Abstract
This phase I study was conducted to determine the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of the heat shock protein 90 (HSP90) inhibitor 17-allyamino-17-demethoxygeldanamycin (17-AAG) in combination with bortezomib, and to provide pharmacokinetic data in relapsed or refractory acute myeloid leukemia (AML). Eleven patients were enrolled. The MTD was 17-AAG 150mg/m2 and bortezomib 0.7mg/m2. Hepatic toxicity and cardiac toxicity were dose limiting. Co-administration on day 4 led to a decrease in clearance (p=0.005) and increase in AUC (p=.032) of 17-amino-17-demethoxygeldanamycin (17-AG) not observed when 17-AAG was administered alone. Pharmacokinetic parameters of patients who developed toxicities and those who did not were not different. The combination of 17-AAG and bortezomib led to toxicity without measurable response in patients with relapsed or refractory AML. Pharmacokinetic data provide insight for studies of related agents in AML; next generation HSP90 inhibitors are appealing for further development in this area.
Keywords: Relapsed AML, bortezomib, 17-AAG, heat shock protein inhibition
INTRODUCTION
The biologic heterogeneity of acute myeloid leukemia (AML), a disease characterized by clonal accumulation and expansion of immature myeloid cells within the bone marrow, represents a major challenge in the advancement of treatment for patients with the disease.[1] The development of agents that target aberrant signaling pathways believed to promote leukemogenesis is an attempt to diverge from anthracycline and cytarabine based regimens which result in a long term remission in only 40% of younger patients (age <60 years) and <10% of older patients (age ≥ 60 years).[2]
Heat shock protein 90 (HSP90) is an abundant 90-kDa protein that functions as a molecular chaperone to regulate the conformation, stability, and activation of “client” proteins, many of which are important for intracellular signaling and the adaptive response to stress.[3] Among the many proteins regulated by HSP90 are several oncoproteins with known aberrant activity in AML including FLT3, c-KIT, AKT, and others.[4] In AML, mutations and/or over-expression of genes encoding the tyrosine kinase receptors FLT3 and c-KIT are relatively frequent and ultimately lead to activation of downstream signaling that promotes proliferation and survival of myeloid blasts.[5,6] These and related pathways are excellent targets for novel therapies in AML.
Inhibition of HSP90 function prevents activation and stabilization of client proteins.[7,8] As a result, the inactive protein is ubiquitinated and targeted for proteasomal degradation, preventing its involvement in intended signaling pathways within the cell. Interestingly, mutated proteins may be more sensitive to a loss of chaperone function than their wild type counterparts, rendering tumor cells more susceptible than normal cells to chaperone inhibition.[7] 17-Allylamino-17-demethoxygeldanamycin (17-AAG), a member of the benzoquinone ansamycin family, became the first HSP90 inhibitor to enter clinical trials and has been studied in a number of advanced solid tumors and in multiple myeloma.[9-13] In vitro, 17-AAG has demonstrated cytotoxic effects in AML cell lines harboring mutated FLT3 or BCR-ABL and induced apoptosis in primary AML cells.[14-16]
There is an important relationship between the molecular chaperone function of HSP90 and the ubiquitin-proteasome pathway. IkB kinase, a client protein of HSP90, phosphorylates a key regulator of the transcription factor NF-κB, Iκβ.[17] Following phosphorylation, Iκβ is ubiquitinated and targeted to the proteasome allowing NF-κB to translocate to the nucleus and induce gene transcription. In AML, constitutive expression of NF-κB contributes to growth and resistance to apoptosis.[18-20] Bortezomib, a dipeptidyl boronic acid proteasome inhibitor, blocks proteasome mediated degradation of Iκβ and prevents activation of NF-κB.[21] Although this agent has demonstrated activity in lymphoid malignancies, it has shown only transient effects when used as a single agent in patients with relapsed or refractory AML, though combination studies have suggested efficacy.[22-26] When added to cytarabine and anthracycline based induction chemotherapy, bortezomib administration has been tolerable, inducing clinical responses in patients with previously untreated or relapsed AML.[24,25] In multiple myeloma (MM), proteasome inhibition is a key therapeutic target and bortezomib based therapies are utilized during induction, consolidation and maintenance.[27] In patients with relapsed or refractory MM, bortezomib in combination with 17-AAG was found to be well tolerated with anti-tumor activity. [12,28]
Given the importance of HSP90 and its client proteins in key oncogenic processes in AML, sequential administration of the HSP90 inhibitor 17-AAG followed by the proteasome inhibitor bortezomib may result in a pronounced accumulation of ubiquitinated proteins within the cell, thus triggering apoptosis. We report a phase I dose escalation study to determine the maximum tolerated dose (MTD) of 17-AAG and bortezomib in patients with relapsed or refractory AML.
PATIENTS AND METHODS
Eligibility criteria and study design
Patients (age ≥ 18 years) with relapsed or refractory non-M3 AML, not candidates for curative therapy with stem cell transplantation, were eligible. Patients were required to have a stable white blood cell (WBC) count ≤ 40 x 109/L for five days prior to initiation of therapy, total bilirubin ≤ 1.5 mg/dL, AST/ALT ≤ 2.5 X upper limit of normal (ULN), creatinine ≤ 2.0 mg/dL, Eastern Cooperative Oncology Group performance status of ≤ 2, and life expectancy of at least 12 weeks. Initially, a resting ejection fraction (EF) ≥ 50% on a pre-treatment echocardiogram (ECHO) or multigated acquisition (MUGA) and a QTc of ≤ 500 msec on an electrocardiogram (EKG) were the only eligibility criteria used to define adequate cardiac function. Three months after the trial opened these criteria were modified because of observed cardiac toxicities that were possibly associated with 17-AAG administration in this and other trials sponsored by the National Cancer Institute's (NCI) Cancer Therapy Evaluation Program (CTEP). As a result, patients with significant heart disease, including heart failure that met New York Heart Association (NYHA) class III and IV definitions, myocardial infarction within one year of entry, uncontrolled dysrhythmias, a history of serious ventricular arrhythmias, or poorly controlled angina, were now excluded. The QTc requirement was also changed. Men were required to have a baseline QTc ≤ 450 msec and women ≤ 470 msec. At trial opening, the use of concomitant medications that could prolong the QTc was not prohibited, however with the change in eligibility requirements, all ancillary medications known to prolong the QTc were excluded.
Adequate pulmonary function with a pre-treatment diffusion lung carbon monoxide (DLCO) ≥ 60% and an exercise oxygen saturation by pulse oximetry of ≥ 90% were required. Patients with symptomatic pulmonary disease were not eligible.
Patients with a pre-existing ≥ grade 2 sensory or motor peripheral neuropathy, history of allergic reaction to eggs (17-AAG is formulated using egg phospholipids), or active or untreated CNS leukemia were not eligible. Concurrent treatment with hydroxyurea (maximum dose 1gm TID) was allowed during cycle 1 only. No other chemotherapy, radiation therapy, or other investigational agents were allowed within 14 days of initiation of therapy.
17-AAG was administered intravenously (IV) over 60 minutes on days 1, 4, 8 and 11 of each 21 day cycle and bortezomib was administered IV push over 3 to 5 minutes immediately following 17-AAG on days 1, 4, 8 and 11. 17-AAG only was administered on day 1 of cycle 1 to permit pharmacokinetic (PK) evaluations of single agent 17-AAG and to test for potential PK interactions between the two agents (via comparison of PK parameters on day 1 and day 4). Dose escalation was as follows: dose level 1, 17-AAG 150 mg/m2 and bortezomib 0.7 mg/m2; dose level 2, 17-AAG 200mg/m2 and bortezomib 0.7 mg/m2. EKGs were checked pre-treatment and following completion of the 17-AAG infusion on day 1 of cycle 1 in triplicate, and therapy was held if the QTc was ≥ 500 msec.
Dose limiting toxicities (DLT) were evaluated and defined for dose escalation decisions during cycle 1 of treatment. The National Cancer Institute Common Terminology Criteria of Adverse Events (CTCAE) version 3.0 was used to characterize toxicity. Nonhematologic toxicity of grade 3 or 4, with the exception of alopecia, nausea and vomiting controllable with anti-emetic therapy, felt to be drug-related, was considered a DLT. Hematologic toxicity was defined as a failure to recover neutrophil and/or platelet counts by day 42 in patients with < 5% blasts in the bone marrow, absence of myelodysplastic changes, and/or absence of evidence of disease by flow cytometry in the bone marrow. Febrile neutropenia and infection were not necessarily assessed as DLT given the frequency of these events in this population irrespective of therapy administered.
The planned determination of the MTD was to utilize a standard cohorts-of-three phase I design of dose escalation using 3 patients per cohort with 3 to 6 patients at each dose level. Patients received full supportive care including blood and platelet transfusions, antibiotics, hydration, etc. when appropriate.
Clinical response was defined according to International Working Group Criteria.[29] Patients with progressive disease discontinued treatment.
Pharmacokinetic and pharmacodynamic analysis
Plasma concentrations of 17-AAG and its active metabolite, 17-amino-17-demethoxygeldanamycin (17-AG), were determined by a validated liquid chromatography-tandem mass spectrometry method described previously.[30] Plasma samples were obtained on days 1 and 4 of cycle 1 at the following time points: pre-infusion, immediately prior to the end of 17-AAG infusion, and 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24 and 48 hours after the end of the 17-AAG infusion. Pharmacokinetic parameter estimates for 17-AAG and 17-AG were generated using non-compartmental analysis in WinNonlin Professional version 5.2 (Pharsight Corporation, Mountain View, CA, USA). The area under the concentration vs. time curve (AUC) was determined with the linear up/log down method.
Given that bortezomib rapidly exits the intravascular compartment and is widely distributed, conventional pharmacokinetic parameters are difficult to follow; we used pharmacodynamic evaluation of 20S proteasome inhibition to characterize the activity of bortezomib at its target site as a PK surrogate. To assess the effect of bortezomib on proteasome activity and to confirm a lack of effect of 17-AAG on proteasome activity, 10 mL whole blood were collected pre-treatment, 1, 4, 8, 24, and 48 hours after the end of 17-AAG administration on days 1 and 4 of cycle 1 (bortezomib was administered immediately following 17-AAG end-of-infusion on Day 4 only). 20S proteasome activities were measured using an assay similar to that which has been previously described. [31]
Statistical analysis
A standard cohorts-of-three phase I study design was used to direct dose escalation decisions. The number of DLTs and the frequency of adverse events and toxicities were summarized across and within dose levels. Secondary endpoints of patient characteristic factors and clinical outcomes were also summarized to characterize the cohorts and obtain preliminary data on efficacy of this regimen in this patient population; however, formal evaluation and hypothesis testing were largely avoided given the inherent limitations of phase I studies and the limited number of patients in this trial. Pharmacokinetic endpoints were explored in relation to dose level, weight, and other PK parameters of interest in order to identify potential relationships and differences. Nonparametric Wilcoxon signed rank (paired sample) tests were used to evaluate in an exploratory manner any differences in PK parameters from day 1 to day 4 as well as between 17-AAG vs. 17-AG based PK markers.
RESULTS
Patient characteristics and treatment groups
Eleven patients with relapsed or refractory AML were enrolled in this single-center phase I study over 34 months (Table I). The median age was 63 years (range 42-77 years). Four patients had AML arising from an antecedent hematologic disorder (AHD), either myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML). One had relapsed after prior allogeneic transplantation. According to the European LeukemiaNet (ELN) genetic groups [32], seven patients were classified as ELN intermediate-II risk, and four had adverse genetic risk. Four patients enrolled with primary refractory disease; of the seven patients with relapsed AML, six had a CR duration of less than one year following initial induction chemotherapy.
Table I.
Patient characteristics
| Patient characteristic | Result |
|---|---|
| Median Age (years) | 63 years (range, 43-77) |
| Male/Female (n) | 6/5 |
| AML rising from AHD (n) | 4 |
| t-AML (n) | 1 |
| Refractory AML (n) | 4 |
| Relapsed AML (n) | 7 |
| ELN* genetic group | |
| Intermediate II | 7 |
| Adverse | 4 |
| Prior anthracycline exposure (n) | 10 |
| Median number prior regimens (n) | 2 (range, 1-4) |
AHD, antecedent hematologic disorder, t-AML, therapy-related AML,
See ref.32
Dose escalation, toxicities, and clinical outcomes
At dose level 1, eight patients were treated. Two patients were replaced due to disease-related complications: the first, who required hydroxyurea treatment prior to starting therapy, was removed from study due to early disease progression, the second had a hemorrhagic stroke in the setting of disease-related thrombocytopenia. Though this was not felt to be drug related, the patient was not evaluable for assessment of drug-related toxicities. In order to provide a better safety signal, dose level 1 was expanded to include a total of six evaluable patients. One patient developed grade 3 QTc prolongation on day 3 of cycle 1 that was felt to possibly be related to the investigational agents and met criteria for DLT. The patient had also been receiving other medications with the potential to prolong the QTc interval. Central review of the ECGs for this patient, performed by three cardiologists outside of our institution, provided expert opinion that there was no prolongation of the QTc interval and that the tracing findings were artifactual. All medications with the potential to prolong the QTc interval were discontinued, the QTc normalized, and the patient was able to complete the therapy without further complications. As no other patient experienced DLT at this dose level, and given the discordant opinions regarding whether QTc prolongation was actual or artifactual, dose escalation proceeded to dose level 2.
At dose level 2, the dose of 17-AAG was increased to 200mg/m2 while the dose of bortezomib remained the same; three patients were enrolled. All three experienced DLTs. The first patient in the cohort developed grade 4 transaminitis on day 7 of cycle 1 that resolved following cessation of study drug administration. The second patient, who did not have a history of glucose intolerance, developed grade 3 hyperglycemia that was asymptomatic and resolved without sequelae on day 5 of cycle 1. The third patient (who began therapy 4 days after the second patient) developed QTc prolongation and torsades de pointes on day 9 of cycle 1. This patient did have a remote history of paroxysmal supraventricular tachycardia (SVT), but was not considered to be at an increased risk for the development of additional arrhythmias with chemotherapy, and indeed had received an allogeneic transplant with reduced intensity conditioning without any serious cardiac toxicity. Pretreatment QTc was 453. At the time of the study-related adverse event, the telemetry reading was consistent with torsades de pointes and subsequent 12 lead ECGs showed a prolonged QTc of 635 msec (Figures 1 and 2). She was asymptomatic and responded to treatment with electrolyte supplementation. The QTc ultimately returned to normal several days later, and echocardiographic evaluation was unchanged from screening. Her course was further complicated by the development of methicillin-resistant Staphylococcus epidermidis (MRSE) bacteremia and hypoxic respiratory failure in the setting of rapidly progressive AML and the patient expired on day 21 of the cycle. A review of all available electrocardiographic data for this patient including ECGs performed 1 year prior to the event confirmed that she did not have an underlying long QT syndrome that placed her at risk for QTc prolongation, and it is likely that this was related to the investigational agents. This patient was the only participant who had a brief decrease in peripheral blood blast percentage from 79.7% on day 1 to 9.2% on day 9. All other patients had either stable disease or disease progression. Other grade 3 or higher nonhematologic toxicities are summarized in Table II.
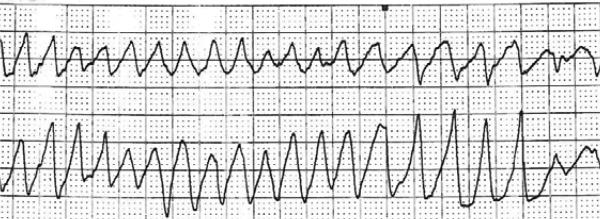
Figure 1.
Torsades de pointes in third patient on dose level 2 on day 9 of cycle 1.
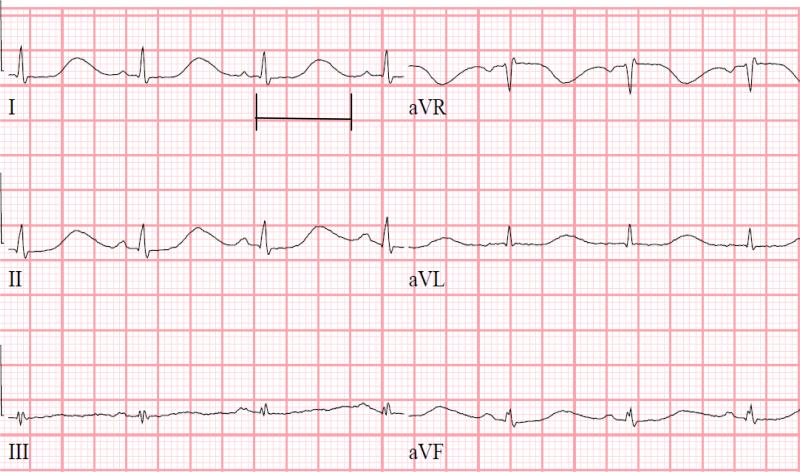
Figure 2.
Prolonged QTc interval of 635 msec in third patient on dose level 2 on day 9 of cycle 1.
Table II.
Grade 3 or Higher Non Hematologic Toxicities*
| No. of Patients | |
|---|---|
| Febrile Neutropenia | 4 |
| Hyperglycemia | 2 |
| AST | 2 |
| ALT | 2 |
| Lung infection | 2 |
| Prolonged QTc | 2 |
| Hypertension | 1 |
| Cardiac Troponin | 1 |
| Hypoglycemia | 1 |
| Hyponatremia | 1 |
| Intracranial Hemorrhage (Grade 5) | 1 |
| Hypoxia | 1 |
| Dyspnea | 1 |
| Maculopapular rash | 1 |
| Vomiting | 1 |
| Fatigue | 1 |
| Confusion | 1 |
| Skin Infection | 1 |
| Infections and Infestations-Urine | 1 |
| Infections and Infestations-Blood | 1 |
Regardless of attribution
Given that three patients at dose level 2 had DLTs, dose escalation was stopped and dose level 1 was declared the MTD.
Pharmacokinetic and pharmacodynamic analysis
Pharmacokinetic results from days 1 and 4 for 17-AAG and 17-AG are shown in tables III and IV, respectively. When comparing 17-AAG pharmacokinetics between dose levels we observed a significantly higher Cmax for the 200 mg/m2 dose on day 1 only (p=0.03, 2-tailed t-test, Table III). No other parameters were found to be significantly different for 17-AAG. When comparing 17-AG PK between the two dose levels, significant differences were observed in the mean T1/2 (terminal half-life), Cmax (the maximum concentration), Tmax (time to reach Cmax), AUC and AUCcombined (combined AUC of 17-AAG and 17-AG) on day 4 only (Table IV). No differences in 17-AG PK were observed between dose levels on day 1. However, these findings are limited by the small number of patients who were treated at the higher dose level (n=3).
Table III.
Pharmacokinetics of 17AAG
| Day 1 (17-AAG alone) | Day 4 (17-AAG+PS-341) | ||||||
|---|---|---|---|---|---|---|---|
| Dose Level (mg/m2) No. of patients | 150 n=8 | 200 n=3 | P1 | 150 n=8 | 200 n=3 | P4 | P |
| Cmax (ng/mL) | 2480 ± 836 | 3814 ± 578 | 0.030 | 2588 ± 562 | 2785 ± 656 | 0.677 | 0.700 |
| AUCtot (ng/mL*h) | 5871± 2508 | 9002 ± 1970 | 0.096 | 6717 ± 2631 | 9142 ± 1358 | 0.086 | 0.170 |
| Terminal T1/2 (h) | 4.77 ± 1.15 | 4.59 ± 0.21 | 0.684 | 4.04 ± 0.86 | 3.78 ± 1.29 | 0.773 | 0.033 |
| Clearance (L/h) | 29.36 ± 11.11 | 22.92 ± 4.89 | 0.220 | 24.94 ± 8.10 | 21.92 ± 3.91 | 0.435 | 0.068 |
| Vss (L) | 98.10 ± 37.46 | 73.30 ± 16.81 | 0.169 | 82.36 ± 19.91 | 86.83 ± 24.37 | 0.795 | 0.460 |
P1 : p-value of parameters between dose levels (150 vs 200 mg/m2) on cycle 1, day 1.
P4 : p-value of parameters between dose levels (150 vs 200 mg/m2) on cycle 1, day 4.
P : p-value of parameters between day 1 and day 4.
Table IV.
Pharmacokinetics of 17-AG
| Day 1 (17-AAG alone) | Day 4 (17-AAG+PS-341 | ||||||
|---|---|---|---|---|---|---|---|
| Dose Level (mg/m2) No. of patients | 150 n=8 | 200 n=3 | P1 | 150 n=8 | 200 n=3 | P4 | P |
| Cmax (ng/mL) | 821 ± 450 | 1300 ± 421 | 0.198 | 1025 ± 445 | 1412 ± 26 | 0.044 | 0.054 |
| AUCtot (ng/mL*h) | 6334 ± 6244 | 9957 ± 4258 | 0.323 | 7751 ± 5028 | 13261 ± 1216 | 0.02 | 0.032 |
| Terminal T1/2 (h) | 11.16 ± 1.40 | 7.92 ± 1.76 | 0.064 | 10.53 ± 2.20 | 7.47 ± 0.73 | 0.009 | 0.41 |
| Clearance (L/h) | 41.35 ± 26.48 | 23.07 ± 12.01 | 0.155 | 29.87 ± 23.29 | 14.86 ± 1.55 | 0.113 | 0.005 |
| Tmax (h) | 1.53 ± 0.42 | 1.53 ± 0.46 | 0.992 | 1.85 ± 1.10 | 3.72 ± 0.64 | 0.013 | 0.068 |
| AUC exposed (ng/mL*h) | 12205 ± 8530 | 18959 ± 3169 | 0.092 | 14468 ± 7179 | 22403 ± 1036 | 0.019 | 0.083 |
P1 : p-value of parameters between dose levels (150 vs 200 mg/m2) on cycle 1, day 1.
P4 : p-value of parameters between dose levels (150 vs 200 mg/m2) on cycle 1, day 4.
P : p-value of parameters between day 1 and day 4.
In order to compare PK parameters for 17-AAG and 17-AG on day 1 (17-AAG alone) vs day 4 (17-AAG+bortezomib), we combined the PK parameters of all 11 patients. For 17-AAG, only T1/2 was found to be different between the two days (p=0.033, Wilcoxon signed rank test, last column in Table III). For the metabolite 17-AG, both AUC and apparent clearance (CL) were different between days (p=0.032 and 0.005, respectively, last column in Table IV). Given that bortezomib was administered on day 4 and not on day 1, it is possible that the observed differences in 17-AAG and 17-AG PK for days 1 and 4 are related to bortezomib co-administration.
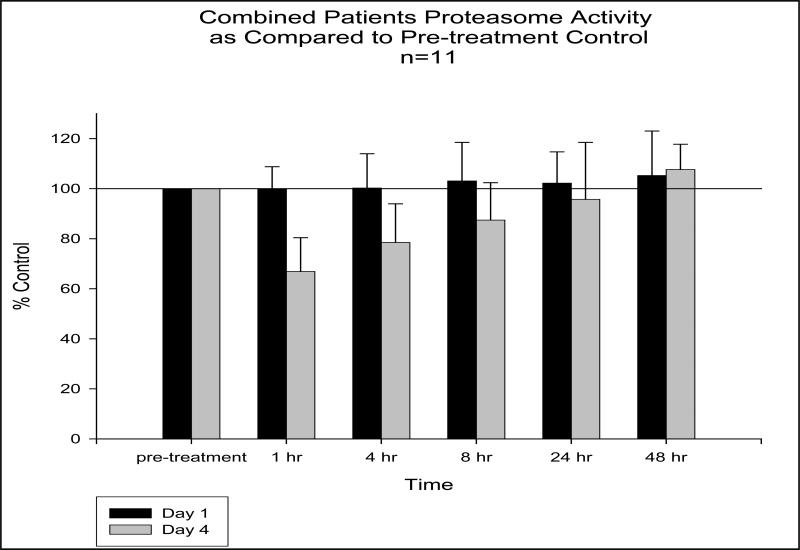
The pharmacodynamic evaluation of the 20S proteasome activity is shown graphically in figure 3. A notable decrease in proteasome activity is observed within the first hour following bortezomib administration, and activity appears to recover to pre-treatment levels within 24 to 48 hours. Proteasome activity on day 4 at 1 hour after bortezomib administration ranged from 51% to 90% (n=11). No apparent relationships were observed between proteasome inhibition and 17-AAG pharmacokinetics or clinical outcome. In those patients who experienced grade 3 or higher toxicities, no significant differences in the measured PK parameters were observed.
Figure 3.
Proteasome activity as compared to pre-treatment control
DISCUSSION
In this phase I dose escalation of 17-AAG and bortezomib in patients with relapsed or refractory AML, the MTD of combination therapy was 17-AAG 150mg/m2 and bortezomib 0.7mg/m2, dose limited by the development of serious cardiac and hepatic adverse events.
In previous studies of patients receiving single agent 17-AAG, diarrhea, nausea and vomiting, and reversible hepatotoxicity were the most frequent non-hematologic toxicities reported.[9-13,33] We observed similar toxicities in our study participants. The most frequent grade 3 or higher nonhematologic toxicity was febrile neutropenia occurring in four of the 11 patients, though the combination of 17-AAG and bortezomib did not appear to increase the expected frequency or severity of this adverse event.
Reports of possible QTc prolongation in studies of 17-AAG by NCI CTEP led to the extensive ECG monitoring and requirements for adequate cardiac function that became part of the eligibility requirements for this trial. Cardiac toxicity has been reported with another HSP90 inhibitor, alvespimycin, where myocardial infarction and an elevation in troponin were seen in two AML patients treated with that agent, although both had an extensive history of cardiac disease which made attribution to alvespimycin difficult.[35] Nonetheless, the dose at which these events occurred was determined to be the primary DLT of that study. In our study, two patients developed QTc prolongation. In the first patient it was not clear whether the ECG findings were actual or artifact, and it is possible that concurrent medications also contributed to this variance. Clearly, the second patient described developed QTc prolongation and torsades de pointes on day 9 of therapy that was likely related to the study medications. Notably, ten of the eleven patients had received prior anthracycline therapy. Though cardiac function based on pre-treatment ejection fraction and ECG evaluation was normal, prior exposure to cardiotoxic therapy may have placed patients at an increased risk for toxicity. Interestingly, the patient who developed torsades de pointes had not had prior anthracycline exposure.
Richardson and colleagues reported a phase II trial of bortezomib (1.3mg/m2 on days 1,4,8,11) and three different doses of 17-AAG (50, 175, 340 mg/m2 on days 1,4,8,11) in patients with relapsed/refractory MM that was well tolerated, even at the 340mg/m2 dose of 17-AAG.[12] Cardiac arrhythmias or QTc prolongation were not reported despite the higher doses of both agents utilized as compared to our study.[12] In a separate phase 1/2 dose escalation study of 17-AAG (100-340 mg/m2 on days 1, 4, 8, 11) and bortezomib (0.7-1.3 mg/m2 on days 1, 4, 8, 11) in patients with relapsed/refractory MM, Richardson et al again found the combination to be well tolerated and cardiac toxicities were not observed.[28] It may be important to note, however, that the formulation of 17-AAG was quite different between these trials and the current trial we report on here. The 17-AAG used in the Richardson trials was provided by Bristol Myers Squibb; the excipients used in the formulation of 17-AAG were Cremophor, propylene glycol, and ethanol. In our CTEP-sponsored trial, the excipients were egg phospholipids and DMSO. It is unknown whether the formulation type might impact the development of specific toxicities of 17-AAG.
An important observation from our study was the significant increase in 17-AG AUC following combined administration of 17-AAG and bortezomib (p=.032), as compared to when 17-AAG was administered alone. This is in contrast to PK results reported by Richardson et al where a decrease in AUC of both 17-AAG and 17-AG following co-administration was observed, though the interpretation of these findings are limited by sample size.[28] While we did not observe differences in 17-AG PK on day 1 between the 17-AAG 150 mg/m2 and 200 mg/m2 dose levels, we did observe significant increases in 17-AG Cmax, AUC, and Tmax, and a decrease in 17-AG T½ between the two dose levels on day 4. Our data may suggest that bortezomib affects the pharmacokinetics of 17-AAG and/or 17-AG. Both bortezomib and 17-AAG are metabolized by cytochrome P450 3A4, which provides one mechanism for a potential drug-drug interaction.[36-38] However, given the limited number of patients in this study, further evaluation in larger cohorts would be needed to explore this possible interaction further. No other notable pharmacokinetic interactions or associations with outcomes were observed, including a lack of difference in 17-AAG or 17-AG pharmacokinetics between patients with low vs. high (grade 3 or higher) levels of toxicity.
No patient received treatment beyond cycle 1 because of disease progression (or toxicity). Though the serious nature of the DLTs that occurred and the uniform disease progression at the MTD prevented further development of this regimen, heat shock protein inhibition as a way to target multiple oncogenic signaling pathways has been pursued in AML with promising results. Indeed, in the previously mentioned phase I trial of the more potent HSP90 inhibitor alvespimycin, a water-soluble analog of 17-AAG, three patients achieved a complete remission with incomplete count recovery after one cycle of therapy.[35] Furthermore, an additional patient was able to proceed to allogeneic transplantation following a greater than 50% reduction in bone marrow blasts. Next-generations agents such as alvespimycin, with improved tolerability and clinical activity, support continued evaluation of HSP90 inhibitors in this disease.
Acknowledgements
This work was supported by: NIH/NCI K23CA120708 (WB), K12CA133250 (JCB and AW) NIH/NCI U01CA76576 (MRG), K23CA109004 (KB) and CALGB Young Investigator Award (KB). AW is a Paul Calabresi Clinical Scholar and a scholar of the American Society of Hematology-Amos Medical Faculty Development Program.
Footnotes
Declaration of Interest The authors do no report a conflict of interest.
REFERENCES
- 1.Lowenberg B DJ, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Applebaum F GH, Head D, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao R, Houry WA. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol. 2005;83:703–10. doi: 10.1139/o05-158. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl) 2004;82:488–99. doi: 10.1007/s00109-004-0549-9. [DOI] [PubMed] [Google Scholar]
- 5.Kottaridis PD, Gale RE, Linch DC. Flt3 mutations and leukaemia. Br J Haematol. 2003;122:523–38. doi: 10.1046/j.1365-2141.2003.04500.x. [DOI] [PubMed] [Google Scholar]
- 6.Sritana N, Auewarakul CU. KIT and FLT3 receptor tyrosine kinase mutations in acute myeloid leukemia with favorable cytogenetics: two novel mutations and selective occurrence in leukemia subtypes and age groups. Exp Mol Pathol. 2008;85:227–31. doi: 10.1016/j.yexmp.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 8.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. others. [DOI] [PubMed] [Google Scholar]
- 10.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–87. doi: 10.1200/JCO.2005.09.119. others. [DOI] [PubMed] [Google Scholar]
- 11.Grem JL, Morrison G, Guo XD, Agnew E, Takimoto CH, Thomas R, Szabo E, Grochow L, Grollman F, Hamilton JM. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. others. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M, Berman D, Messina M, Anderson KC. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–37. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, Kelly WK, DeLaCruz A, Curley T, Heller G. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13:1775–82. doi: 10.1158/1078-0432.CCR-06-1863. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008;13:357–64. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, Naoe T. Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia. 2002;16:1535–40. doi: 10.1038/sj.leu.2402558. [DOI] [PubMed] [Google Scholar]
- 16.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–93. [PubMed] [Google Scholar]
- 17.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–74. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 18.Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, Manzoli L, Cocco L, Martelli AM. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–89. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 19.Bueso-Ramos CE, Rocha FC, Shishodia S, Medeiros LJ, Kantarjian HM, Vadhan-Raj S, Estrov Z, Smith TL, Nguyen MH, Aggarwal BB. Expression of constitutively active nuclear-kappa B RelA transcription factor in blasts of acute myeloid leukemia. Hum Pathol. 2004;35:246–53. doi: 10.1016/j.humpath.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 21.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 22.Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–75. doi: 10.1200/JCO.2005.03.108. others. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–84. doi: 10.1200/JCO.2005.02.050. others. [DOI] [PubMed] [Google Scholar]
- 24.Attar EC, De Angelo DJ, Supko JG, D'Amato F, Zahrieh D, Sirulnik A, Wadleigh M, Ballen KK, McAfee S. Miller KB and others. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14:1446–54. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 25.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, Deangelo DJ, Kolitz JE, Powell BL, Voorhees P, Wang ES. Bortezomib Added to Daunorubicin and Cytarabine During Induction Therapy and to Intermediate-Dose Cytarabine for Consolidation in Patients With Previously Untreated Acute Myeloid Leukemia Age 60 to 75 Years: CALGB (Alliance) Study 10502. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.45.2177. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S, Garcia-Manero G, McConkey D, Ruiz SL. Guerciolini R and others. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–6. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M, Anderson KC. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729–40. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. others. [DOI] [PubMed] [Google Scholar]
- 30.Johnston JS, Phelps MA, Blum KA, Blum W, Grever MR, Farley KL, Dalton JT. Development and validation of a rapid and sensitive high-performance liquid chromatography-mass spectroscopy assay for determination of 17-(allylamino)-17-demethoxygeldanamycin and 17-(amino)-17-demethoxygeldanamycin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:15–21. doi: 10.1016/j.jchromb.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46:673–83. [PubMed] [Google Scholar]
- 32.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. others. [DOI] [PubMed] [Google Scholar]
- 33.Nowakowski GS, McCollum AK, Ames MM, Mandrekar SJ, Reid JM, Adjei AA, Toft DO, Safgren SL, Erlichman C. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin Cancer Res. 2006;12:6087–93. doi: 10.1158/1078-0432.CCR-06-1015. [DOI] [PubMed] [Google Scholar]
- 34.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–37. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 35.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. doi: 10.1038/leu.2009.292. others. [DOI] [PubMed] [Google Scholar]
- 36.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 1998;58:2385–96. [PubMed] [Google Scholar]
- 37.Lorusso PM, Venkatakrishnan K, Ramanathan RK, Sarantopoulos J, Mulkerin D, Shibata SI, Hamilton A, Dowlati A, Mani S, Rudek MA. Pharmacokinetics and Safety of Bortezomib in Patients with Advanced Malignancies and Varying Degrees of Liver Dysfunction: Phase 1 NCI Organ Dysfunction Working Group Study NCI-6432. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2873. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatakrishnan K, Rader M, Ramanathan RK, Ramalingam S, Chen E, Riordan W, Trepicchio W, Cooper M, Karol M, von Moltke L. Effect of the CYP3A inhibitor ketoconazole on the pharmacokinetics and pharmacodynamics of bortezomib in patients with advanced solid tumors: a prospective, multicenter, open-label, randomized, two-way crossover drug-drug interaction study. Clin Ther. 2009;31(Pt 2):2444–58. doi: 10.1016/j.clinthera.2009.11.012. others. [DOI] [PubMed] [Google Scholar]