Abstract
Intrinsic laryngeal muscle investigations, especially those of the interarytenoid (IA) muscle, have been primarily teleologically based. We determined IA muscle anatomy and histochemical and immunohistochemical classification of extrafusal and intrafusal (muscle spindle) fibers in 5 patients. Extrafusal fibers were oxidative type I and glycolytic types IIA and IIX. Intrafusal fibers of muscle spindles were identified by the presence of tonic and neonatal myosin. The results demonstrate that the IA muscle has a phenotype similar to that of limb skeletal muscle. Myosin coexpression, the absence of intrafusal fibers, and fiber type grouping were unusual features found previously in the thyroarytenoid and posterior cricoarytenoid muscles, but they were not present in the IA muscle. These findings lead to the conclusion that the IA muscle has functional significance beyond its assumed importance in maintaining vocal fold position during phonation. The presence of spindles demonstrates differences in motor control as compared to the thyroarytenoid and posterior cricoarytenoid muscles. Further, extrafusal fiber characteristics implicate IA muscle involvement in muscle tension dysphonia and adductor spasmodic dysphonia. Given the unique physiologic characteristics of the human IA muscle, further research into the role of the IA muscle in voice disorders is warranted.
Keywords: fiber type, interarytenoid muscle, laryngeal muscle, muscle fatigue, muscle spindle, voice disorder
INTRODUCTION
Intrinsic laryngeal muscles are commonly thought to perform simple general tasks as either vocal fold “adductors” or “abductors”; however, current research indicates that this classification may be misleading.1 Although each of the intrinsic laryngeal muscles has primary roles in laryngeal function, all intrinsic laryngeal muscles are needed for what have been defined as traditional adductor tasks (ie, phonation) and abductor tasks (ie, rapid breathing). For example, the interarytenoid (IA) muscle, a largely unstudied laryngeal muscle, was previously thought to be primarily used in the closure of the posterior glottis during adduction of the vocal folds.2,3 Findings of laryngeal electromyography (EMG) performed during different phonatory and vegetative tasks, however, show that the IA muscle has a major role in vocal fold positioning associated with extended phonation and even stabilizes the cricoarytenoid joint during abduction tasks (ie, forceful active breathing).1 The IA muscle also appears to function independently of the other traditional adductors — the thyroarytenoid (TA) and lateral cricoarytenoid muscles — during some glottic closure tasks such as throat clearing and swallowing. Although laryngeal EMG can provide some information about intrinsic laryngeal muscle activation, quantification of muscle activation varies according to electrode placement and the effort given by the subject and is not possible with laryngeal EMG.1 Another method, therefore, is needed to provide further insight into the functioning of the IA muscle.
Animal experimentation has provided a substantial basis for understanding mammalian laryngeal function, but the highly adapted nature of the human larynx and the functional requirements of speech necessitate some direct human experiments to enhance our knowledge of laryngeal muscle function. Recently, there has been renewed interest in characterizing contractile proteins, fiber types, and function in laryngeal muscle. A variety of new investigative methods can be used on normally functioning laryngeal muscle to biochemically isolate contractile and regulatory proteins4,5 and gene message (m-RNA),6 to determine the physiological properties of single muscle fibers or fiber bundles,7,8 and to establish fiber type amount and distribution from frozen tissue sections.9–11 These techniques taken together give a more complete estimate of how intrinsic laryngeal muscles behave physiologically during laryngeal function. This information forms an important basis for characterization of normal contraction speeds and fatigue rates, and whether these characteristics change in voice disorders such as vocal fold paralysis, paresis, atrophy, and muscle tension dysphonia. Results from such investigations may also contribute to a more comprehensive understanding of normal voice production and vocal disorders. Furthermore, this knowledge will likely enhance the future research of laryngeal reinnervation and the development of a laryngeal pacemaker.
These techniques have been used to further describe fiber types and function in the human TA and posterior cricoarytenoid (PCA) muscles. In mammals, skeletal muscle fiber types are classified as slow-contracting type I fibers and fast-contracting type II fibers, which are further subclassifed as types IIA, IIB, and IIX.12 Each of these fiber types expresses a different myosin heavy chain (MHC) isoform, which is the principal regulator of contraction speed.13 An important distinction in large mammals, including humans, is that the type IIB fiber (predominant in small mammals) is not present. Type IIB fibers are the fastest-contracting and most fatigable of the fast subtypes. A continuum from slow to fast contraction speed for type II muscle fibers is IIA > IIX > IIB. The tension cost for type IIB fibers (the total amount of adenosine triphosphate [ATP] that must be utilized per unit of force), however, is dramatically higher than for type IIA and IIX fibers.14 Large mammals have probably lost the IIB fiber type in accordance with energy conservation. In normal adult human skeletal muscle, therefore, type I, IIA, and IIX fibers predominate, but vary with functional differences in individual muscles.
In determining fiber types in specialized cranial muscles, highly adapted functions have led to the evolution of novel myosins and fiber types, as seen in the extraocular (globe-rotating) muscles and the jaw-closing muscles. For example, an extraocular MHC isoform is present in mammalian extraocular muscles and is associated with a fast contraction speed.15 The jaw-closing muscles of carnivores and primates contain a type II masticatory fiber type with a “IIM” MHC isoform that is thought to be necessary for carnivorous behavior and aggressive, predatory biting.16 Controversy still exists regarding the presence of these or other unique isoforms in human laryngeal muscles. An initial observation in rat TA and PCA muscles indicated the presence of an additional MHC isoform after protein separation with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). This isoform was termed “IIL” because of its presence in laryngeal muscle, but with the additional caveat that this band comigrated with the protein species known to be the extraocular MHC isoform in rat rectus muscles.17 Since this report, several laboratories have attempted to definitively identify a novel MHC protein in laryngeal muscle, but this goal has remained elusive for 2 reasons: first, it is doubtful that an antibody with reactivity specific to extraocular MHC currently exists, and second, there is no solid molecular evidence of a novel MHC gene or protein.18 At present, human laryngeal muscles are thought to express the typical type I, IIA, and IIX isoforms similar to fiber types seen in limb skeletal muscle.
Human PCA and TA muscles have recently been characterized for fiber type and protein expression. Even previously, the PCA muscle was thought to be subdivided into 2 bellies, the horizontal and oblique (vertical),19 as was confirmed by recent results that describe different proportions of fiber types between these compartments. The horizontal compartment is predominantly slow, with 75% to 80% of the fibers being type I,5,10 whereas the vertical belly has a more even distribution of fast and slow fibers. Wu et al5 report 60% fast fibers, whereas Brandon et al10 report about 50% fast fibers. In comparing reports from these two studies, an important distinction must be made. Wu et al took muscle samples and dissected single fibers that were subsequently analyzed for protein content by SDS-PAGE. Brandon et al, however, froze and stained serial sections of muscle to determine fiber type. In a comparison of the results from the vertical compartment of the PCA muscle, single fiber dissection showed that about 40% of the fibers coexpressed MHC isoforms (most commonly IIA and IIX),5 whereas tissue staining from serial sections showed only a modest amount of coexpression. These differing results suggest one of two possibilities: either 1) dissection did not produce single fibers, or 2) MHC isoform coexpression varies along the fiber length such that upon sectioning, some sections will yield only type IIA MHC and others only type IIX. Further work is needed to resolve these uncertainties.
In characterizing skeletal muscle, “fiber type” is a term generally used to describe the skeletal muscle fiber cells themselves: however, an additional group of muscle fiber cells are present in a sensory organ. termed the muscle spindle. Muscle spindles are stretch receptors that contain their own skeletal muscle fiber types associated with unique MHC isoform expression, commonly known as intrafusal fibers. Intrafusal fibers are classified into 3 types: bag1 fibers containing elevated levels of tonic MHC, bag2 fibers containing elevated levels of neonatal MHC, and chain fibers also containing neonatal myosin. These fibers are surrounded by a connective tissue capsule and are innervated by both motor and sensory afferent nerves. Work has been done to characterize the presence of intrafusal fibers in human TA muscle. Hematoxylin staining has previously established that human TA muscle is richly endowed with muscle spindles, especially in regions near the vocal folds.20 We recently attempted to confirm the results of Sanders et al20 by immunohistochemical staining with antibodies reactive for neonatal and tonic myosin, but we were unable to identify muscle spindles in either the TA or PCA muscles.10,11 Given the close proximity of muscle fiber cells to the vocal fold and the abundant connective tissue component of TA muscle, it is possible to confuse several isolated skeletal muscle fibers associated with muscle connective tissue with a true muscle spindle. Staining with tonic and neonatal myosin easily separates true spindles from structures that morphologically appear to be spindles after routine cell staining.
Only one study to date has observed myosin composition in the IA muscle.6 The MHC types I, IIA, and IIB were found in single fibers by SDS-PAGE and Western blot techniques, with a reportedly higher percentage of type 1 than type II fibers, but these results are questionable, because type IIB MHC protein is not expressed in humans. In addition to more fully describing fiber type composition, knowledge of the presence and anatomy of muscle spindles is necessary for understanding IA muscle function. The IA muscle’s overall importance to laryngeal functioning is only partially appreciated at present, given its very complex and variable roles in vocal fold adduction, maintenance of vocal fold adduction during extended phonation, stabilization of the cricoarytenoid joint during throat clearing and swallowing, and abduction during vigorous active breathing. The purpose of this study, therefore, was to provide a more complete view of the IA muscle with regard to general anatomy, muscle fiber type, and muscle spindle presence and characterization.
MATERIALS AND METHODS
Whole IA muscle was obtained from 5 subjects who underwent total laryngectomy as part of their cancer treatment in the Department of Otolaryngology, University of Pittsburgh Medical Center. Appropriate institutional review board approval for experiments involving human subjects was obtained. Of these subjects, 4 were male and 1 was female; the average age was 68 years. Before surgical intervention, vocal fold function was assessed by videolaryngoscopy by one author (C.R.) so that normal vocal fold function could be ascertained. Immediately after removal of the larynx, the IA muscle was dissected free from the arytenoid cartilages and oriented on gauze moistened with cooled saline solution so that the native in vivo orientation of the sample could be maintained. The right arytenoid cartilage attachment was identified with a suture, and the dorsal surface of the muscle was marked with India ink. The samples were transported on ice to the laboratory, in which the muscle was oriented and snap-frozen in isopentane cooled by dry ice to −70°C. Serial 10-μm cryosections were obtained along the entire length of the samples.
To determine extrafusal (skeletal muscle fibers) and intrafusal (muscle spindle fibers) fiber types, we stained mini-series of sections at regular intervals throughout the entire section with anti-MHC antibodies, with adenosine triphosphatase (ATPase) histochemical stains after preincubation of tissue in a variety of pH buffers, and with metabolic histochemical stains that could determine the relative oxidative and glycolytic tissue capacities.
Antibody staining was used to help determine the type and amount of MHC isoform contained in each muscle fiber by an indirect immunoperoxidase method. Primary antibody reactivity was detected with either a peroxidase conjugated secondary antibody or a biotin-labeled secondary antibody visualized by extravidin peroxidase. The following MHC-specific antibodies were used: anti–type I monoclonal (slow skeletal MHC; Sigma Aldrich clone NOQ7), anti-fast monoclonal (fast skeletal IIA, IIB, and IIX MHC; Sigma Aldrich clone MY-32), anti-IIA monoclonal (fast IIA; American Type Culture Collection clone SC-71), anti-neonatal polyclonal,21 anti-tonic polyclonal,21 anti-cardiac monoclonal (cardiac α MHC; Sera Lab clone MAS-366), and anti-masticatory polyclonal.4 The slow and fast antibodies were used to help determine extrafusal fiber type, the tonic and neonatal antibodies were used to determine intrafusal fiber type (as explained in Results), and the cardiac α and type II masticatory antibodies were used to determine whether atypical myosins were also present in IA muscle.
Myofibrillar ATPase histochemical staining was used to determine the actual ATPase enzyme activity of the respective MHC isoforms. This assessment assists in fiber typing, because each isoform has a distinct reactivity for ATP that varies by intracellular pH levels. The original protocol for histochemical determination of fiber type after preincubation in buffers of different pH for human muscle was developed by Brooke and Kaiser.22 We modified the protocol as previously described by Snow et al23 as method A, except that the incubation time in ATP was doubled to obtain maximal reactivity. This was necessary because the skeletal muscle of large mammals tends to have less overall ATPase activity than that of smaller mammals.
As with our previous work on human skeletal muscle, a fiber type classification was possible for IA muscle (Fig 1). The metabolic capacity of fibers was determined by histochemical staining to detect the reactivity, presence, and amounts of 2 key metabolic enzymes: glyceraldehyde 3-phosphate dehydrogenase (α-GPDH), which is active in glycolysis, and succinate dehydrogenase (SDH), which is active in oxidative phosphorylation (Fig 2). The staining method used in the present study for α-GPDH was similar to that first described by Padykula,24 and the SDH staining protocol was similar to the original method of Nachlas et al.25 In skeletal muscle, there is a tradeoff in energy production. During rapid contraction, muscle fibers use the glycolytic pathway, because ATP production must also be rapid. This process quickly produces a limited amount of ATP for energy use along with the additional metabolic by-product, lactic acid. With rapid contraction, therefore, ATP stores diminish rapidly, and lactic acid and acidic pH levels increase, causing muscle fiber fatigue. Elevated levels of α-GPDH are found in these “fast-contracting–fatigable” fibers. During slow to moderately fast contractions, muscle fibers do not require extremely rapid production of energy. These fibers, therefore, rely on the citric acid cycle and oxidative phosphorylation to produce relatively large amounts of ATP. This metabolic pathway avoids lactic acid buildup and provides adequate energy for sustained excitation-contraction coupling. Hence, fibers of this variety are commonly termed “slow-contracting–fatigue-resistant” or “fast-contracting–fatigue-resistant” and contain elevated levels of SDH. Taking the myofibrillar ATPase and metabolic histochemical staining profiles together with previous physiological results on mammalian skeletal muscle, a common classification of fiber types is as follows: type I fibers, slow-contracting–fatigue-resistant; type IIA fibers, fast-contracting–fatigue-resistant; and type IIX fibers, fast-contracting–fatigable.
Fig. 1.
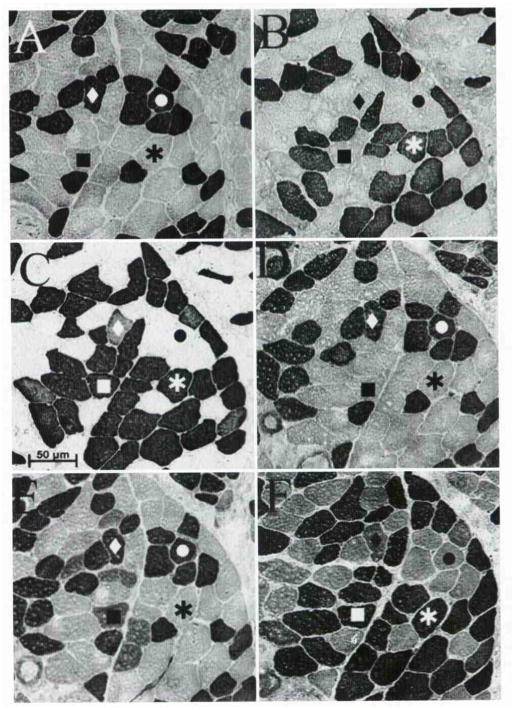
Myofibrillar adenosine triphosphatase (ATPase) histochemical stain used to determine enzyme reactivity of muscle fiber types after preincubation in buffers of varying pH. Shadings differentiate fiber types present in muscle. Dark and intermediate shadings indicate positive reactivity. Pale shadings indicate negative reactivity. Fast-contracting (type II) fibers are reactive at alkali pH, and slow-contracting (type I) fibers are reactive at mild acid pH. After preincubation in buffer pH 4.6, all 3 fiber types may be identified by differential staining: type I as highly reactive, type IIX as intermediately reactive, and IIA as non-reactive.
Fig. 2.
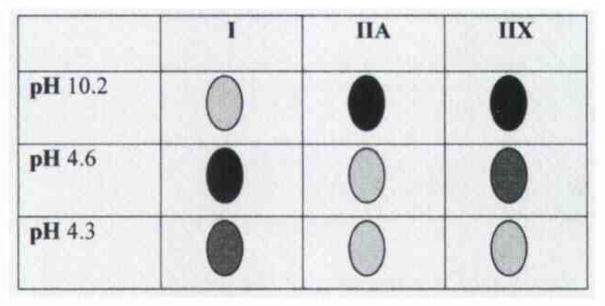
Metabolic histochemical staining used to detect reactivity, presence, and amounts of 2 metabolic enzymes: glyceraldehyde 3-phosphate dehydrogenase (α-GPDH) and succinate dehydrogenase (SDH). Dark shadings indicate elevated levels of enzyme. Intermediate shadings indicate medium levels of enzyme, and pale shadings indicate no reactivity.
RESULTS
General Anatomy
The IA muscle consists of a series of muscle bellies oriented in a generally transverse direction. The dorsal bellies are oblique and extend from the superior aspect of the vocal process of one arytenoid cartilage to the inferior aspect of the vocal process of the contralateral arytenoid cartilage. The ventral (main) belly consists of muscle fibers that run transversely from the concave posterolateral face of one arytenoid cartilage to that of the other. In situ, the IA muscle assumes a U shape. Contraction of the IA muscle should slide the arytenoid cartilages toward one another, as well as pull them into a more upright position — in effect, tensing the vocal folds.
Extrafusal Fiber Types
Through a combination of antibody and myofibrillar ATPase histochemical analysis, we were able to classify fibers in accordance with type I, IIA, and IIX fibers as typically found in human skeletal muscle. Slow fibers were reactive for anti–type I MHC antibody and had ATPase activity at pH 4.6 and 4.3. Type IIA fibers were reactive for anti–type IIA and anti-fast MHC antibody and had ATPase activity at pH 10.2. Type IIX fibers were reactive only for anti-fast MHC antibody and had strong ATPase activity at pH 10.2 and moderate ATPase activity at pH 4.6 (Fig 3E). Of particular interest are fibers that coexpress type I, IIA, and IIX myosins, as previous groups have reported significant fiber populations with this phenotype. In the present study, however, only a relatively few fibers showed this hybrid coexpression. In Fig 3,3 fibers coexpressed both type I and IIX MHC isoforms (diamond in Fig 3), which is a very unusual combination, because myosin transitions and/or coexpressions tend to follow the continuum I > I + IIA > IIA > IIA + IIX > IIX. Although morphometric analysis was not a goal of the present work, the human IA muscle fiber type composition was estimated from the tissue staining and was found to be approximately 35% type I fibers, 45% type IIA fibers, 15% type IIX fibers, and 5% or fewer fibers that coexpress MHC isoforms.
Fig. 3.
Serial sections from normally functioning human interarytenoid (IA) muscle stained for A–C) myosin heavy chain (MHC) content and D–F) myofibrillar ATPase enzyme activity. Immunohistochemical identification of protein was possible with A) anti–type I antibody, B) anti–type IIA antibody, and C) general anti–type II antibody. ATPase activity demonstrated fiber type–specific reactivity for MHC isoforms after preincubation in buffers of D) pH 4.3. E) pH 4.6. and F) pH 10.2. Circle — type I fiber; asterisk — type IIA fiber; square — type IIX fiber; diamond — fiber expressing both type I and type IIX MHCs. IA muscle has phenotype similar to typical limb skeletal muscle without unusual MHC isoform expression and very limited cellular coexpression of myosin.
After the proportion of types was determined, serial sections were processed for metabolic histochemical staining (Fig 4). Type I fibers, as determined by immunohistochemical and ATPase staining, had very low levels of the glycolytic metabolic enzyme α-GPDH and tended to have the highest levels of the oxidative metabolic enzyme SDH. There were, however, instances in which a type I fiber ex pressed more α-GPDH than SDH, which were an indication that these particular fibers were more glycolytic than oxidative (arrows in Fig 4). Such a finding is seldom reported in the literature. In a similar comparison, fibers typed as IIA and IIX had much higher α-GPDH activity than did type I fibers and had lower levels of SDH activity. Occasionally, a type IIA fiber had only modest levels of α-GPDH, indicating a fiber classification of “fast-contracting–fatigue-resistant,” but this finding was exceedingly rare. In addition, there were generally very low levels of SDH activity throughout the tissue. This result, as well as the characterization of most type IIA fibers as “fast-contracting–fatigable” (asterisk in Fig 4), led us to conclude that the IA muscle could be rapidly fatigued if called upon for quick and forceful contraction. These findings are in sharp contradistinction to those in other cranial muscles such as the extraocular and jaw-closing groups, in which even the fastest-contracting fibers have exceedingly high levels in SDH and rely on oxidative metabolism.26
Fig. 4.
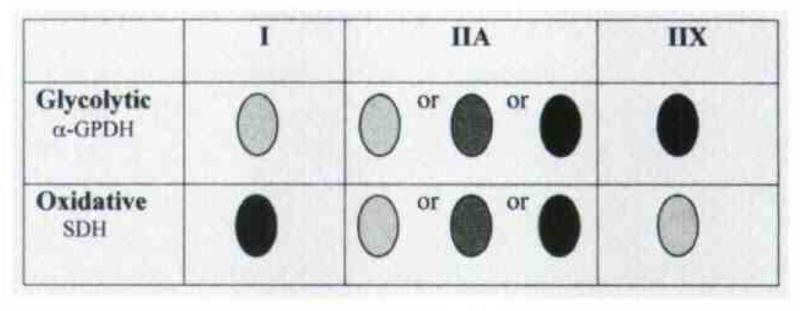
Levels of cellular metabolites in comparison to fiber type composition in human IA muscle. Circle — type I fiber; asterisk — type IIA fiber; square — type IIX fiber. A) Glycolytic capacity was determined by reactivity for α-GPDH, enzyme in glycolytic pathway. B) Myofibrillar ATPase histochemical staining after preincubation in buffer of pH 4.6 allowed determination of fiber type (confirmed with additional staining as in Fig 1). C) Oxidative capacity was determined by reactivity for SDH, enzyme in citric acid cycle. IA muscle contained robust stores of glycolytic enzymes in type IIA and type IIX fibers, and type I fibers had diminished levels of oxidative enzymes. Some type I fibers, however, had increased levels of α-GPDH (arrows). IA muscle appears to be more glycolytic and less oxidative than other cranial muscles studied previously.
Intrafusal Fiber Types and Muscle Spindle Anatomy
Typical mammalian muscle spindles usually contain a larger-diameter bag1 fiber type, identified by expression of tonic MHC, and 2 other fiber types, bag2 and chain, which express neonatal MHC. These fibers may be distinguished by their relative size: bag2 fibers have a larger diameter than do chain fibers. The fiber type distribution, at least in simple muscle spindles in smaller mammals, usually consists of 1 bag1 fiber, 1 bag2 fiber, and 2 or more chain fibers. In previous investigations, we were unable to identify muscle spindles in human PCA10 and TA11 muscles. In the current study, however, muscle spindles were easily identified in human IA muscle, at an average of 7 spindles per muscle. In general, the spindles spanned the entire length of the muscle and were located in the central portion of the muscle belly. Some spindles had a simple anatomy, in that they existed as a single entity surrounded by a solitary spindle capsule of connective tissue. Other spindles had a much more complex anatomy. As seen in Fig 5, these spindles have separate encapsulations at their ends, but in the equatorial areas they share the same connective tissue capsule. A highly unusual anatomic arrangement seen in some spindles with a complex anatomy was the presence of extrafusal fibers along at least a portion of their length (Fig 5F). When present, these extrafusal fiber types were both fast and slow. The fiber type distribution in most spindles was as expected, with 1 bag1 fiber, 1 bag2 fiber, and a variable number of chains (Fig 5), but in some spindles no bag2 fibers could be identified (Fig 6). Finally, a common feature in all spindles was that toward the ends of the muscle, their orientation in the tissue changed such that they could only be identified in an oblique section (Fig 6C, D). This change in orientation represented an anatomic bending of the spindles as the muscle wrapped around the lateral aspect of the arytenoid cartilages.
Fig. 5.
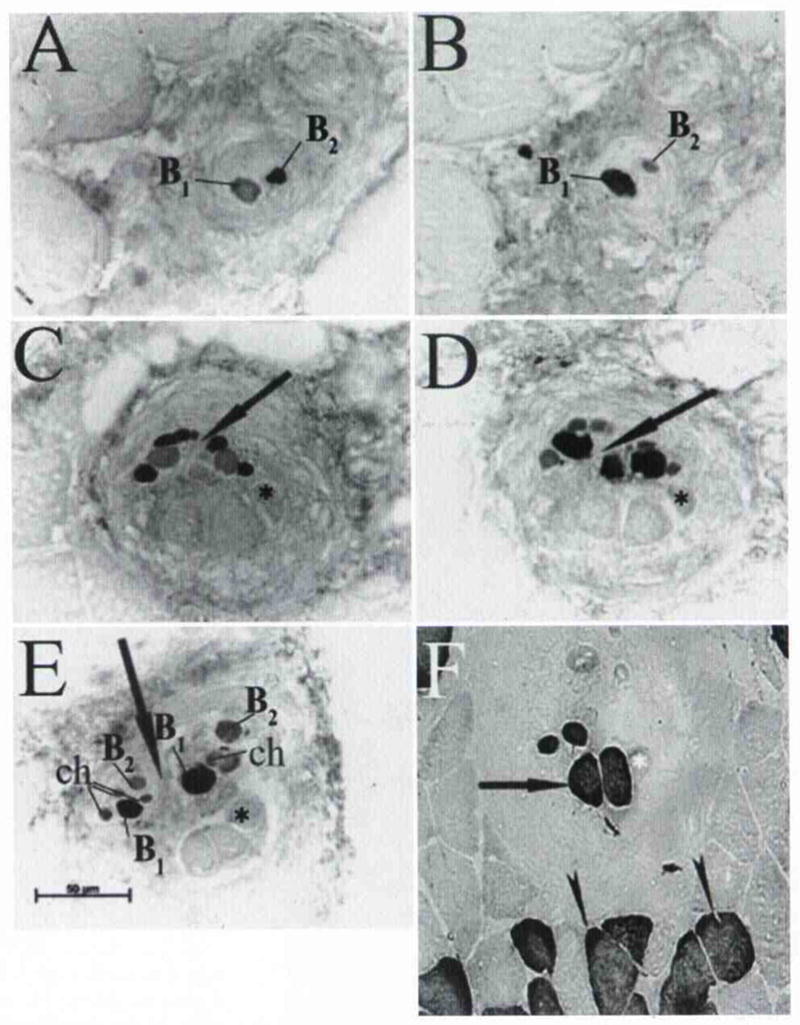
Two spindles in IA muscle that share common connective tissue capsule through only portion of their length are demonstrated by staining with tonic and neonatal MHC–specific isoforms. Origin of one of these spindles starts with identification of 1 bag1 fiber (B1) and 1 bag2 fiber (B2). A) Bag2 fiber was identified by staining with anti–neonatal MHC antibody. B) Bag1 fiber was identified by staining with anti–tonic MHC antibody. C, D) In equatorial area, this spindle shares its encapsulation with another spindle (arrow), asterisk — fast extrafusal fiber contained in spindle capsule. E) Further along their length, spindles again separate into 2 distinct capsules (arrow). Each spindle contained 1 bag1 fiber, 1 bag2 fiber, and 2 to 4 chain fibers. Additional unusual feature in this spindle arrangement was inclusion of 3 extrafusal fibers in spindle capsule. ch — chain fibers. F) Two extrafusal fibers with positive reactivity for slow MHC antibody (arrow). Arrowheads — normal slow extrafusal fibers.
Fig. 6.
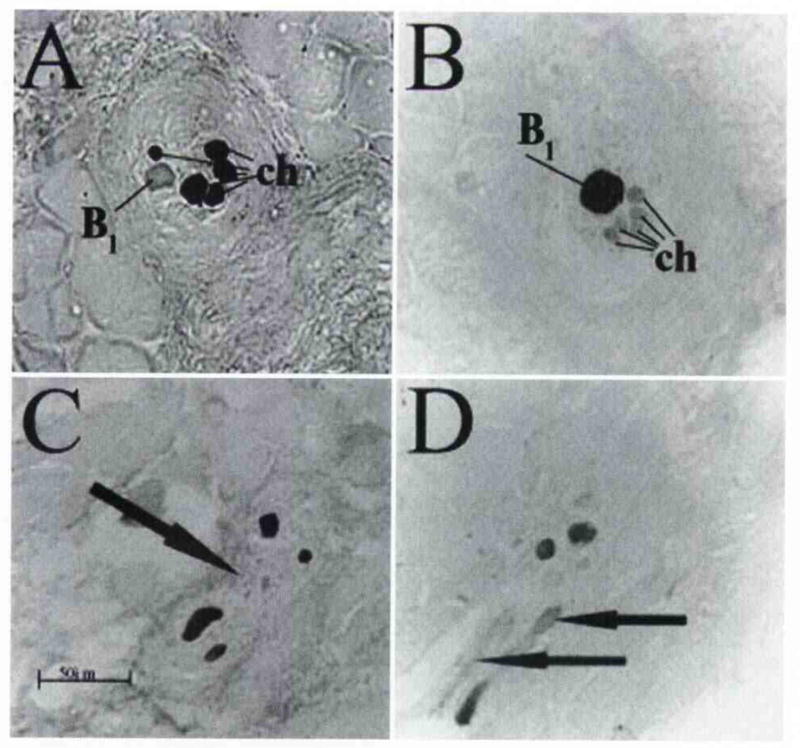
Muscle spindle containing A, C, D) 5 chain fibers as detected with neonatal MHC antibody, and B) 1 bag1 fiber as detected with tonic MHC antibody. D) Feature common to most spindles was change to oblique orientation at their beginning and end, which represents bending of muscle belly as it attaches to arytenoid cartilages. Arrow in C marks separation of spindle into two distinct capsules. Arrows in D mark oblique fibers in spindle.
Lack of Unusual Muscle Features
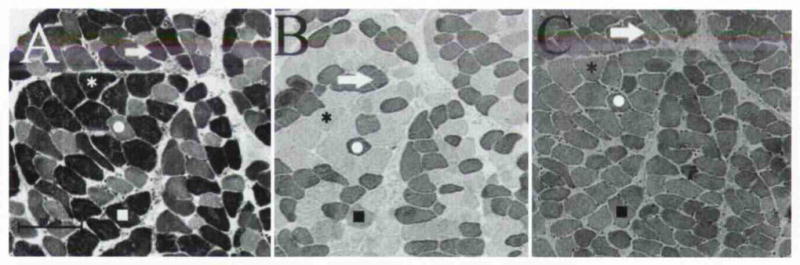
Intrinsic laryngeal muscles may be easily damaged, mostly because of problems with innervation. Such damage is usually seen at the tissue level in 2 forms: fiber type grouping and expression of neonatal MHC in the extrafusal fibers. Fiber type grouping occurs when a local area of extrafusal fibers loses its original motor nerve supply from a number of motor neurons. With subsequent regeneration, a single motor neuron from the local area begins sprouting nerve terminal endings and eventually forms motor end plates on all of the skeletal muscle fibers. These muscle fibers are therefore “grouped,” as they are all innervated by the same motor neuron. Because MHC expression is controlled in part by the propagative activity of the motor nerve, all of these muscle fibers modify their MHC expression to match the innervating motor neuron type. In the PCA muscle, we found fiber type grouping both in patients with vocal fold immobilization and in those with normal vocal function (Fig 7). In the IA muscle, no histologic signs of disrupted innervation and no fiber type grouping were found. Average- and small-diameter neonatal MHC–positive fibers, however, were identified in the IA muscle (Fig 8). The presence of these fibers is extremely rare. Neonatal MHC is expressed in muscle development, but may also be found in adult extrafusal fibers during denervation, regeneration, or atrophy. A small number of extrafusal fibers with neonatal MHC is a normal skeletal muscle phenotype, especially in areas close to connective tissue and muscle fascicles.27 The IA muscle apparently escapes a predisposition to damage, most likely because of innervation differences between it and a muscle such as the PCA.
Fig. 7.
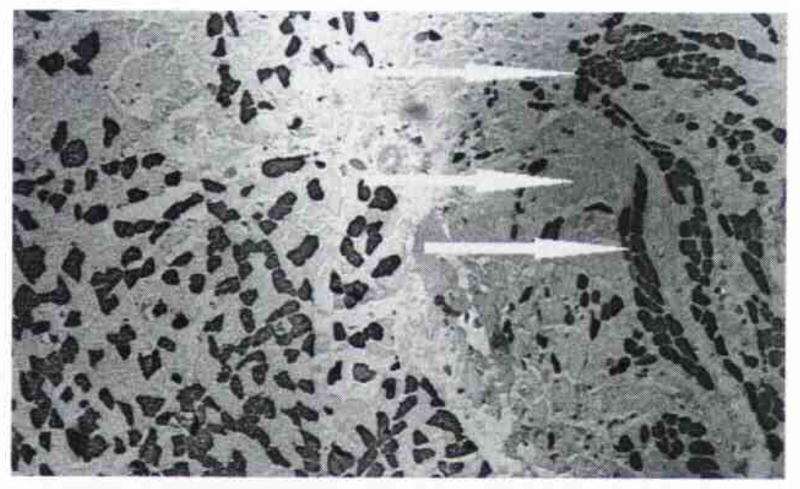
Infrahyoid muscle (right) and PCA muscle frozen, sectioned, and stained together for comparative purposes with anti–fast MHC antibody. Infrahyoid muscle demonstrates mosaic pattern of fiber type distribution typical of normal limb skeletal muscle. PCA muscle, however, had significant numbers of fast fibers distributed together (arrows), indicative of motor nerve sprouting and fiber type grouping subsequent to nerve and muscle damage.
Fig 8.
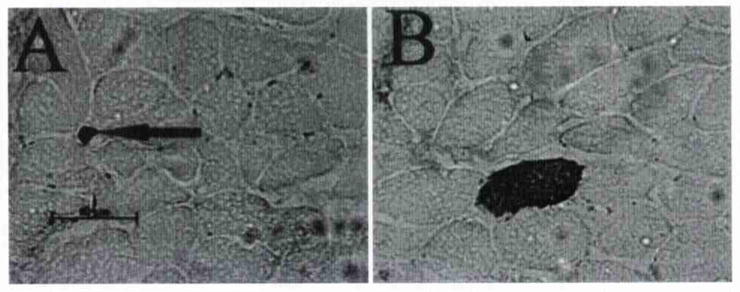
Staining of 2 different areas of 1 human IA muscle sample. Neonatal MHC could be detected in extrafusal fibers of A) small size and B) normal size. Neonatal fibers were very rare and were not indicative of muscle tissue damage. Arrow in A points to small neonatal positive fiber.
DISCUSSION
Interarytenoid Extrafusal Fiber Types Resemble Normal Skeletal Muscle
Our work establishes that substantial information may be obtained about laryngeal function from sampling tissue from fresh laryngectomy specimens. Characterization of human intrinsic laryngeal muscles is in its infancy, yet holds great promise of providing physiological information that presently is lacking or at very best limited. Here we describe for the first time the histologic features of the human IA muscle and suggest how these results apply to a fuller understanding of laryngeal function. Although the laryngeal muscles are specialized cranial muscle, the IA muscle has many phenotypic features similar to those of typical limb skeletal muscle. In IA extrafusal fibers, there is very little coexpression of myosin and no appreciable expression of atypical myosins. Type II masticatory, α-cardiac, and tonic MHCs could not be detected in these fibers. Neonatal myosin could be detected, but only in a very few fibers per muscle sampled. The only human MHC isoform we could not detect with antibodies was extraocular MHC, because there is no antibody with adequate specificity to this isomyosin. Although there has been much speculation about the presence of an extraocular MHC in human laryngeal muscles, it is doubtful that extraocular MHC exists in these muscles. We are currently concluding a study that compares isomyosin protein levels and the MHC message from their corresponding genes in human PCA and TA muscles and have found no extraocular gene message in these muscles (preliminary data). We intend to test for the presence of an extraocular gene message in the human IA muscle in the future, but do not anticipate positive findings.
Extrafusal Fibers Are Highly Glycolytic
Although extrafusal fibers can be characterized as typical skeletal muscle fibers, one unusual component to their phenotype is elevated levels of glycolytic metabolites relative to oxidative metabolites. Type I fibers are known to have principally an oxidative metabolism, yet we could only detect modest levels of the oxidative enzyme SDH. Another surprising finding was that some type I fibers had elevated levels of glycolytic enzymes. As expected, type IIX fibers were always associated with elevated levels of glycolytic enzymes. Type IIA fibers can either be fast-contracting–fatigue-resistant or fast-contracting–fatigable and, accordingly, can have elevated levels of oxidative or glycolytic metabolites. In the IA muscle, type IIA fibers almost always had elevated levels of glycolytic enzymes. Given that all fast-contracting type II fibers are glycolytic, a total of at least 65% of IA muscle fiber content may be characterized as fatigable.
Interarytenoid Muscle Contains Anatomically Complex Muscle Spindles
Although some investigators have purported to find muscle spindles in the human TA muscle,20 recent research has not found spindle structures by antibody staining for spindle-specific myosin antibodies.11 Likewise, no spindle structures were identified in the PCA muscle.10 In the IA muscle, however, we were able to identify muscle spindles easily, at an average of 7 spindles per muscle. Some of these spindles had complex anatomy, in that for part of their length they shared a connective capsule with another spindle. The functional consequences of such structures are unknown, but other specialized cranial muscle spindles have similar arrangements. For example, in human28 and cat29 masseter muscles, deeper anatomic compartments have a number of simple spindles fused together into complex units. Again, the functional significance of these spindle complexes remains unknown. Another unusual feature was the presence of extrafusal fibers found along part of the spindle capsule length. These structures were found previously by Okamura and Katto30 in a scanning electron microscopic study of the IA muscle. In our results, all spindles with complex anatomic arrangements had as their ends independent encapsulated structures with an average of 1 bag1 fiber, 1 bag2 fiber, and 4 or 5 chain fibers. This finding agrees nicely with previous reports on human muscle spindles, in which the most common distribution was 1 of each bag fiber and 5 to 8 chain fibers.31 Another interesting finding in some IA spindles was the presence of a bag1 fiber and a few chains, but no bag2 fibers. These results do imply a functional significance, because there is a well-established physiology literature on the functional differences between intrafusal fibers. Bag2 and chain fibers detect “static sensitivity”; ie, they monitor the resting length and postural activity of the muscle.32 Conversely, bag1 fibers detect “dynamic sensitivity” and provide afferent information about the rate of shortening during active muscle contraction. Given the presence of spindles with only bag1 fibers and the elevated number of fast-contracting–fatigable fibers, the IA muscle must have exquisite ability to contract rapidly and be able to modify the actual rate of shortening for different tasks or situations. These findings are in contrast to the sole “vocal position maintenance” role that has previously been ascribed to the IA muscle.
Innervation May Protect Interarytenoid Muscle From Tissue Damage
The fiber type grouping observed previously in the PCA muscles of normal and unilaterally immobile vocal folds was not found in the IA muscle. Isolated neonatal MHC–positive fibers were noted in the IA muscle, but there were too few of these fibers to indicate any damage. These findings lead to the assumption that the IA muscle may have the ability to escape damage (eg, viral, ischemic, senescence-related), possibly because of differences in innervation between the IA and the PCA muscles. The 2 bellies of the PCA muscle are each innervated by 1 separate branch of the recurrent laryngeal nerve (RLN). The IA muscle is a midline muscle that is innervated bilaterally by the RLN and the internal branch of the superior laryngeal nerve (SLN), which create an intramuscular plexus of nerves contributing to the motor innervation of the IA muscle.33 The IA muscle may even be considered the central site in which all laryngeal nerves meet.33
There is general consensus that the RLN is more prone to damage than the SLN because of its long trunk and circuitous pathway to the intrinsic laryngeal muscles. In the PCA muscle, damage to the RLN appears at the tissue level in the form of fiber type grouping. The IA muscle may therefore be protected from the fiber type grouping seen in response to injury in the PCA muscle when even a portion of the RLN is damaged because of the dual innervation of the IA muscle. According to the current literature, however, it is unclear whether the internal branch of the SLN provides sensory or motor innervation to the IA muscle. If, however, it does provide motor innervation, then innervation by the internal branch of the SLN may spare the IA muscle when there is damage to the RLN.
Clinical Importance of Findings
Many of the findings in this study relate to the current view of the IA muscle in laryngeal function; however, the results also lead to other hypotheses about the principal role of the IA muscle. Hillel1 stated that IA muscle activation was primarily for maintenance of glottic closure during phonation. This “static” or postural activity is supported by the presence of slow-contracting type I fibers and muscle spindles that contain bag2 intrafusal fibers. The majority of the fibers we observed in the IA, however, were fast-contracting and fatigable, as demonstrated by the highly glycolytic properties of the type II muscle fibers. This finding, as well as the finding that some muscle spindles provide mostly dynamic sensitivity (ie, they have bag1 but no bag2 fibers), indicates that the IA muscle has an important role in rapid or brisk adduction or abduction tasks (ie, swallowing and vigorous active breathing). Perlman et al,34 using laryngeal EMG, indicated that the IA muscle has electromyographic activation that is shorter in duration than that of other pharyngeal muscles used in deglutition. Hillel1 also found activation of the IA muscle with swallowing, throat clearing, coughing, and laughing.
We believe that both the type IIA and type IIX groups are highly fatigable, resulting in overall IA muscle fatigue soon after recruitment to maximal force levels. During the functions described above, the muscle probably behaves normally, well below maximal force and fiber fatigue levels, but during functional voice disorders, physiologic demands may exceed muscle performance. In muscle tension dysphonia, for example, intrinsic laryngeal muscles are recruited to contract at a maximal rate during phonation, causing effortful vocalizations during speech and subsequent muscle fatigue.
Adductor spasmodic dysphonia is another voice disorder that has patterns of intrinsic laryngeal muscle contraction different from that seen in normal phonation. This voice disorder is the most common form of laryngeal dystonia and is characterized by closure spasms of the glottis that cause phonatory breaks, vowel initiation difficulties, and an overall sensation of glottic tightness during speech.1 On laryngeal EMG, the IA muscle also has been shown to have postphonatory activity in patients with adductor spasmodic dysphonia. This muscle’s contraction preceding phonation may contribute to increased fatigue in the IA muscle, because the IA muscle does not have a resting period after phonation in which to recover.
In comparison to the PCA and TA muscles, the IA muscle is distinctive, given its fiber type composition and the presence of muscle spindles. It is involved in both postural and rapidly contracting laryngeal functions and seems to contribute to clinical dysfunction in ways that have not been previously considered. A lack of pathological features occurring with age and characterization of multiple and bilateral innervation patterns are preliminary findings that must be explored further to understand IA muscle function. Ongoing investigations with larger patient samples are needed to determine variability in the IA muscle phenotype in normal laryngeal function with age and in voice disorders, but the current findings are sufficient to formulate a number of important concepts necessary for exploration.
CONCLUSIONS
The IA muscle is phenotypically similar to typical limb skeletal muscle and does not share fiber type specializations common to other cranial muscles, including the PCA and TA muscles.
The IA muscle possesses a diverse fiber type population that enables physiology at the extremes of function for both slow, sustained contractions and rapid, forceful contractions.
The fast fibers of the IA muscle are prone to fatigue, given their metabolic composition, and may be implicated in voice disorders such as muscle tension dysphonia and adductor spasmodic dysphonia.
The presence of muscle spindles in the IA muscle and their absence in the PCA and TA muscles indicate that motor control of intrinsic laryngeal muscles varies significantly with the independent tasks of each muscle.
The IA muscle is less prone to nerve damage and dysfunction than other intrinsic laryngeal muscles, but may easily be overworked if called upon to compensate for weakness, synkinesis, or other damage to agonist or antagonist muscles.
Footnotes
Presented at the meeting of the American Laryngological Association, Nashville, Tennessee, May 2-3, 2003.
References
- 1.Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111:1–47. doi: 10.1097/00005537-200104001-00001. [DOI] [PubMed] [Google Scholar]
- 2.Choi HS, Ye M, Berke GS. Function of the interarytenoid (IA) muscle in phonation: in vivo laryngeal model. Yonsei Med J. 1995;36:58–67. doi: 10.3349/ymj.1995.36.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Nasri S, Beizai P, Sercarz JA, Kreiman J, Graves MC, Berke GS. Function of the interarytenoid muscle in a canine laryngeal model. Ann Otol Rhinol Laryngol. 1994;103:975–82. doi: 10.1177/000348949410301208. [DOI] [PubMed] [Google Scholar]
- 4.Sciote JJ, Rowlerson AM, Carlson DS. Myosin expression in the jaw-closing muscles of the domestic cat and American opossum. Arch Oral Biol. 1995;40:405–13. doi: 10.1016/0003-9969(94)00181-a. [DOI] [PubMed] [Google Scholar]
- 5.Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ. New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Arch Otolaryngol Head Neck Surg. 2000;126:857–64. doi: 10.1001/archotol.126.7.857. [DOI] [PubMed] [Google Scholar]
- 6.Shiotani A, Westra WH, Flint PW. Myosin heavy chain composition in human laryngeal muscles. Laryngoscope. 1999;109:1521–4. doi: 10.1097/00005537-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Sciote JJ, Horton MJ, Rowlerson AM, Link J. Specialized cranial muscles: how different are they from limb and abdominal muscles? Cells Tissues Organs. 2003;174:73–86. doi: 10.1159/000070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Antona G, Megighian A, Bortolotto S, et al. Contractile properties and myosin heavy chain isoform composition in single fibre of human laryngeal muscles. J Muscle Res Cell Motil. 2002;23:187–95. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- 9.Sciote JJ, Rowlerson AM, Hopper C, Hunt NP. Fibre type classification and myosin isoforms in the human masseter muscle. J Neurol Sci. 1994;126:15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Muscle fiber type composition and effects of vocal fold immobilization on the two compartments of the human posterior cricoarytenoid: a case study of four patients. J Voice. 2003;17:63–75. doi: 10.1016/s0892-1997(03)00027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–54. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990;38:257–65. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- 13.Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50:197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol. 1996;493(Pt 2):299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartore S, Mascarello F, Rowlerson A, et al. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil. 1987;8:161–72. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- 16.Rowlerson AM, Mascarello F, Veggetti A, Carpené E. The fibre type composition of the first branchial arch muscles in Carnivora and Primates. J Muscle Res Cell Motil. 1983;4:443–72. doi: 10.1007/BF00711949. [DOI] [PubMed] [Google Scholar]
- 17.DelGaudio JM, Sciote JJ, Carroll WR, Esclamado RM. Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol. 1995;104:237–45. doi: 10.1177/000348949510400310. [DOI] [PubMed] [Google Scholar]
- 18.Merati AL, Bodine SC, Bennett T, Jung HH, Furuta H, Ryan AF. Identification of a novel myosin heavy chain gene expressed in the rat larynx. Biochim Biophys Acta. 1996;1306:153–9. doi: 10.1016/0167-4781(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 19.Bryant NJ, Woodson GE, Kaufman K, et al. Human posterior cricoarytenoid muscle compartments. Anatomy and mechanics. Arch Otolaryngol Head Neck Surg. 1996;122:1331–6. doi: 10.1001/archotol.1996.01890240039009. [DOI] [PubMed] [Google Scholar]
- 20.Sanders I, Han Y, Wang J, Biller H. Muscle spindles are concentrated in the superior vocal is subcompartment of the human thyroarytenoid muscle. J Voice. 1998;12:7–16. doi: 10.1016/s0892-1997(98)80070-2. [DOI] [PubMed] [Google Scholar]
- 21.Rowlerson A, Mascarello F, Barker D, Saed H. Muscle-spindle distribution in relation to fibre-type composition of masseter in mammals. J Anat. 1988;161:37–60. [PMC free article] [PubMed] [Google Scholar]
- 22.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–79. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 23.Snow DH, Billeter R, Mascarello F, Carpene E, Rowlerson A, Jenny E. No classical type IIB fibres in dog skeletal muscle. Histochemistry. 1982;75:53–65. doi: 10.1007/BF00492533. [DOI] [PubMed] [Google Scholar]
- 24.Padykula HA. The localization of succinic dehydrogenase in tissue sections of the rat. Am J Anat. 1952;91:107–45. doi: 10.1002/aja.1000910104. [DOI] [PubMed] [Google Scholar]
- 25.Nachlas MM, Tsou KC, de Souza E, Cheng CS, Seligman AM. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957;5:420–36. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- 26.Rowlerson AM. Neurophysiology of the jaws and teeth. In: Taylor A, editor. Specialization of mammalian jaw muscles: fibre type compositions and the distribution of muscle spindles. London, England: Macmillan Press; 1990. pp. 1–44. [Google Scholar]
- 27.Dubowitz V, Brooke MH. Muscle biopsy: a modern approach. London, England: WB Saunders; 1973. [Google Scholar]
- 28.Eriksson PO, Thornell LE. Relation to extrafusal fibre-type composition in muscle-spindle structure and location in the human masseter muscle. Arch Oral Biol. 1987;32:483–91. doi: 10.1016/s0003-9969(87)80009-2. [DOI] [PubMed] [Google Scholar]
- 29.Sciote JJ. Fibre type distribution in the muscle spindles of cat jaw-elevator muscles. Arch Oral Biol. 1993;38:685–8. doi: 10.1016/0003-9969(93)90008-a. [DOI] [PubMed] [Google Scholar]
- 30.Okamura H, Katto Y. Fine structure of muscle spindle in interarytenoid muscle of human larynx. In: Fujimura O, editor. Vocal physiology: voice production, mechanisms, and functions. New York, NY: Raven Press; 1988. pp. 135–44. [Google Scholar]
- 31.Saghal V, Subramani V, Saghal S, Kochar H. Morphology and morphometry of human muscle spindles. In: Boyd IA, Gladden MH, editors. The muscle spindle. London, England: Macmillan; 1985. pp. 107–14. [Google Scholar]
- 32.Bakker GJ, Richmond FJR. Two types of muscle spindles in cat neck muscles: a histochemical study of intrafusal fiber composition. J Neurophysiol. 1981;45:973–86. doi: 10.1152/jn.1981.45.6.973. [DOI] [PubMed] [Google Scholar]
- 33.Sanders I, Wu BL, Mu L, Li Y, Biller HF. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg. 1993;119:934–9. doi: 10.1001/archotol.1993.01880210022003. [DOI] [PubMed] [Google Scholar]
- 34.Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–9. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]