Abstract
Purpose
In an attempt to reduce treatment time in corneal collagen cross-linking (CXL) with riboflavin and ultraviolet-A (UV-A), recent protocol modifications include shorter irradiation times at higher fluence, while maintaining constant total applied energy (Bunsen-Roscoe law of reciprocity). While such parameter changes might produce similar biological results within a certain range, the limits of reciprocity are unknown. Limitations in the corneal oxygen diffusion capacity and its potential impact on the efficacy of CXL, raise concerns regarding the efficiency of high-fluence CXL, and also of transepithelial CXL.
Methods
Porcine corneas were treated with an epithelium-off CXL at a fluence of 9 mW/cm2 under two different atmospheres: one with a regular oxygen content (21%) and another in a helium-supplemented, low-oxygen environment (<0.1%). Untreated corneas served as controls (n = 20 each). Five-millimeter corneal stripes were prepared and biomechanical stiffness was measured using an extensometer.
Results
Corneas cross-linked under normal oxygen levels showed a significant increase in biomechanical stability (14.36 MPa ± 2.69 SD), whereas corneas treated similarly, but in a low-oxygen atmosphere showed a Young's modulus similar to untreated controls (11.72 MPa ± 2.77 SD).
Conclusions
The biomechanical effect of CXL seems to be oxygen dependent. This dependency will be of particular importance in high-fluence and transepithelial CXL and will most likely require major protocol modifications to maintain the efficiency of the method.
Translational Relevance
The oxygen dependency of CXL shown here raises concerns about the effectiveness of high-fluence and transepithelial CXL. Both methods were introduced to clinical ophthalmology without thorough validation.
Keywords: corneal collagen cross-linking, high fluence, transepithelial, helium, UV-A absorption, oxygenation
Introduction
Corneal collagen cross-linking (CXL) with riboflavin and ultraviolet-A (UV-A) is a method that uses the reaction between a photo-activatable substance (riboflavin, vitamin B2), and UV-A light to create additional covalent bonds between and within the collagen fibers of the cornea.1,2 This photopolymerization process increases the biomechanical rigidity of the cornea.
Within a mere 15 years, CXL has emerged from a laboratory procedure to the gold standard for the treatment of progressive ectasia. The procedure has been successfully used to stabilize keratoconus, pellucid marginal degeneration, and ectasia after refractive laser surgery with excellent long-term results in adults and children.3–10 The original protocol developed in Dresden, Germany includes the removal of the epithelium (“epi-off”) allowing a 0.1% riboflavin solution to penetrate the corneal stroma, followed by UV-A irradiation at 365 nm at 3 mW/cm2 for 30 minutes.4,11,12
More recently, however, two trends have emerged that depart from the Dresden protocol. The first is a tendency to shorten treatment times. The justification for this approach is based on the Bunsen-Roscoe law of reciprocity that states the irradiation time and intensity can be varied without a change in the overall effect as long as the total energy is the same. In other words, treatment at 3 mW/cm2 for 30 minutes is equivalent to 9 mW/cm2 for 10 minutes. Commercial devices now offer ultrafast settings such as 18 mW/cm2 for 5 minutes and even 43 mW/cm2 for 2 minutes. While shorter treatment times are certainly attractive to clinicians and possibly their patients, commercial development has outpaced the scientific underpinnings that would support such a trend. Clinical validation in the form of peer-reviewed results is still missing despite most irradiation systems including high-fluence settings.
A second recent trend in the field has been to develop an “epi-on” approach, such that the epithelium remains intact during CXL. Again, this approach shortens the overall procedure time; however, both experimental and clinical findings show that the efficacy of transepithelial CXL is lower than in conventional CXL.13,14
In CXL, free radicals created by irradiation of applied substances on corneal tissue create new covalent bonds between collagen fibrils, which strengthens the cornea.1,15,16 This process requires oxygen to be present in sufficient quantities to participate in the reaction. It is conceivable that increasing the speed of the entire process (high fluence) or preventing oxygen from easily penetrating the stroma (“epi-on”) would create a relatively hypoxic microenvironment. These changes to the original Dresden protocol might affect the effectiveness of covalent bonds creation and biomechanical efficiency of the treatment. Here, we perform CXL in ambient and oxygen-poor conditions and demonstrate that oxygen is required for corneal CXL. The implications for recent high-fluence and “epi-on” procedures are discussed.
Methods
CXL Procedure
Freshly enucleated pig eyes with intact epithelium were obtained from a slaughterhouse and randomly assorted into three different groups (n = 20 for each group). One group was subjected to CXL in the presence of normal oxygen levels (room air, 21% oxygen). A second group underwent CXL in a low-oxygen environment (<0.1% oxygen, helium atmosphere). A third group served as controls and was treated identically to those eyes in the first group, but without UV-A irradiation. Prior to treatment, the epithelium was removed using a hockey knife, corneas were saturated with riboflavin 0.1% drops without dextran every minute for 25 minutes, and the epi-off CXL procedure was performed using the Schwind CXL-365 Vario system (Schwind eye-tech-solutions GmbH & Co., Kleinostheim, Germany) as described previously,17 with the only modification of a fluence of 9 mW/cm2 (5.4 J/cm2) for 10 minutes. No additional riboflavin was applied during irradiation. Corneal thickness was assessed using ultrasound pachymetry. Only corneas with a minimal central thickness of 700 μm and a maximal central thickness of 800 μm were used.
Helium Environment
The choice of helium was based on its properties as an inert monoatomic gas and that it is widely used for controlled-atmosphere experimentations. We used a hermetically sealed, Plexiglas box (39.2 × 16.2 × 16.2 cm) linked to a helium bottle (purity > 99.9%) with a humidification bottle of distilled water (Respiflo; Tyco Healthcare, Neustadt, Germany) (Fig. 1). In order to control the oxygen concentration, we used an oxygen sensor indicating O2 percentage and temperature (Firesting; PyroScience GmbH, Aachen, Germany) at the surface of the corneal stroma. The cornea was first exposed to a normal atmosphere. Immediately after initiation of UV-A irradiation, the helium atmosphere was instilled and oxygen concentration dropped from 21% to less than 0.5% within 30 seconds. The temperature remained stable at 22°C during all experiments.
Figure 1.

Experimental setup for the low-oxygen/helium environment. CXL is performed in a sealed chamber in a helium environment.
Biomechanical Measurements
After the CXL procedure, the corneas were excised and a central corneal strip of 5 × 10 mm was prepared. Young's modulus was examined at 10% strain using an extensometer (Zwicki-Line Testing Machine; Zwick, Ulm, Germany). Measurements were performed 20 minutes after the end of irradiation. Data analysis was performed with the Xpert II-Testing Software for Static Testing Systems (Zwick).
Statistical Analysis
Statistical analysis was performed using the Kruskal–Wallis one-way analysis of variance (ANOVA). P values below 0.05 were considered statistically significant.
Results
Corneas that underwent CXL in the presence of normal oxygen levels (21% oxygen, room air) successfully increased the stiffness of the corneas. Young's modulus (elasticity modulus) in these corneas was 14.36 MPa ± 2.69 SD (Fig. 2). The second group of corneas was exposed to normal oxygen levels (21% oxygen, room air) for the first 30 seconds of UV-A irradiation only. Oxygen was then replaced with a helium atmosphere, bringing the oxygen level in the chamber to less than 0.1%. Young's modulus was 11.72 MPa ± 2.77 SD, significantly lower than the corneas treated under normal oxygen levels (P = 0.001 versus normal oxygen levels [room air]; Kruskal-Wallis ANOVA).
Figure 2.
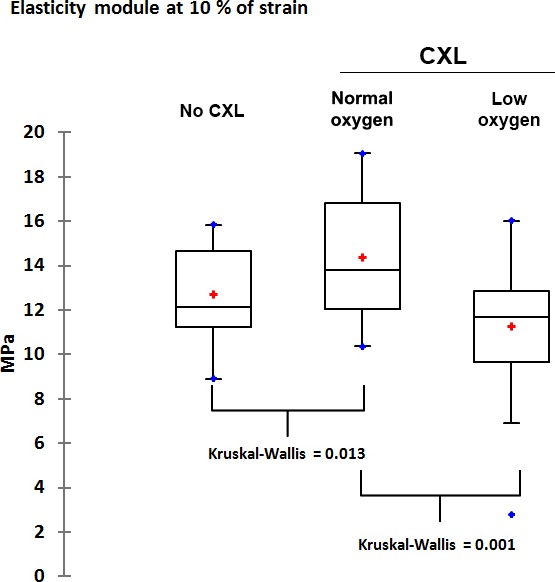
Mean stress values (Young's modulus) at 10% strain measured by extensometer. The left boxplot shows values for untreated control corneas. The middle boxplot shows the stress values of corneas that underwent CXL at 9 mW/cm2 for 10 minutes at normal oxygen concentration. The right boxplot shows stress values at 9 mW/cm2 for 10 minutes under a low-oxygen/helium atmosphere.
Controls were treated similarly, but were not subjected to UV-A irradiation. Controls had a low average Young's modulus of 12.68 MPa ± 2.17 SD (P = 0.013 versus normal oxygen levels [room air]; Kruskal-Wallis ANOVA).
Discussion
The oxygen distribution through the whole cornea is not homogenous, and oxygen permeability and consumption vary between different layers. In 1973, Fatt and colleagues18 reported the oxygen tension distribution of the rabbit cornea as an increasing curve starting at 55 mm Hg in the endothelium and progressively reaching more than 120 mm Hg at the basal epithelium. Likewise, oxygen diffusion through corneal tissue layers is a time-dependent process.
Recently, Kamaev et al.19 measured the oxygen concentration in porcine cornea below a 100-μm thick corneal flap during a standard CXL irradiation (0.1% riboflavin, 3 mW/cm2, 30 minutes, 25°C). Their results demonstrate that the initial oxygen concentration at this depth decreases rapidly during the first 10 to 15 seconds, subsequently reaching an initial steady state. After 10 minutes, oxygen concentration rises again as oxygen diffuses through the tissue. Since the authors were unable to measure oxygen within the cornea after 15 seconds of irradiation, they assume that oxygen was depleted and that remaining photopolymerization reactions are induced by “[riboflavin] triplets and reactive groups of corneal proteins, which leads to the cross-linking of the proteins mainly through radical reactions.”19
We have directly tested this hypothesis by initially saturating the cornea with oxygen and maintaining an oxygen-rich atmosphere during the first 30 seconds of treatment. According to Kamaev and colleagues,19 this should be sufficient to initiate CXL and additional reactions should take place without the need for oxygen. Our results show the contrary. Corneas treated with CXL at 9 mW/cm2 in a controlled, low-O2 environment had an average Young's modulus (i.e., stiffness) similar to that of untreated controls. Removal of oxygen after 30 seconds of irradiation essentially halts the photopolymerization process.
We believe that the steady state that occurs after several seconds of irradiation corresponds to a dynamic oxygen-dependent phase during which oxygen transportation through the cornea is matched by its consumption. As generally supposed, the strengthening effect of CXL on the cornea is likely the result of the creation of reactive oxygen species (free radicals) leading to the formation of covalent bonds between collagen and proteoglycan molecules. Our results suggest that oxygen is essential for the photochemical polymerization reaction in CXL and is probably the limiting factor of this reaction.
Based on our results, we demonstrate that the biomechanical increase in CXL is oxygen dependent. This may have direct implications for high-fluence and transepithelial CXL for ectasia. In the former case, it is possible that increasing fluence in an attempt to accelerate photopolymerization will not allow sufficient time for oxygen to diffuse and participate in the reaction. In the latter, the intact epithelium acts as a barrier to rapid oxygen diffusion into the corneal stroma and result in suboptimal cross-linking. It is very likely that a simple arithmetical modification of the parameters is not appropriate. Rather, proper protocol modifications for each technique should be developed to ensure adequate oxygen supply and biomechanical efficiency of CXL.
Acknowledgments
Supported in part by a grant of the Swiss National Science Foundation and by the Castier Foundation, Geneva, Switzerland.
Disclosure: O. Richoz, None; A. Hammer, None; D. Tabibian, None; Z. Gatzioufas, None; F. Hafezi, spin-off company from Geneva University (I), PCT/CH 2012/000090 (P)
Footnotes
Oliver Richoz and Arthur Hammer contributed equally to this work.
References
- 1.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 2.Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28:510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 3.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Vinciguerra P, Albe E, Mahmoud AM, Trazza S, Hafezi F, Roberts CJ. Intra- and postoperative variation in ocular response analyzer parameters in keratoconic eyes after corneal cross-linking. J Refract Surg. 2010;26:669–676. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]
- 7.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–231. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 8.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–758. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 9.Richoz O, Schutz JS, Pajic B, Coskunseven E, Hafezi F. Crosslinking for recurrent keratoconus. Ophthalmology. 2012;119:878–878. doi: 10.1016/j.ophtha.2011.11.007. e872. [DOI] [PubMed] [Google Scholar]
- 10.Richoz O, Mavrakanas N, Pajic B, Hafezi F. Corneal collagen cross-linking for ectasia after LASIK and photorefractive keratectomy: long-term results. Ophthalmology. 2013;120:1354–1359. doi: 10.1016/j.ophtha.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 12.Coskunseven E, Jankov MR, II, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009;25:371–376. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 13.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38:1000–1005. doi: 10.1016/j.jcrs.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 16.McCall AS, Kraft S, Edelhauser HF. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–138. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coskunseven E, Janko MR, II, Hafezi F, Atun S, Arslan E, Kymionis GD. Effect of treatment sequence in combined intrastromal corneal rings and corneal collagen crosslinking for keratoconus. J Cataract Refract Surg. 2009;35:2084–2091. doi: 10.1016/j.jcrs.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Fatt I, Freeman RD, Lin D. Oxygen tension distributions in the cornea: a re-examination. Exp Eye Res. 1974;18:357–365. doi: 10.1016/0014-4835(74)90112-2. [DOI] [PubMed] [Google Scholar]
- 19.Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53:2360–2367. doi: 10.1167/iovs.11-9385. [DOI] [PubMed] [Google Scholar]


