be investigated. Analysis of the retina from 4.1G-knockout mice would greatly enhance our understanding of the role of 4.1G in photoreceptor outer segment structure and function.
Materials and Methods
Animals
All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. All protocols and procedures conformed to the University of British Columbia policy and were approved by the UBC Committee on Animal Care.
RT-PCR and amplification of full-length cDNAs
Total RNA was isolated from bovine (Bos taurus) retinal tissue using RNeasy mini kit (Qiagen, Maryland, MA) and was reverse transcribed using a One-Step RT-PCR kit (Qiagen) according to manufacturer's instructions. The primers used for the amplification of 4.1G were: bovine forward, 5′-ATAAGCTGTGACCATGACTACTGAAG-3′; bovine reverse, 5′-CTCTTCCCCCTCCTCCAACTC-3′. Cycling conditions were 30 minutes at 50°C, 15 minutes at 95°C, 35 cycles of 30 seconds at 94°C, 30 seconds at 62°C, and 3 minutes at 68°C, and a final elongation step of 10 minutes at 68°C. The resulting PCR products were cloned into pcDNA3 and all clones were sequenced at GENEWIZ, Inc. (Seattle, WA). The GenBank accession numbers for the bovine short, middle and long 4.1G cDNAs are KF306276, KF306277 and KF306278 respectively.
Generation of antibodies to 4.1G
The full-length bovine 4.1G with a 1D4 tag cloned in pcDNA3 was expressed in HEK-293 cells and purified with an anti-1D4 antibody column. Purified bovine 4.1G-1D4 was used to immunize mice and the spleen cells were fused with NS-1 mouse myeloma cells to generate hybridoma cell lines producing monoclonal antibodies against 4.1G. Culture fluid from hybridoma cells (Epb41l2-13B2) was used at a dilution of 1∶10 for western blotting and undiluted for immunofluorescence labeling studies. The polyclonal antibody against 4.1G was generated by immunizing rabbits with a synthetic peptide, HDQALAQAIREAREQHPDMS, at YenZym Antibodies (South San Francisco, CA). The selected region of amino acids is conserved between human, bovine and mouse. For immunoprecipitation studies, purified 4.1G polyclonal antibodies were covalently coupled to CNBr-activated Sepharose 2B (GE Healthcare, Mississauga, ON) at 2 mg protein/ml of packed beads.
Antibodies
The generation of anti-CNGA1 (PMc-1D1), anti-CNGB1 (Garp-8G8) and anti-peripherin-2 (Per-2B6) monoclonal antibodies has been described previously (Cook et al., 1989; Molday et al., 1987; Poetsch et al., 2001). For the detection of the 1D4 and 3F4 tags, Rho-1D4 and Rim-3F4 antibodies were used, respectively (Illing et al., 1997; MacKenzie et al., 1984). Polyclonal antibody to the GARP part of CNGB1 has been previously described (Colville and Molday, 1996). Anti-GST monoclonal antibody was purchased from Rockland Immunochemical (Gilbertsville, PA) and anti-MBP antibody was purchased from New England BioLabs (Ipswich, MA).
Truncation of 4.1G
Deletion constructs of 4.1G with a 3F4 tag at the C-terminus were generated by PCR and subcloned into pcDNA3. Primers for ΔU1 were: forward, 5′-AAAACTGTCCAGTGTAAAG-3′; reverse, 5′-CTCTTCCCCCTCCTCCAACTC-3′. Primers for ΔFERM were: forward, 5′-CAGCCACCAAAGGCCAA-3′; reverse 5′-CTCTTCCCCCTCCTCCAACTC-3′. Primers for ΔCTD were: forward, 5′-ATAAGCTGTGACCATGACTACTGAAG-3′; reverse, 5′-TTAGGAGATTTCTGTCCTTTG-3′. All constructs were sequenced at GENEWIZ, Inc.
For mapping the epitope of the Epb41l2-13B2 monoclonal antibody, the U1 domain of 4.1G was further truncated into smaller fragments which were subcloned into pGEX-4T1 to make GST-fusion proteins. Primers used were: U1-210aa forward, 5′-AAAACTGTCCAGTGTAAAG-3′; U1-148aa forward, 5′-CAGAAGAAAGAGAAAGAT-3′; U1-98aa forward, 5′-TTAGACAAGGAGGAATCTCT-3′; U1-48aa forward, 5′-GAAGAGAAGGTGAAGGAAAT-3′. Reverse primer, 5′-TTA-TTTACACTGGACAGTTTTGGT-3′ was used for all U1 domain truncated constructs. The identity of all constructs was confirmed by sequencing at GENEWIZ, Inc.
Cell culture techniques
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Carlsbad, CA) containing 8% (v/v) bovine growth serum (Life Technologies) in 5% CO2. Cells were transfected with calcium phosphate and BES-buffered system. After 48 hours, cells were harvested and experiments were performed.
Preparation of retinal tissue
Bovine ROSs were isolated from frozen bovine retinas by sucrose gradient centrifugation as described previously (Molday and Molday, 1987; Holopainen, Cheng et al., 2010). The procedure for isolating a sample of a more pure bovine ROS membranes by Ficoll flotation method was described previously (Smith and Stubbs et al., 1975). Briefly, the crude ROS preparation was subjected to hypotonic lysis in buffer with 10 mM Tris with or without 2 mM MgCl2 and subsequently purified by flotation on 5% Ficoll solution.
Preparation of mouse retinal membranes has been described previously (Molday et al., 2013). Briefly, six dissected mouse retinas were immersed in 400 ml Tris buffer saline (TBS: 20 mM Tris, pH 7.4, 0.1 M NaCl and Complete protease inhibitor; Roche) for 20 minutes on ice. The suspension was then passed through a 22 gauge and 28 gauge needle, layered on top of 1.6 ml of 60% (w/w) sucrose/TBS, and centrifuged at 41,000 g for 30 minutes in a TLS-55 rotor using a Beckman TLX Optima centrifuge. The retinal membrane fraction which banded on top of the 60% sucrose was removed, diluted with three volumes of TBS, and pelleted by centrifugation at 60,000 g for 10 minutes in a TLA 100.4 rotor.
Immunoprecipitation and immunoblotting
Co-immunoprecipitation experiments were performed with retinal tissue lysate and lysate from HEK-293 cells transfected with various combinations of 4.1G truncation and CNG constructs. Total protein from retinal tissues or transfected cells was extracted with lysis buffer (20 mM Tris, 150 mM NaCl, 1% Triton X-100), and lysate was incubated with either anti-4.1G, anti-CNGA1 or anti-CNGB1 antibody column for 2 hours at 4°C. The column was washed extensively and bound proteins were eluted with 2% (w/v) SDS. The eluted proteins were analyzed by SDS-PAGE and immunoblotted with antibodies to 4.1G, CNGA1 and CNGB1. Detection was performed with anti-mouse Ig conjugated with Alexa Fluor 680 and anti-rabbit Ig conjugated with Alexa Fluor 800 (dilution 1∶20,000; Life Technologies) and imaged by the LI-COR Odyssey system (Lincoln, NE).
In-gel digestion of proteins for proteomics analysis
The procedure for handling samples for proteomic analysis has been described previously (Kwok et al., 2008). In brief, each lane of an SDS gel containing the eluate from the immunoprecipitation studies was cut into small pieces and digested with trypsin. Digested samples were submitted to the Proteomics Core Facility of Center for High Throughput Biology (CHiBi) at the University of British Columbia. Peptide mass fingerprinting was performed by Fourier transform mass spectrometry with a MASCOT database search for protein identification.
Immunofluorescence microscopy
The procedures for the fixation and labeling of retinal tissue have been described previously (Cheng et al., 2013; Holopainen et al., 2010). In brief, whole mouse eye cups were fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4 for 2 hours and subsequently rinsed and cryoprotected in 0.1 M phosphate buffer containing 20% sucrose (w/v). Cryosections were labeled overnight with primary antibody, rinsed, then labeled for 1 hour with secondary antibodies conjugated with either Alexa Fluor 488 or Alexa Fluor 568 (Life Technologies) and counterstained with DAPI nuclear stain (Life Technologies). The stained sections were examined under a Zeiss LSM700 confocal microscope (Carl Zeiss, Toronto, ON) and processed with Zeiss Zen Image Browser.
GST/MBP-pulldown technique
The U1 domain and CTD of 4.1G were cloned into pGEX-4T1 to generate GST–U1 and GST–CTD fusion proteins. The primers used to amplify the U1 domain were: forward, 5′-AAAACTGTCCAGTGTAAAG-3′; reverse, 5′-TTA-TTTACACTGGACAGTTTTGGT-3′. The primers used to amplify the CTD domain were: forward, 5′-CAGCCACCAAAGGCCAA-3′; reverse, 5′-CTCTTCCCCCTCCTCCAACTC-3′. The FERM domain was cloned into pMAL-C2 to generate maltose-binding protein (MBP)-FERM fusion protein. The primers used to amplify the FERM domain were: forward, 5′-GTCCTGGCCAAAGTGACCCTT-3′; reverse, 5′-TTAAGACACAAGCCTGTAGAAAGT-3′. The GST–U1, GST–CTD, MBP–FERM fusion proteins were expressed in BL21 bacterial cells, purified and immobilized on glutathione (GE Healthcare) or amylose (NEB) resin. Bovine retinal lysate was added to the resin and it was incubated for 3 hours at 4°C. The resin was washed extensively and bound proteins were eluted with 2% (w/v) SDS. The eluted proteins were analyzed by SDS-PAGE and immunoblotted with antibodies to GST, MBP, CNGA1 and CNGB1. Immunoreactive bands were visualized after incubation with secondary antibodies coupled to horseradish peroxidase (GE Healthcare) and with the chemiluminescence detection kit (GE Healthcare).
Acknowledgments
We thank Dr Ming Zhong for help with initial cloning of 4.1G and Theresa Hii for technical assistance with 4.1G antibody production. We also thank Laurie Molday and Hidayat Djajadi for their expertise and assistance with the subretinal injection. R.S.M. holds a Canada Research Chair in Vision and Macular Degeneration.
Footnotes
Author contributions
C.L.C. designed and performed experiments, interpreted data and wrote the manuscript. R.S.M. designed and interpreted data, and edited the manuscript.
Funding
This work was supported by the National Institutes of Health [grant no. EY002422]. Deposited in PMC for release after 12 months.
References
- Baines A. J., Bennett P. M., Carter E. W., Terracciano C. (2009). Protein 4.1 and the control of ion channels. Blood Cells Mol. Dis. 42, 211–215 10.1016/j.bcmd.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Djajadi H., Molday R. S. (2013). Cell-specific markers for the identification of retinal cells by immunofluorescence microscopy. Methods Mol. Biol. 935, 185–199 10.1007/978-1-62703-080-9_12 [DOI] [PubMed] [Google Scholar]
- Coleman S. K., Cai C., Mottershead D. G., Haapalahti J-P., Keinänen K. (2003). Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. Neuroscience 23, 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville C. A., Molday R. S. (1996). Primary structure and expression of the human beta-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J. Biol. Chem. 271, 32968–32974 10.1074/jbc.271.51.32968 [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. (1991). Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J. Biol. Chem. 266, 8273–8280 [PubMed] [Google Scholar]
- Cook N. J., Molday L. L., Reid D., Kaupp U. B., Molday R. S. (1989). The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J. Biol. Chem. 264, 6996–6999 [PubMed] [Google Scholar]
- Gibson A. W., Li X., Wu J., Baskin J. G., Raman C., Edberg J. C., Kimberly R. P. (2012). Serine phosphorylation of FcγRI cytoplasmic domain directs lipid raft localization and interaction with protein 4.1G. J. Leukoc. Biol. 91, 97–103 10.1189/jlb.0711368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen J. M., Cheng C. L., Molday L. L., Johal G., Coleman J., Dyka F., Hii T., Ahn J., Molday R. S. (2010). Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptor cells. Biochemistry 49, 7439–7447 10.1021/bi1005249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttl S., Michalakis S., Seeliger M., Luo D-G., Acar N., Geiger H., Hudl K., Mader R., Haverkamp S., Moser M. et al. (2005). Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. Neuroscience 25, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing M., Molday L. L., Molday R. S. (1997). The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303–10310 10.1074/jbc.272.15.10303 [DOI] [PubMed] [Google Scholar]
- Ivanovic A., Horresh I., Golan N., Spiegel I., Sabanay H., Frechter S., Ohno S., Terada N., Möbius W., Rosenbluth J. et al. (2012). The cytoskeletal adapter protein 4.1G organizes the internodes in peripheral myelinated nerves. J. Cell Biol. 196, 337–344 10.1083/jcb.201111127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., McCarty J. H. (2012). Band 4.1 proteins regulate integrin-dependent cell spreading. Biochem. Biophys. Res. Commun. 426, 578–584 10.1016/j.bbrc.2012.08.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel C., Vogt A., Campagna A., Chatr-aryamontri A., Swiatek-de Lange M., Beer M., Bolz S., Mack A. F., Kinkl N., Cesareni G. et al. (2011). Structural and functional protein network analyses predict novel signaling functions for rhodopsin. Mol. Syst. Biol. 7, 551 10.1038/msb.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K., Baker S. A., Arshavsky V. Y., Bennett V. (2009). Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science (New York) 323, 1614–1617 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein D. R., Kappeler F., Hauri H. P. (1998). A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 17, 6168–6177 10.1093/emboj/17.21.6168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok M. C. M., Holopainen J. M., Molday L. L., Foster L. J., Molday R. S. (2008). Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol. Cell. Proteomics 7, 1053–1066 10.1074/mcp.M700571-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Yan H., Othman T., Rivkees S. A. (2004a). Cytoskeletal protein 4.1G is a binding partner of the metabotropic glutamate receptor subtype 1 alpha. J. Neurosci. Res. 78, 49–55 10.1002/jnr.20230 [DOI] [PubMed] [Google Scholar]
- Lu D., Yan H., Othman T., Turner C. P., Woolf T., Rivkees S. A. (2004b). Cytoskeletal protein 4.1G binds to the third intracellular loop of the A1 adenosine receptor and inhibits receptor action. Biochem. J. 377, 51–59 10.1042/BJ20030952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie D., Arendt A., Hargrave P., McDowell J. H., Molday R. S. (1984). Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 10.1021/bi00321a041 [DOI] [PubMed] [Google Scholar]
- Molday R. S., Molday L. L. (1987). Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J. Cell Biol. 105, 2589–2601 10.1083/jcb.105.6.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R. S., Molday L. L. (1998). Molecular properties of the cGMP-gated channel of rod photoreceptors. Vision Res. 38, 1315–1323 10.1016/S0042-6989(97)00409-4 [DOI] [PubMed] [Google Scholar]
- Molday R. S., Hicks D., Molday L. (1987). Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest. Ophthalmol. Vis. Sci. 28, 50–61 [PubMed] [Google Scholar]
- Molday L. L., Djajadi H., Yan P., Szczygiel L., Boye S. L., Chiodo V. A., Gregory-Evans K., Sarunic M. V., Hauswirth W. W., Molday R. S. (2013). RD3 gene delivery restores guanylate cyclase localization and rescues photoreceptors in the Rd3 mouse model of Leber congenital amaurosis 12. Hum. Mol. Genet. 22, 3894–3905 10.1093/hmg/ddt244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Ji C., Cao G., Cheng H., Guo L., Gu S., Ying K., Zhao R. C., Mao Y. (2003). Molecular cloning and characterization of the protein 4.1O gene, a novel member of the protein 4.1 family with focal expression in ovary. J. Hum. Genet. 48, 101–106 10.1007/s100380300015 [DOI] [PubMed] [Google Scholar]
- Nunomura W., Jinbo Y., Isozumi N., Ohki S., Izumi Y., Matsushima N., Takakuwa Y. (2013). Novel mechanism of regulation of protein 4.1G binding properties through Ca(2+)/calmodulin-mediated structural changes. Cell Biochem. Biophys. 66, 545–558 [DOI] [PubMed] [Google Scholar]
- Ohno N., Terada N., Tanaka J., Yokoyama A., Yamakawa H., Fujii Y., Baba T., Ohara O., Ohno S. (2005). Protein 4.1 G localizes in rodent microglia. Histochem. Cell Biol. 124, 477–486 10.1007/s00418-005-0058-0 [DOI] [PubMed] [Google Scholar]
- Ohno N., Terada N., Yamakawa H., Komada M., Ohara O., Trapp B. D., Ohno S. (2006). Expression of protein 4.1G in Schwann cells of the peripheral nervous system. J. Neurosci. Res. 84, 568–577 10.1002/jnr.20949 [DOI] [PubMed] [Google Scholar]
- Okumura K., Mochizuki E., Yokohama M., Yamakawa H., Shitara H., Mburu P., Yonekawa H., Brown S. D. M., Kikkawa Y. (2010). Protein 4.1 expression in the developing hair cells of the mouse inner ear. Brain Res. 1307, 53–62 10.1016/j.brainres.2009.10.039 [DOI] [PubMed] [Google Scholar]
- Parra M., Gascard P., Walensky L. D., Snyder S. H., Mohandas N., Conboy J. G. (1998). Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics 49, 298–306 10.1006/geno.1998.5265 [DOI] [PubMed] [Google Scholar]
- Parra M., Gascard P., Walensky L. D., Gimm J. A., Blackshaw S., Chan N., Takakuwa Y., Berger T., Lee G., Chasis J. A. et al. (2000). Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J. Biol. Chem. 275, 3247–3255 10.1074/jbc.275.5.3247 [DOI] [PubMed] [Google Scholar]
- Peters L. L., Weier H. U., Walensky L. D., Snyder S. H., Parra M., Mohandas N., Conboy J. G. (1998). Four paralogous protein 4.1 genes map to distinct chromosomes in mouse and human. Genomics 54, 348–350 10.1006/geno.1998.5537 [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Taylor-Harris P. M., Bennett P. M., Carter E., Hayes N. V. L., King M. D. A., Holt M. R., Maggs A. M., Gascard P., Baines A. J. (2012). Isoforms of protein 4.1 are differentially distributed in heart muscle cells: relation of 4.1R and 4.1G to components of the Ca2+ homeostasis system. Exp. Cell Res. 318, 1467–1479 10.1016/j.yexcr.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Poetsch A., Molday L. L., Molday R. S. (2001). The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J. Biol. Chem. 276, 48009–48016 [DOI] [PubMed] [Google Scholar]
- Ramez M., Blot-Chabaud M., Cluzeaud F., Chanan S., Patterson M., Walensky L. D., Marfatia S., Baines A. J., Chasis J. A., Conboy J. G. et al. (2003). Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 63, 1321–1337 10.1046/j.1523-1755.2003.00870.x [DOI] [PubMed] [Google Scholar]
- Reidel B., Thompson J. W., Farsiu S., Moseley M. A., Skiba N. P., Arshavsky V. Y. (2011). Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol. Cell Proteomics 10, M110 002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter L. M., Khattree N., Tam B., Moritz O. L., Schmitz F., Goldberg A. F. X. (2011). In situ visualization of protein interactions in sensory neurons: glutamic acid-rich proteins (GARPs) play differential roles for photoreceptor outer segment scaffolding. Neuroscience 31, 11231–11243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Dütting E., Enz R. (2008). Band 4.1 proteins are expressed in the retina and interact with both isoforms of the metabotropic glutamate receptor type 8. J. Neurochem. 105, 2375–2387 10.1111/j.1471-4159.2008.05331.x [DOI] [PubMed] [Google Scholar]
- Saito M., Sugai M., Katsushima Y., Yanagisawa T., Sukegawa J., Nakahata N. (2005). Increase in cell-surface localization of parathyroid hormone receptor by cytoskeletal protein 4.1G. Biochem. J. 392, 75–81 10.1042/BJ20050618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer A., Schauf H., Bauer P. J. (2000). Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J. Biol. Chem. 275, 13448–13454 10.1074/jbc.275.18.13448 [DOI] [PubMed] [Google Scholar]
- Scott C., Phillips G. W., Baines A. J. (2001). Properties of the C-terminal domain of 4.1 proteins. Eur. J. Biochem. 268, 3709–3717 [DOI] [PubMed] [Google Scholar]
- Skiba N. P., Spencer W. J., Salinas R. Y., Lieu E. C., Thompson J. W., Arshavsky V. Y. (2013). Proteomic identification of unique photoreceptor disc components reveals the presence of PRCD, a protein linked to retinal degeneration. J. Proteome Res. 12, 3010–3018 10.1021/pr4003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. (1975). The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp. Eye Res 20, 211–217 [DOI] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr (1990). Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J. Cell Biol. 110, 617–624 10.1083/jcb.110.3.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateyama M., Kubo Y. (2007). Coupling profile of the metabotropic glutamate receptor 1alpha is regulated by the C-terminal domain. Mol. Cell. Neurosci. 34, 445–452 10.1016/j.mcn.2006.11.021 [DOI] [PubMed] [Google Scholar]
- Taylor-Harris P. M., Felkin L. E., Birks E. J., Franklin R. C. G., Yacoub M. H., Baines A. J., Barton P. J. R., Pinder J. C. (2005). Expression of human membrane skeleton protein genes for protein 4.1 and betaIISigma2-spectrin assayed by real-time RT-PCR. Cell. Mol. Biol. Lett. 10, 135–149 [PubMed] [Google Scholar]
- Terada N., Ohno N., Saitoh S., Saitoh Y., Komada M., Kubota H., Ohno S. (2010). Involvement of a membrane skeletal protein, 4.1G, for Sertoli/germ cell interaction. Reproduction 139, 883–892 10.1530/REP-10-0005 [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. (1979). Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc. Natl. Acad. Sci. USA 76, 5192–5196 10.1073/pnas.76.10.5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. (1979). In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature 280, 811–814 10.1038/280811a0 [DOI] [PubMed] [Google Scholar]
- Vedrenne C., Klopfenstein D. R., Hauri H-P. (2005). Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol. Biol. Cell 16, 1928–1937 10.1091/mbc.E04-07-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky L. D., Gascard P., Fields M. E., Blackshaw S., Conboy J. G., Mohandas N., Snyder S. H. (1998). The 13-kD FK506 binding protein, FKBP13, interacts with a novel homologue of the erythrocyte membrane cytoskeletal protein 4.1. J. Cell Biol. 141, 143–153 10.1083/jcb.141.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky L. D., Blackshaw S., Liao D., Watkins C. C., Weier H. U., Parra M., Huganir R. L., Conboy J. G., Mohandas N., Snyder S. H. (1999). A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. Neuroscience 19, 6457–6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu C., Debnath G., Baines A. J., Conboy J. G., Mohandas N., An X. (2010). Comprehensive characterization of expression patterns of protein 4.1 family members in mouse adrenal gland: implications for functions. Histochem. Cell Biol. 134, 411–420 10.1007/s00418-010-0749-z [DOI] [PubMed] [Google Scholar]
- Xia W., Liang F. (2012). 4.1G promotes arborization and tight junction formation of oligodendrocyte cell line OLN-93. J. Cell. Physiol. 227, 2730–2739 10.1002/jcp.23017 [DOI] [PubMed] [Google Scholar]
- Yang S., Weng H., Chen L., Guo X., Parra M., Conboy J., Debnath G., Lambert A. J., Peters L. L., Baines A. J. et al. (2011). Lack of protein 4.1G causes altered expression and localization of the cell adhesion molecule nectin-like 4 in testis and can cause male infertility. Mol. Cell. Biol. 31, 2276–2286 10.1128/MCB.01105-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Molday L. L., Molday R. S., Sarfare S. S., Woodruff M. L., Fain G. L., Kraft T. W., Pittler S. J. (2009). Knockout of GARPs and the β-subunit of the rod cGMP-gated channel disrupts disk morphogenesis and rod outer segment structural integrity. J. Cell Sci. 122, 1192–1200 10.1242/jcs.042531 [DOI] [PMC free article] [PubMed] [Google Scholar]