Abstract
CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily, is broadly expressed on antigen-presenting cells (APCs) and other cells, including fibroblasts and endothelial cells. Binding of CD40 and its natural ligand CD40L (CD154) triggers cytokine secretion and increased expression of costimulatory molecules is required for T cell activation and proliferation. However, to our knowledge, the use of agonistic antibodies to CD40 to boost adoptively transferred T cells in vivo has not been investigated. The purpose of this study was to determine whether anti-CD40 monoclonal antibody (mAb) in combination with interleukin (IL)-2 could improve the efficacy of in vitro-activated T cells to enhance antitumor activity. Mice bearing B16 melanoma tumors expressing the gp100 tumor antigen were treated with cultured, activated T cells transgenic for a T-cell receptor specifically recognizing gp100, with or without anti-CD40 mAb. In this model, the combination of anti-CD40 mAb with IL-2 led to expansion of adoptively transferred T cells and induced a more robust antitumor response. Furthermore, the expression of CD40 on bone marrow (BM)-derived cells and the presence of CD80/CD86 in the host were required for the expansion of adoptively transferred T cells. The use of neutralizing mAb to IL-12 provided direct evidence that enhanced IL-12 secretion induced by anti-CD40 mAb was crucial for the expansion of adoptively transferred T cells. Collectively, these findings provide a rationale to evaluate the potential application of anti-CD40 mAb in adoptive T cell therapy for cancer.
Keywords: anti-CD40 mAb, adoptive cell transfer (ACT), immunotherapy
INTRODUCTION
Adoptive cell transfer (ACT) therapy, which may directly provide large numbers of in vitro-selected, highly active and tumor-antigen-specific T lymphocytes to the autologous host, is one of the most promising immunotherapeutic approaches for cancer treatment (1). Studies have shown that ACT therapy can induce T cell-mediated antitumor responses in patients with melanoma, metastatic renal cell carcinoma and lymphoma (1–5). However, many of the clinical responses observed have been transient (6–8). The rapid disappearance of in vitro-expanded, adoptively transferred T cells has been frequently observed in these patients, suggesting that inadequate persistence of tumor-antigen-specific T cells in vivo results in the lack of antitumor efficacy (9, 10).
To induce a productive antitumor response while avoiding deletion and/or tolerance, CD8+ T cells require three signals. Studies have indicated that in addition to T-cell receptor (TCR) complex and costimulation (most notably from CD28), interleukin (IL)-12 and interferon (IFN) α/β are the major sources of the third signal (11–14). CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is broadly expressed on B cells, T cells, dendritic cells (DCs), monocytes, macrophages, and nonhematopoietic cells (15–17). Activation of DCs or macrophages with an agonist of CD40 results in secretion of IL-12 and other cytokines, and also induces the upregulation of costimulatory molecules such as MHC II, CD80, and CD86, which are required for host naïve T cell activation and proliferation (15, 18, 19). However, it is not known whether the CD40/CD40L interaction can also effectively induce expansion of adoptively transferred, in vitro-activated T cells and whether the combination of anti-CD40 monoclonal antibody (mAb) with ACT can enhance antitumor activity.
The purpose of this study was to determine whether anti-CD40 mAb in concert with IL-2 can improve the efficacy of in vitro-activated, adoptively transferred T cells to enhance antitumor activity. For our animal model, we used C57BL/6 mice harboring established subcutaneous B16 tumors expressing the melanoma tumor antigen gp100. For tumor treatment, we adoptively transferred ex vivo-cultured, activated, transgenic T cells (pmel-1 cells) expressing a TCR that specifically recognizes an H-2Db-restricted epitope of gp100, in conjunction with anti-CD40 mAb administration. Although adoptive transfer of pmel-1 T cells with IL-2 alone failed to induce tumor regression, the addition of anti-CD40 mAb resulted in expansion of transferred gp100-specific T cells in vivo and significantly improved antitumor responses.
MATERIALS AND METHODS
Cell Lines, Reagents, and Mice
We cultured B16 melanoma cells and MC38 colon adenocarcinoma cells in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), L-glutamine, sodium pyruvate, nonessential amino acids, and penicillin-streptomycin (all from Invitrogen, Inc., Carlsbad, CA). Recombinant human IL-2 (rhIL-2) was provided by TECIN (National Cancer Institute Biological Resources Branch, Bethesda, MD). The anti-CD40 (FGK4.5) and anti-IL-12 (C17.8) mAbs purified by protein G affinity chromatography were purchased from Bio X Cell (West Lebanon, NH). Antimouse mAbs used for flow cytometry analysis were purchased from BD Biosciences (San Jose, CA). Female C57BL/6 (B6) mice, μMT (B-cell-deficient) mice, CD11c-diptheria toxin receptor (DTR) mice, CD40 knockout (KO) mice and CD80/86 KO mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Thy1.1+ Pmel-1 transgenic mice express a TCR specific for an H-2Db–restricted epitope of the melanoma tumor antigen gp100 (gp10025–33) on a C57BL/6 background as described previously (20, 21). All mice were maintained in a specific pathogen-free barrier facility at The University of Texas MD Anderson Cancer Center (Houston, TX). Mice were handled in accordance with protocols approved by our institutional animal care and use committee. Experiments were started when mice were 8–10 weeks of age.
Tumor Treatment and Monitoring
C57BL/6 mice were subcutaneously inoculated with 3 × 105 B16 tumor cells on day -7. On day 0, 3–5 × 106 in vitro-activated Thy1.1+ pmel-1 T cells were adoptively transferred into tumor-bearing mice by intravenous injection, as described previously (20). RhIL-2 (600,000 IU/mouse) was administered directly following ACT and twice daily for the next 3 days. Anti-CD40 mAb (200 μg/mouse) was also administered intraperitoneally on day 0. Rat immunoglobulin G (22) was used as control antibody. B16 tumor growth was monitored by measuring the perpendicular diameters of the tumors in order to calculate the area of the tumor base.
Bone Marrow Chimera
All the recipient mice (B6) were irradiated with 1,000 rads 16 hours before the bone marrow (BM) cells were transferred. BM cells were harvested from the tibias and femurs of donor B6 (wild-type [WT]) or CD40 KO mice; about 8 × 106 cells were injected intravenously into the recipient mice. After 8 weeks, mice were bled from their tails to assess the reconstitution by flow cytometric analysis.
Cytokine Array
The concentration of cytokines in the serum was measured with a cytokine array (Luminex) according to the manufacturer's protocol (Millipore, Billerica, MA).
IL-12 Neutralization and DC depletion
For the IL-12 neutralization studies, B6 mice were injected intraperitoneally with 100 μg of anti-IL-12 mAb before and during treatment. Rat IgG was used as the control. For DC depletion, B6 mice or CD11c-DTR mice were intraperitoneally injected with 10 ng of diphtheria toxin (DT) per gram of body weight 24 hours before T cell adoptive transfer. This dose of DT can eliminate 86% of splenic CD11c+ cells in CD11c-DTR mice (data not shown).
Flow Cytometric Analysis
After depletion of erythrocytes using ACK lysing buffer (Invitrogen, Grand Island, NY). The remaining peripheral blood lymphocytes (PBLs) or splenocytes were treated with Fc blocking mAbs (anti-CD16/32 2.4G2) and then stained with mAbs against CD40, CD3e, CD8, Thy1.1, CD19, CD11c, F4/80, CD80 and CD86(BD Biosciences or eBiosciences). Samples were analyzed using a FACSCalibur (BD Biosciences).
Statistical Analysis
The data were represented as mean ± standard error of the mean (SEM). We used Student's t test to compare tumor sizes and percentages of cells. P values are based on two-tailed tests, with P < 0.05 considered statistically significant.
RESULTS
Anti-CD40 mAb Leads to the Expansion of Adoptively Transferred pmel-1 T Cells and Enhanced Antitumor Activity In Vivo
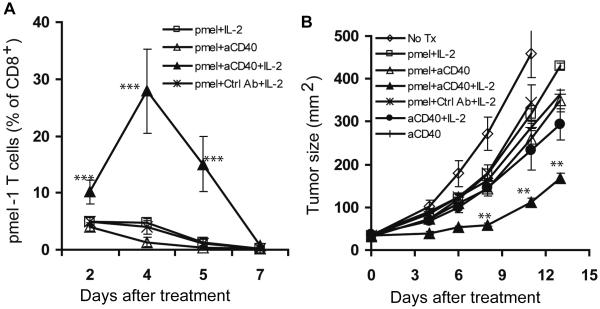
In ACT therapy, transferred in vitro-expanded T cells do not usually expand and persist in the absence of further in vivo stimulation (23). We therefore sought to determine if anti-CD40 mAb can lead to proliferation of activated, adoptively transferred T cells and enhance antitumor activity in vivo. We chose to address this issue using murine B16 melanoma, an aggressive tumor with low immunogenicity, as a tumor model. Mice were inoculated subcutaneously with B16 tumor cells, and established tumors were treated 7 days later with adoptive transfer of pmel-1 T cells, transgenic T cells that express a TCR specifically recognizing an H-2Db-restricted epitope of gp100, followed by IL-2 administration, and in conjunction with anti-CD40 mAb. PBLs were harvested by tail-bleeding for flow cytometric analysis at the indicated time points following T cell adoptive transfer and B16 tumor growth was monitored by measuring the perpendicular diameters of tumors. As shown in Fig. 1A, the expansion of adoptively transferred pmel-1 T cells was only observed in mice treated with anti-CD40 mAb in combination with IL-2, and that resulted in 10-fold higher numbers of transferred T cells in peripheral blood 5 days after treatment compared with mice treated with anti-CD40 mAb or IL-2 alone. As expected, the longer persistence of tumor-antigen-specific T cells led to more robust antitumor responses (Fig. 1B). These results demonstrate that administration of anti-CD40 mAb with IL-2 can induce expansion of in vitro activated, adoptively transferred pmel-1 T cells in vivo and in turn enhance the antitumor response.
FIGURE 1.
Anti-CD40 monoclonal antibody (mAb) induces expansion of adoptively transferred T cells and enhances antitumor activity. B6 mice (5–10 mice per group) were subcutaneously inoculated with B16 tumor cells on day -7 and treated by intravenous injection of pmel-1 T cells on day 0. Recombinant human IL-2 (rhIL-2) was administered intraperitoneally on days 0–3. Anti-CD40 mAb was injected intraperitoneally on day 0. A, Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells at the indicated time points. B, Tumor growth is shown as the area calculated by multiplying the perpendicular diameters of the tumors. Results shown are representative of three independent experiments with similar results. **P < 0.01, *** P < 0.001.
Anti-CD40 mAb Leads to the Expansion of Adoptively Transferred pmel-1 T Cells Independent of Tumor Antigen Cross-presentation
Since anti-CD40 and IL-2 treatment may induce tumor-specific, endogenous CD8+ T cell responses (22), next we wanted to determine whether the expansion of adoptive transferred pmel-1 T cells was induced by tumor antigen cross-presentation. A gp100 negative colon adenocarcinoma, MC38 was inoculated as a control of B16 melanoma on day-7. Mice were treated as described above; PBLs were harvested and stained with CD3-FITC, CD8-APC and Thy1.1-PE for flow cytometric analysis. As shown in Fig. 2, the expansion of adoptively transferred pmel-1 T cells was not significantly different in mice inoculated with B16 or MC38, indicating that administration of anti-CD40 mAb and IL-2 can induce in vitro-activated T cell expansion independent of tumor antigen cross-presentation.
FIGURE 2.
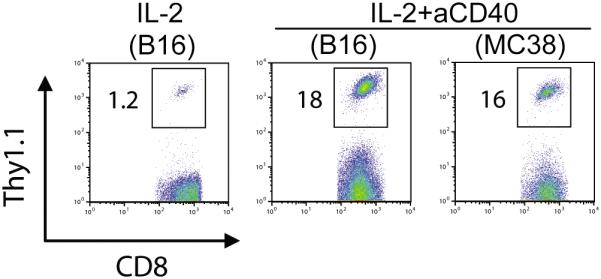
Anti-CD40 monoclonal antibody (mAb) induces expansion of adoptively transferred T cells independent of tumor antigen cross-presentation. B6 mice (5 mice per group) were subcutaneously inoculated with B16 or MC38 tumor cells on day -7 and treated by intravenous injection of pmel-1 T cells on day 0. Recombinant human interleukin-2 (RhIL-2) was administered intraperitoneally for 3 days after T cell transfer. Anti-CD40 mAb was injected intraperitoneally on day 0. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 4. Results shown are representative of two independent experiments with similar results.
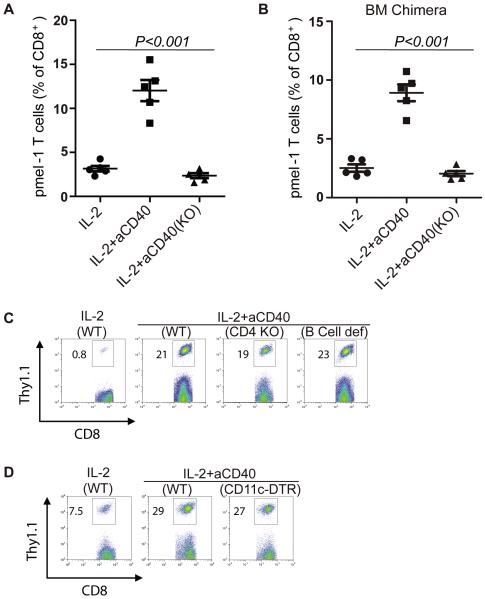
CD40 Expressed on BM-derived Cells is Necessary for Anti-CD40 mAb Induced Expansion of Adoptively Transferred pmel-1 T Cells
The expression of CD40 is not restricted to immune cells such as T cells and APCs but extends to a variety of nonhematopoietic cells, including endothelial cells and fibroblasts (24, 25). To understand what cells played a role in the expansion of adoptively transferred pmel-1 T cells induced by the administration of anti-CD40 mAb and IL-2, B6 mice or CD40 KO mice were adoptively transferred with in vitro expanded pmel-1 T cells, followed by IL-2 and anti-CD40 Ab administration as described in Fig. 1. PBLs were harvested for flow cytometric analysis 3 days after T cell adoptive transfer. As shown in Fig. 3A, the expansion of adoptively transferred pmel-1 T cells induced by anti-CD40 mAb could only be observed in WT mice and was absent from CD40 KO mice. This finding indicates that the CD40 expressed on host cells plays a role in mediating the expansion of adoptively transferred pmel-1 T cells, and that anti-CD40 mAb cannot induce the expansion of pmel-1 T cells by binding to CD40 expressed on the transferred T cells themselves.
FIGURE 3.
Expansion of adoptively transferred pmel-1 T cells is dependent on CD40 expressed on bone marrow (BM)-derived cells. A, B6 mice or CD40 knockout (KO) mice (5 mice per group) were treated as described in Fig. 2. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 3. B, Eight weeks after BM reconstitution (as described in Materials and Methods), we treated BM chimera mice (WT or CD40 KO) as described above. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 3. C, B6 mice, CD4 KO mice, and B cell-deficient mice (5 mice per group) were treated as described above. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 5. D, B6 mice or CD11c-DTR mice were injected with 10 ng of DT per gram of body weight 24 hours before T cell adoptive transfer and then treated as described above. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 3. Results shown are representative of two or three independent experiments with similar results.
To further define the cells expressing CD40 mediated the T cell expansion were hemopoietic or non- hemopoietic cells, we also performed BM chimera experiments where lethally-irradiated B6 hosts were reconstituted with BM cells from WT or CD40 KO mice. 8 weeks later, the mice were treated as above. PBLs were harvested for flow cytometric analysis 3 days after T cell adoptive transfer. As shown in Fig. 3B, the T cell expansion was observed in mice that were reconstituted with BM cells from WT mice but not from CD40 KO mice. This finding indicates that the BM-derived cells were necessary to mediate the expansion of adoptively transferred T cells induced by anti-CD40 mAb, although CD40 is broadly expressed on hematopoietic and nonhematopoietic cells.
We also found that the expansion of pmel-1 T cells was not impaired in CD4 KO mice or B cell-deficient mice (Fig. 3C). In addition, by administering DT to deplete DCs in CD11c-DTR mice, we found that DCs were dispensable for the expansion of adoptively transferred pmel-1 T cells induced by anti-CD40 mAb (Fig. 3D).
CD80/86 are Indispensable for Anti-CD40 mAb-Induced Expansion of Adoptively Transferred pmel-1 T Cells
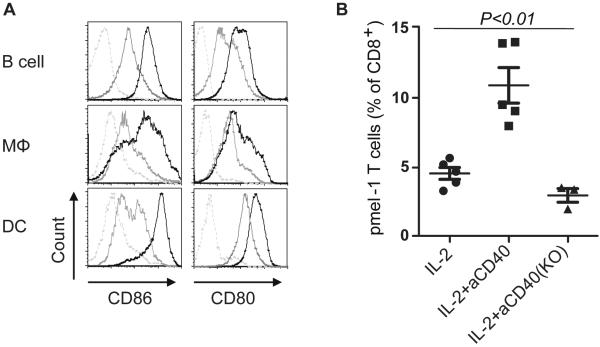
The importance of CD80 and CD86 as the ligands of CD28 in T cell activation and priming has been extensively investigated (26). Mice deficient in CD28 or both of its ligands (CD80 and CD86) have been shown to have severely impaired proliferation of CD4+ T cells (27, 28). To investigate whether CD80 and CD86 are important in the expansion of adoptively transferred pmel-1 T cells induced by anti-CD40 mAb, first, we showed that administration of anti-CD40 Ab can upregulate the expression of CD80/86 on B cells, macrophages and DCs (Fig. 4A). And then WT and CD80/86 KO mice were treated as described in Fig. 2. Mice were bled 3 days after T cell adoptive transfer; PBLs were harvested for flow cytometric analysis. As shown in Fig. 4B, the lack of anti-CD40-induced T-cell expansion in the KO mice demonstrates that CD80 and CD86 are critical for the expansion of adoptively transferred pmel-1 T cells induced by anti-CD40 mAb.
FIGURE 4.
Expansion of adoptively transferred pmel-1 T cells is dependent on CD80/86. A, Splenocytes were harvested at 36 hrs after treatment with anti-CD40 Ab or control Ab, and stained with CD11c, CD19, F4/80, CD86 and CD80 for flow cytometric analysis (dashed: isotype, gray: control Ab, black: anti-CD40 Ab). B, B6 mice or CD80/86 knockout (KO) mice (5 mice per group) were treated as described in Fig. 2. Peripheral blood was subjected to flow cytometric analysis for percentage of pmel-1 cells on day 3. Results shown are representative of two independent experiments with similar results.
Anti-CD40 mAb-induced IL-12 Is Vital for the Full Expansion of Adoptively Transferred pmel-1 T Cells
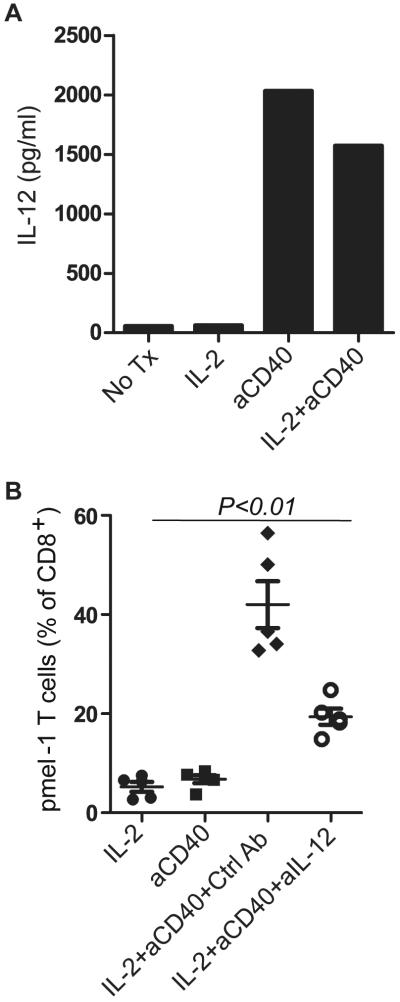
We have shown that costimulatory molecules CD80 and CD86 are indispensable in the expansion of adoptively transferred pmel-1 T cells induced by anti-CD40 mAb. Next, we wanted to explore whether cytokines are also involved in the pmel-1 T cell expansion in vivo. B6 mice were treated as described in Fig. 2. Mouse serum was harvested and pooled 1 day after adoptive T cell transfer for Luminex-based cytokine analysis. As shown in Fig. 5A, administration of anti-CD40 mAb led to increased IL-12 secretion in addition to IL-1α and TNF-α (data not shown). To directly address whether IL-12 was responsible for mediating the pmel-1 T cell expansion, we administered anti-IL-12 mAb or control mAb intraperitoneally before and during treatment. Mice were then bled 4 days after adoptive transfer of pmel-1 T cells and peripheral blood was analyzed by flow cytometric analysis. As shown in Fig. 5B, our results indicate that IL-12 secretion was an important mediator for the expansion of the adoptively transferred pmel-1 T cells.
FIGURE 5.
Interleukin-12 (IL-12) is required for the expansion of adoptively transferred pmel-1 T cells. B6 mice were treated as described in Fig. 2. A, Mouse serum was harvested via tail bleeding on day 1 for the Luminex assay. B, Anti-IL-12 or control antibody (15) was administered intraperitoneally on days −1, 0, and 2. Peripheral blood lymphocytes (PBLs) were subjected to flow cytometric analysis for percentage of pmel-1 cells on day 4. Data are representative of two independent experiments with similar results.
DISCUSSION
When using in vitro-expanded T cell clones for ACT therapy, a major consideration is that the iterative expansion of a single T cell clone into the billions can result in a shortened cellular lifespan, and therefore the cells promptly disappear in vivo after adoptive transfer.(29) However, the persistence of adoptively transferred T cells correlates with cancer regression in patients receiving ACT (30). To this end, designing rational strategies to enhance the persistence of adoptively transferred T cells may augment the therapeutic effectiveness of ACT therapy.
In this study, we showed that anti-CD40 mAb in conjunction with IL-2 can enhance the expansion of adoptively transferred T cells in vivo and boost their antitumor activity. Other approaches have also been used for activation and expansion of T cell clones in vivo. For example, using anti-CD3/CD28 beads to expand T cell clones may support maintenance of CD28 expression in T cell clones, which may in turn increase the postinfusion survival of T cells in patients (29, 31). It was recently shown in macaques that antigen-specific CD8+ T cell clones derived from a central memory population retain an intrinsic capacity that enables them to have long-term in vivo survival after adoptive transfer and revert to the memory cell pool (32). In a B16 mouse tumor model, we previously found that DC/gp100 vaccination led to enhanced proliferation and increased tumor infiltration of adoptively transferred pmel-1 T cells in vivo and significantly improved antitumor response (20). Although anti-CD40 mAb/IL-2 treatment can enhance the antitumor activity of pmel-1 T cells, the B16 tumors eventually escaped. The role of repeated cycles of combination therapy and whether this could further expand pmel-1 T cells to enhance antitumor activity should be evaluated in future studies.
CD40 expressed on APCs plays a critical role in the priming and expansion of antigen-specific CD4+ and CD8+ T cells (33). But in the absence of a tumor antigen vaccination, Kedl et al. showed that the administration of anti-CD40 mAb resulted in accelerated depletion of CD8+ T cells in tumor-bearing mice (34). Interestingly, we showed here that administration of anti-CD40 agonistic mAb in combination with IL-2 in the absence of antigen-specific vaccination can expand adoptively transferred T cells in vivo. Our results and those of others (22) strongly suggest that cytokines or other stimuli may also be required when administering anti-CD40 mAb to treat patients with cancer. In addition, we observed that endogenous CD8+ T cells were also expanded by the combinational therapy (data not shown), though the 2–3 fold expansion for endogenous T cells in blood was not as robust as that of activated T cells (5–10 fold). It is likely that the differentiation status of the T cells is critical for the expansion induced by treatment with anti-CD40 Ab. Besides inducing the proliferation of T cells, a previous study has shown that the ligation of CD40 on DCs can increase the capacity of DCs to induce IFN-γ production by T cells (35), suggesting that treatment with anti-CD40 Ab could enhance the cytotoxic activity of T cells.
This broad expression of CD40 on B cells, DCs, macrophages, endothelial cells, and fibroblasts accounts for the central role it plays in the regulation of immune response and host defense.(33) Using CD40 KO mice and BM-reconstituted mice, we demonstrated that BM-derived cells mediated the expansion induced by CD40 agonistic mAb; however, when using CD4 KO mice, B cell-deficient mice, and CD11c-DTR mice for depletion of DCs, we could not further define which cell population is critical for T cell expansion—possibly because of the overlapping functions among cell types. Alternatively, it is also possible that other APCs such as macrophages are involved in promoting anti-CD40 induced T-cell expansion. A recent study has shown that administration of CD40 agonists can activate macrophages that can infiltrate tumor and become tumoricidal (36). Depletion of macrophages is needed to determine their role in this model.
Cytokines such as IL-12 and costimulatory molecules such as CD80 and CD86 triggered by CD40/CD40L interaction on immune cells are required for host naïve T cell activation and proliferation (16, 18, 19). Our results indicate that, in addition to costimulatory molecules, CD40 agonistic mAb-induced IL-12 secretion is also important to expand in vitro-activated, adoptively transferred T cells.
In conclusion, our data indicate that administration of CD40 agonistic mAb, along with IL-2, can expand adoptively transferred T cells and boost their antitumor response. Thus, the use of anti-CD40 mAb to enhance adoptively transferred T cells may represent a promising new approach for cancer treatment.
ACKNOWLEDGMENTS
Grant support: This work was supported in part by the following National Cancer Institute grants: R01 CA123182 (P. H), P01 CA128913 (P. H), R01 CA116206 (P. H) and R01 CA143077 (W. W. O). This research was also supported in part by the National Institutes of Health through University of Texas MD Anderson Cancer Center's Support Grant CA016672.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plautz GE, Bukowski RM, Novick AC, et al. T-cell adoptive immunotherapy of metastatic renal cell carcinoma. Urology. 1999;54:617–23. doi: 10.1016/s0090-4295(99)00303-9. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 3.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–63. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–21. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 5.Kwak LW, Neelapu SS, Bishop MR. Adoptive immunotherapy with antigen-specific T cells in myeloma: a model of tumor-specific donor lymphocyte infusion. Semin Oncol. 2004;31:37–46. doi: 10.1053/j.seminoncol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 8.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtsinger JM, Schmidt CS, Mondino A, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–62. [PubMed] [Google Scholar]
- 12.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 22:333–40. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikora AG, Jaffarzad N, Hailemichael Y, et al. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–9. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 15.Saemann MD, Diakos C, Kelemen P, et al. Prevention of CD40-triggered dendritic cell maturation and induction of T-cell hyporeactivity by targeting of Janus kinase 3. Am J Transplant. 2003;3:1341–9. doi: 10.1046/j.1600-6143.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 16.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 18.Tong AW, Stone MJ. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 2003;10:1–13. doi: 10.1038/sj.cgt.7700527. [DOI] [PubMed] [Google Scholar]
- 19.van Mierlo GJ, den Boer AT, Medema JP, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–6. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou Y, Wang G, Lizee G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–90. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy WJ, Welniak L, Back T, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol. 2003;170:2727–33. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 23.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 25.Loskog AS, Eliopoulos AG. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21:301–7. doi: 10.1016/j.smim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 28.Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 29.Yee C. Adoptive therapy using antigen-specific T-cell clones. Cancer J. 16:367–73. doi: 10.1097/PPO.0b013e3181eacba8. [DOI] [PubMed] [Google Scholar]
- 30.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JA, Figlin RA, Sifri-Steele C, Berenson RJ, Frohlich MW. A phase I trial of CD3/CD28-activated T cells (Xcellerated T cells) and interleukin-2 in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2003;9:3562–70. [PubMed] [Google Scholar]
- 32.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonsatti E, Maio M, Altomonte M, Hersey P. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 37:517–23. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci U S A. 2001;98:10811–6. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guizetti J, Schermelleh L, Mantler J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–20. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]