Abstract
Objective
To assess the effect of antiviral therapy for hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) after radical hepatectomy.
Methods
A total of 478 HBV-related HCC patients treated by radical hepatectomy were retrospectively collected. Patients in the treatment group (n=141) received postoperative lamivudine treatment (100 mg/d), whereas patients in the control group (n=337) did not. Recurrence-free survival (RFS) rates, overall survival (OS) rates, treatments for recurrent HCC and cause of death were compared between the two groups. Propensity score matching (PSM) analysis was also conducted to reduce confounding bias between the two groups.
Results
The 1-, 3-, and 5-year RFS rates didn't significantly differ between the two groups (P=0.778); however, the 1-, 3-, and 5-year OS rates in the treatment group were significantly higher than those in the control group (P=0.002). Similar results were observed in the matched data. Subgroup analysis showed that antiviral treatment conferred a significant survival benefit for Barcelona Clinical Liver Cancer stage A/B patients. Following HCC recurrence, more people in the treatment group were able to choose curative treatments than those in the control group (P=0.031). For cause of death, fewer people in the treatment group died of liver failure than those in the control group (P=0.041).
Conclusion
Postoperative antiviral therapy increases chances of receiving curative treatments for recurrent HCC and prevents death because of liver failure, thereby significantly prolonging OS, especially in early- or intermedian-stage tumors.
KEYWORDS : Antiviral therapy, hepatocellular carcinoma, propensity score matching, recurrence-free survival rate
Introduction
Hepatocellular carcinoma (HCC) is associated with a poor prognosis, and its incidence has increased dramatically in many countries1. Hepatectomy is a radical therapy for early-stage HCC. However, even after radical resection, the prognosis for HCC patients remains discouraging because of the high recurrence rate and frequent incidence of intrahepatic metastasis. Therefore, preventing HCC recurrence is very important. Many studies investigated the role of adjuvant and preventive chemotherapies for HCC after radical resection. But until now, there is no definite effective adjuvant or chemotherapies to prevent HCC recurrence2-6.
Hepatitis B virus (HBV) infection is the major risk factor for HCC development in China7,8. Some retrospective studies9,10 have shown that lamivudine treatment for HBV-related HCC patients can effectively reduce the HCC recurrence rate and increase the survival rate after hepatectomy. Other retrospective studies11,12 have shown that postoperative lamivudine treatment neither reduces HCC recurrence rate, nor increases the survival rate of patients, but can reduce the death rate caused by postoperative liver failure. Based on these findings, a meta-analysis13 proposes that antiviral therapy based on lamivudine can not only reduce the HCC recurrence rate, but also reduce the death rate caused by liver failure and increase the survival rate of patients. This meta-analysis integrated some retrospective studies with unmatched covariants between groups, otherwise some clinical heterogeneities like different radical therapies, antiviral drugs, dosages, and treatment courses were observed among these studies. Thus, the conclusion from this meta-analysis has limited reliability. Therefore, whether lamivudine antiviral therapy can reduce the postoperative recurrence of HBV-related HCC and extend the survival of patients remains unclear5.
To address this issue, we retrospectively collected the clinical data from the prospective database of all HBV-related HCC patients who were treated by lamivudine therapy after radical hepatectomy in our center, and compared with lamivudine-untreated patients in terms of recurrence-free survival (RFS), overall survival (OS), treatments for recurrent HCC, and cause of death. To more objectively assess the effects of antiviral therapy, we conducted propensity score matching (PSM) in the present study.
Patients and methods
Clinical data
Inclusion criteria: (1) the initial radical hepatectomy of HCC was performed in the Tumor Hospital of Guangxi Medical University. Diagnosis was verified by postoperative pathology; (2) serum Hepatitis B surface antigen (HBsAg) was positive for all patients; (3) Child-Pugh score was from 5 to 6; (4) Eastern Cooperative Oncology Group score was 0; (5) informed consent was signed.
Exclusion criteria: (1) received transarterial chemoembolization (TACE) or other antitumor therapies before operation; (2) received antiviral therapy (including interferon treatment) in the past year; (3) received prophylactic TACE or other antitumor therapies after operation; (4) combined infection of human immunodeficiency virus, hepatitis C virus, or hepatitis D virus; (5) suffered from other malignant tumors or other severe diseases simultaneously; (6) alcoholism; (7) drug abuse; and (8) pregnant or lactating women.
A total of 478 patients meeting the aforementioned requirements were collected from January 2007 to December 2011. Among these patients, 141 treated by lamivudine after operation were integrated into the treatment group, and the remaining 337 patients without lamivudine treatment were integrated into the control group. To avoid the interference of measured confounding factors, PSM was adopted in making group matches. Exactly 141 pairs were matched successfully. The baseline characteristics of both groups before and after matching are shown in Table 1. This research was approved by the Ethics Committee of our hospital.
Table 1. Baseline characteristics of treatment and control groups.
| Covariates | Before propensity matching (n=478) |
After propensity matching (n=282) |
|||||
|---|---|---|---|---|---|---|---|
| Treatment group (n=141) | Control group (n=337) | P | Treatment group (n=141) | Control group (n=141) | P | ||
| Gender, male/female (%) | 129 (91.5)/12 (8.5) | 284 (84.3)/53 (15.7) | 0.036 | 129 (91.5)/12 (8.5) | 127 (90.1)/14 (9.9) | 0.681 | |
| Age (years) | 48.94±10.47 | 49.86±11.95 | 0.432 | 48.94±10.47 | 49.70±12.10 | 0.574 | |
| HBeAg, +/- (%) | 15 (10.6)/126 (89.4) | 41 (12.2)/296 (87.8) | 0.636 | 15 (10.6)/126 (89.4) | 16 (11.3)/125 (88.7) | 0.849 | |
| HBV-DNA (IU/mL) | 1.66×104 (0-1.86×109) | 3.74×103 (0-3.18×106) | 0.041 | 1.66×104 (0-1.86×109) | 1.08×104(0-3.18×106) | 0.321 | |
| TBIL (μmol/L) | 13.51±5.46 | 14.10±6.93 | 0.37 | 13.51±5.46 | 13.28±6.77 | 0.753 | |
| DBIL (μmol/L) | 4.64±2.22 | 4.86±3.46 | 0.413 | 4.64±2.22 | 4.52±3.59 | 0.723 | |
| TP (g/L) | 69.79±6.32 | 69.70±6.61 | 0.819 | 69.79±6.32 | 70.17±6.63 | 0.622 | |
| ALB (g/L) | 40.42±4.57 | 40.17±4.81 | 0.599 | 40.42±4.57 | 40.58±5.14 | 0.779 | |
| ALT (U/L) | 39 (2-504) | 35 (2-341) | 0.019 | 39 (2-504) | 42 (9-341) | 0.878 | |
| AST (U/L) | 37 (1-270) | 38 (1-410) | 0.616 | 37 (1-270) | 39 (17-410) | 0.96 | |
| PT (s) | 13.04±1.51 | 12.86±1.63 | 0.254 | 13.04±1.51 | 13.02±1.78 | 0.928 | |
| AFP, ≥400 ng/mL/<400 ng/mL (%) | 38 (27.0)/103 (73.0) | 98 (29.2)/238 (70.8) | 0.625 | 38 (27.0)/103 (73.0) | 42 (29.8)/99 (70.2) | 0.597 | |
| Tumor number, 1/2/3 (%) | 102 (72.3)/30 (21.3)/9 (6.4) | 240 (71.2)/74 (22.0)/23 (6.8) | 0.967 | 102 (72.3)/30 (21.3)/9 (6.4) | 107 (75.8)/28 (19.9)/6 (4.3) | 0.674 | |
| Size of the largest tumor (cm) | 4.5 (0.8-15) | 5.5 (1.2-26.0) | 0.042 | 4.5 (0.8-15) | 5.0 (1.2-14.9) | 0.818 | |
| Tumor capsule, complete/incomplete (%) | 84 (59.6)/57 (40.4) | 163 (48.4)/174 (51.6) | 0.025 | 84 (59.6)/57 (40.4) | 80 (56.7)/61 (43.3) | 0.629 | |
| Liver cirrhosis, yes/no (%) | 115 (81.6)/26 (18.4) | 267 (79.2)/70 (20.8) | 0.562 | 115 (81.6)/26 (18.4) | 115 (81.6)/26 (18.4) | 1 | |
| BCLC stage, A/B/C (%) | 107 (75.9)/23 (16.3)/11 (7.8) | 256 (76.2)/60 (17.8)/21 (6.2) | 0.781 | 107 (75.9)/23 (16.3)/11 (7.8) | 105 (74.5)/26 (18.4)/10 (7.1) | 0.882 | |
| Follow-up time (months) | 24 (2-65) | 24 (0-73) | 0.057 | 24 (2-65) | 23 (1-73) | 0.184 | |
Methods
Patients of both groups underwent radical hepatectomy for HCC. This procedure followed the Diagnosis management and treatment of HCC (2011 Version)8 of the Ministry of Health of the People’s Republic of China. Liver resection technique was performed as previously described14,15. Patients in the treatment group were treated by oral lamivudine after operation. Indications for antiviral therapy followed the Guideline of prevention and treatment for chronic hepatitis B (2010 Version)16: (1) for Hepatitis B e Antigen (HBeAg)-positive patients, the value of HBV DNA ≥105 copies/mL (equivalent to 20,000 IU/mL); or for HBeAg-negative patients, HBV DNA ≥104 copies/mL (equivalent to 2,000 IU/mL); and alanine aminotransferase (ALT) ≥ two folds the upper limit of normal; (2) for patients with compensated cirrhosis, HBV DNA ≥104 copies/mL for HBeAg-positive patients, and HBV DNA ≥103 copies/mL for HBeAg-negative patients, which dose not matter whether the level of ALT is high or low; (3) for patients with cirrhosis in the decompensation period, they should receive antiviral therapy once HBV DNA is detected. Lamivudine [Heptodin, from GlaxoSmithKline (China) Investment Co. Ltd.] administration: orally taken by the patients once they had left the hospital or from the first week after the operation for the following one year, with a dosage of 100 mg/d.
Follow-up and the main outcome measures
Patients were followed up every two or three months after the operation for their serum HBV immune markers, HBV DNA, liver function, prothrombin time (PT), alpha-fetoprotein (AFP), ultrasonography, computed tomography or magnetic resonance imaging, and so on. Postoperative recurrence (including intrahepatic and extrahepatic recurrence) was confirmed through the appearance of intrahepatic or extrahepatic lesions meeting the features of HCC in any imaging examination. The main outcome measures included RFS and OS. Re-treatment measures for tumor recurrence included radical measures like re-operation, radiofrequency ablation, and palliative measures like TACE, systemic chemotherapy, symptomatic and supporting treatment, and so on. For deceased patients, the causes of death including recurrent progress of tumor and liver failure were recorded.
Statistical analysis
Data analyses were performed using SPSS Statistics 19.0. Variants with normal distribution or approximately normal distribution were described with mean±SD, whereas variants with non-normal distribution were described with median (range). The mean, median, and categorical variant rates between both groups were compared using t-test, Mann-Whitney U test, and χ2 test, respectively. Survival rate was estimated using the Kaplan-Meier method. Log-rank test was used to compare the differences between the two groups. Differences were considered to be statistically significant if P<0.05. PSM was performed using the PSM extension program developed by Felix Thoemmes for SPSS Statistics in 201114.
Results
Changes in the balance of baseline material before and after PSM
Five of 18 covariants, namely, gender, HBV DNA, ALT, maximum tumor diameter, and envelope integrity, in baseline characteristics before matching was unbalanced (P<0.05). After matching, the covariants were balanced (P>0.05, Table 1). The balance of baseline characteristics improved based on PSM.
Cumulative RFS rates before and after PSM
Before matching, the cumulative RFS rates of the treatment (n=141) and control groups (n=337) at 1-, 3-, and 5-year after the operation were 73.1%, 54.7%, 44.5%, and 70.8%, 58.2%, 52.0%, respectively, P=0.778. After matching, the cumulative RFS rates were 73.1%, 54.7%, 44.5%, and 68.8%, 47.8%, 43.0%, respectively (P=0.503, Figure 1).
Figure 1.
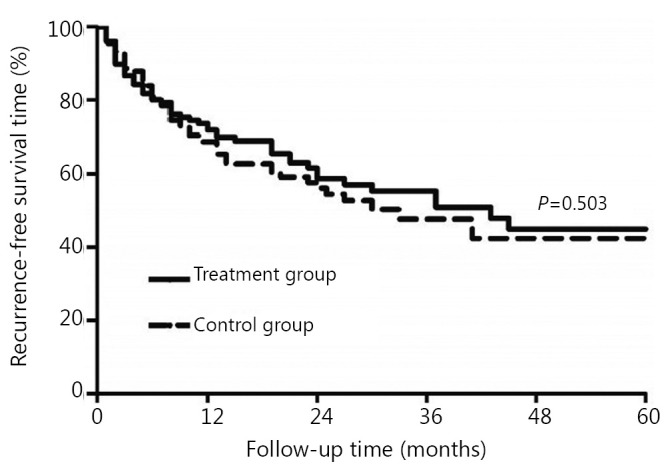
Comparison of cumulative RFS rates between the treatment group and the control group (P>0.05) after PSM.
Cumulative OS rates before and after PSM
Before matching, the cumulative OS rates of the treatment group (n=141) and the control group (n=337) at 1-, 3-, and 5-year after the operation were 92.1%, 84.4%, 79.1%, and 86.9%, 66.1%, 54.5%, respectively, P=0.002. After matching, the cumulative OS rates were 92.1%, 84.4%, and 79.1%, and 89.6%, 66.3%, and 52.1%, respectively (P=0.009, Figure 2).
Figure 2.
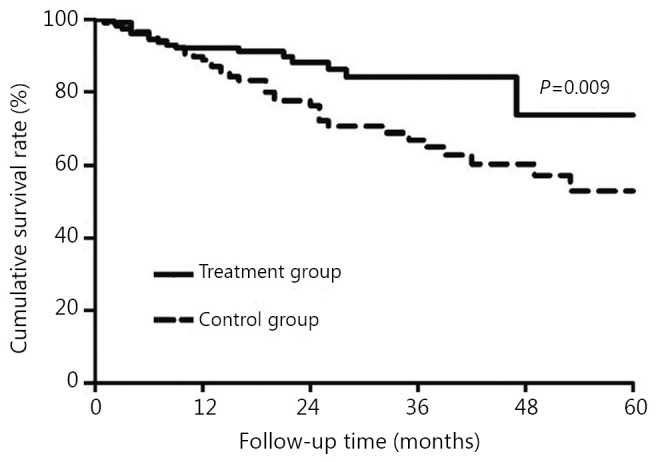
Comparison of cumulative OS rates between the treatment group and the control group (P<0.05) after PSM.
Subgroup analysis of the cumulative OS rates based on Barcelona Clinical Liver Cancer (BCLC) staging
Before matching, the treatment group had 130 cases with BCLC-A/B stages, whereas the control group had 316 cases with the same stages. After matching, the control group had 131 cases, and the cumulative OS rates of patients in the BCLC-A/B stages of the treatment and control groups at 1-, 3-, and 5-year after the operation were 92.2%, 83.9%, 78.5%, and 90.5%, 68.3%, 53.6%, respectively (P=0.035). Prior to matching, the treatment group had 11 cases and the control group had 21 cases in the BCLC-C stage. After matching, the control group had 10 cases in the BCLC-C stage, and the cumulative OS rates of patients in the BCLC-C stages of the treatment and control groups at 1-, 3-, and 5-year after the operation were 81.8%, 61.4%, 43.8%, and 80.0%, 48.0%, 48.0%, respectively (P=0.775).
HBV, liver function, tumor characteristics, and re-treatment measures for recurrence
Before matching, 52 and 117 recurrent cases were recorded in the treatment and control groups, respectively. After matching, 57 were noted as recurrence cases in the control group. After PSM, seven indicators, namely, HBV DNA, total bilirubin (TBIL), direct bilirubin (DBIL), ALT, aspartate transferase (AST), PT, and maximum diameter of the recurrent tumor, in the patients of the treatment group were evidently better than those of the control group (P<0.05) with recurrent HCC. No differences in intrahepatic recurrence or extrahepatic recurrence, single or multinodular recurrence, and vascular invasion as recurrence between the two groups were observed (P>0.05). For recurrent HCC, patients in the treatment group had more opportunities for radical re-treatment (P<0.05, Table 2).
Table 2. Clinical details of recurrent patients in the treatment and control groups.
| Covariates | Treatment group (n=52) | Control group (n=57) | P |
|---|---|---|---|
| HBV-DNA (IU/mL) | 0 (0-4.08×106) | 4.36×103 (0-1.46×107) | 0.013 |
| TBIL (μmol/L) | 12.35 (4.00-113.10) | 23.40 (3.40-298.70) | 0.04 |
| DBIL (μmol/L) | 4.00 (0.40-77.00) | 12.20 (2.80-129.80) | 0.043 |
| TP (g/L) | 70.53±6.51 | 68.43±6.43 | 0.093 |
| ALB (g/L) | 40.29±4.74 | 38.81±5.65 | 0.143 |
| ALT (U/L) | 36.50 (9.00-105.00) | 42.00 (13-218.00) | 0.021 |
| AST (U/L) | 35.50 (12.00-121.00) | 43.00 (15-344.00) | 0.04 |
| PT (s) | 13.27±1.38 | 13.93±1.82 | 0.032 |
| Recurrence in liver/Recurrence out of liver (%) | 48 (92.3)/4 (7.7) | 47 (82.5)/10 (17.5) | 0.125 |
| Tumor number, single nodule/multiple nodules (%) | 24 (46.2)/28 (53.8) | 20 (35.1)/37 (64.9) | 0.24 |
| Size of the largest tumor (cm) | 2.0 (0.4-7.0) | 3.2 (1.0-10.3) | 0.004 |
| Vascular invasion, yes/no (%) | 3 (5.8)/49 (94.2) | 7 (12.3)/50 (87.7) | 0.399 |
| Retreatment for recurrent HCC, radical treatments/palliative treatments (%) | 28 (53.8)/24 (46.2) | 19 (33.3)/38 (66.7) | 0.031 |
Comparison of death causes of patients in both groups
Before matching, 29 and 150 patients died in the treatment and control groups, respectively. After matching, 59 patients died in the control group. Following PSM, 18 (62.1%) and 11 (37.9%) cases died from tumor recurrence and liver failure, respectively, in the treatment group, and 23 (39.0%) and 36 (61.0%), respectively, in the control group (P=0.041).
Discussion
Lamivudine is a type of nucleoside analogue. It can be phosphorylated in cells as lamivudine triphosphate, which is embedded into viral DNA through HBV polymerase by competing with natural substrate deoxyadenosine triphosphate to terminate DNA synthesis. For chronic HBV infection patients, oral lamivudine can inhibit HBV DNA replication, promote ALT normalization, and improve liver inflammation, necrosis, and fibrosis17.
Our results showed that lamivudine treatment for HCC after radical hepatectomy could not effectively increase the cumulative RFS rates of patients, but it significantly increased the postoperative OS rates, which was similar to the results of other studies18,19. HCC recurrence includes primary tumor diffusion and multicentric occurrence7,20. Clinically, HCC recurrence within two years after operation is generally regarded as primary tumor diffusion. After 2 years, the recurrence is mostly multicentric occurrence7,20. Randomized controlled trials (RCT)21 and a multicentric retrospective study with a large sample size22 have shown that lamivudine antiviral therapy can be used for chronic hepatitis B and liver cirrhosis patients to reduce the risk of HCC development. Moreover, HBV infections with continuous high serum titers, as well as continuously or non-continuously increased levels of ALT, are high risk factors of HCC recurrence after radical hepatectomy23,24. So in theory, antiviral therapy can prevent the multicentric recurrence of tumor. However, HCC recurrences in our study mostly occurred within 2 to 3 years after the operation. Such a trend could be attributed to the liver diffusion of the primary tumor and a short follow-up time. This trend could possibly be the important reason for the lack of statistical difference in the cumulative RFS rates between the treatment and control groups, which is the common limitation of this study and other similar studies11,12,18,19. To confirm the effect of lamivudine on the recurrence rate, a clinical study with a larger sample size and a longer follow-up period is warranted.
In fact, the first RCT involving 163 patients investigating the efficacy of adjuvant nucleoside analogs to inhibit HBV-related HCC recurrence after radical hepatectomy has been reported25. In this trial, all patients had serum HBV-DNA concentrations >500 copies/mL and had Child-Pugh class A or B liver function. In the treatment group, 81 patients were given oral lamivudine (100 mg per day) within 1 week after hepatectomy until HBsAg seroconversion. After a median follow-up of 40 months, RFS was found to be significantly higher in the treatment group than in the control group at both 2 years (55.6% vs. 19.5%, P<0.001) and 4 years (37.3% vs. 12.1%, P<0.001). The treatment group also showed significantly higher OS at both 2 years (93.8% vs. 62.2%, P<0.001) and 4 years (86.4% vs. 47.4%, P<0.001), as well as a lower rate of early HCC recurrence (44.4% vs. 80.5%, P<0.001).
Our study introduced BCLC staging into assessment of antiviral efficacy. Patients in the BCLC-A/B stages treated with lamivudine after HBV-related HCC radical hepatectomy obtained obvious benefits in their survival (P<0.05). However, patients in the BCLC-C stage treated with oral lamivudine experienced insignificant effects (P>0.05). This result could be related to the poorer prognosis of patients in the BCLC-C stage, and failure to reflect antiviral efficacy in the short survival time.
When HCC recurrence was diagnosed, patients in the treatment group had lower HBV DNA level and better liver function indices than those in the control group (P<0.05). Most patients had the option of selecting re-operation, radiofrequency ablation, and other radical measures (P=0.031). The number of patients who died from liver failure in the treatment group remarkably decreased (P=0.041), which was similar to the results of other studies11,12,19. This result could be attributed to the ability of lamivudine to inhibit or remove HBV to the maximum, ease liver inflammation and fibrosis, impede and halt the progression of liver disease, reduce and prevent hepatic decompensation17. With liver function improvement after antiviral therapy, patients could obtain more opportunities for radical treatment and bear a larger resection range at recurrence, and the occurrence of liver failure events decreased, which was translated to the survival of patients.
Our study used PSM to reduce pre-existing differences between the groups, and the balance that is expected to create by a RCT design is here established through statistical matching. The results obtained were more reliable than those from previous retrospective studies9-13. Given the limited conditions, the complete information on the resistance rate of the virus and gene mutation rate were not collected. However, the aforementioned factors might be related to the recurrence of HBV-related HCC and the long-term survival of patients.
In summary, this study showed that the application of antiviral treatment with lamivudine after radical hepatectomy of HBV-related HCC could not effectively increase the RFS rate of patients. However, it provided patients with more opportunities for radical treatment during recurrence and reduced the occurrence of liver failure, which significantly enhanced the postoperative OS rates of patients. Antiviral treatment with lamivudine should be considered after radical hepatectomy for HBV-related HCC, particularly in early and intermedian stage. More RCTs with large sample size and long-term follow-up are warranted to prove our results.
Acknowledgments
This work was supported by grants from the National Science & Technology Major Project (Grant No. 2012ZX10002010), Guangxi Scientific Research & Technical Development Project (Grant No.10124001A-4) and the Self-raised Scientific Research Fund of the Ministry of Health of Guangxi (Grant No. Z2011211).
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30 [DOI] [PubMed] [Google Scholar]
- 2.Zhong JH, Mo XS, Xiang BD, Yuan WP, Jiang JF, Xie GS, et al. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS One 2013;8:e58082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong JH, Li H, Li LQ, You XM, Zhang Y, Zhao YN, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol 2012;38:286-295 [DOI] [PubMed] [Google Scholar]
- 4.Zhong JH, Ma L, Wu LC, Zhao W, Yuan WP, Wu FX, et al. Adoptive immunotherapy for postoperative hepatocellular carcinoma: a systematic review. Int J Clin Pract 2012;66:21-27 [DOI] [PubMed] [Google Scholar]
- 5.Zhong JH, Li le Q, Wu LC.Lamivudine with or without adefovir dipivoxil for postoperative hepatocellular carcinoma. Cochrane Database Syst Rev 2011;(12):CD008713. [DOI] [PubMed] [Google Scholar]
- 6.Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res 2010;40:943-953 [DOI] [PubMed] [Google Scholar]
- 7.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55 [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health of the People’s Republic of China Diagnosis management and treatment of hepatocellular carcinoma (2011 Version). J Clin Hepatol 2011;27:1141-1159 [Google Scholar]
- 9.Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906-1914 [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, et al. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg 2011;146:675-681 [DOI] [PubMed] [Google Scholar]
- 11.Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, et al. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma--using an untreated, matched control cohort. Acta Med Okayama 2005;59:217-224 [DOI] [PubMed] [Google Scholar]
- 12.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22:1929-1935 [DOI] [PubMed] [Google Scholar]
- 13.Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104-1112 [DOI] [PubMed] [Google Scholar]
- 14.Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013;8:e68193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic Resection Associated With Good Survival for Selected Patients With Intermediate and Advanced-Stage Hepatocellular Carcinoma. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000236. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Jia JD, Li LJ. The guideline of prevention and treatment for chronic hepatitis B. J Clin Hepatol 2011;27:113-128.
- 17.Shaikh T, Cooper C.Reassessing the role for lamivudine in chronic hepatitis B infection: a four-year cohort analysis. Can J Gastroenterol 2012;26:148-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Lai EC, Shi J, Guo WX, Xue J, Huang B, et al. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection. Ann Surg Oncol 2010;17:179-185 [DOI] [PubMed] [Google Scholar]
- 19.Koda M, Nagahara T, Matono T, Suqihara T, Mandai M, Ueki M, et al. Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function. Intern Med 2009;48:11-17 [DOI] [PubMed] [Google Scholar]
- 20.Li LQ, Peng T, Lin JL, Wu S, Liang ST, Lu YF, et al. The Impact of Cell Source of Recurrent Hepatic Carcinoma on Time of Recurrence. Chin J Clin Oncol 2000;27:635-636 [Google Scholar]
- 21.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-1531 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res 2005;32:173-184 [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol 2012;38:683-691 [DOI] [PubMed] [Google Scholar]
- 24.Cheung YS, Chan HL, Wong J, Lee KF, Poon TC, Wong N, et al. Elevated perioperative transaminase level predicts intrahepatic recurrence in hepatitis B-related hepatocellular carcinoma after curative hepatectomy. Asian J Surg 2008;31:41-49 [DOI] [PubMed] [Google Scholar]
- 25.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of Antiviral Treatment With Nucleotide/Nucleoside Analogs on Postoperative Prognosis of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Two-Stage Longitudinal Clinical Study. J Clin Oncol 2013;31:3647-3655 [DOI] [PubMed] [Google Scholar]


