Abstract
Purpose
To determine the active ingredient in tea tree oil (TTO) responsible for its reported killing effect on Demodex mites, the most common ectoparasite found in the human skin extending to the eye.
Methods
Using a reported in vitro killing assay to measure the survival time of adult Demodex folliculorum up to 150 minutes, we have screened serial concentrations of 13 of the 15 known ingredients of TTO (ISO4730:2004) that were soluble in mineral oil and examined their synergistic relationships in killing mites. The most potent ingredient was then tested for its efficacy in killing Demodex in vivo.
Results
All ingredients exhibited a dose-dependent killing effect. Besides Terpinen-4-ol, the order of relative potency did not correlate with the order of relative abundance in TTO for the remaining 12 ingredients. Terpinen-4-ol was the most potent ingredient followed by α-Terpineol, 1,8-Cineole and Sabinene. Terpinen-4-ol, the most abundant ingredient in TTO, was more potent than TTO at equivalent concentrations and its killing effect was even observable at a mere concentration of 1%. Terpinen-4-ol exhibited a significant synergistic effect with Terpinolene, but an antagonistic effect with α-Terpineol in killing mites (both P < 0.05). In vivo, Terpinen-4-ol was shown to eradicate mites.
Conclusions
The above finding suggests that deployment of Terpinen-4-ol alone should enhance its potency in killing Demodex mites by reducing the adverse and antagonistic effects from other ingredients in TTO.
Translational Relevance
Terpinen-4-ol can be adopted in future formulations of acaricides to treat a number of ocular and cutaneous diseases caused by demodicosis.
Keywords: terpinen-4-ol, tea tree oil, demodex, acaricide, ocular drug delivery
Introduction
Demodex mites (Demodex folliculorum and Demodex brevis) are the most common ectoparasites infesting the pilosebaceous unit of the skin. Unbeknownst to many, Demodex prevalence increases with age and is observed in 84% of the population at age 60 and 100% of the population over the age of 70.1 Uncontrolled skin Demodex infestation (demodicosis) has been implicated in several diseases in the skin, including rosacea, with papulopustular skin lesions and perifollicular inflammatory infiltrate.2–9 Because the eye is surrounded by protruding body parts such as the nose, the brow, and the cheek, it is not as accessible to daily hygiene like the rest of the body. Therefore, once demodicosis occurs in the face, it will spread and flourish in the eyelids. It has been estimated that demodicosis is the most common, but often overlooked, cause of 29% to 74% of eyes with chronic blepharitis,10–17 which constitutes 37% and 47% of the patients seen in clinical practices of ophthalmologists and optometrists, respectively,18 and has a high incidence in elderly populations.12,19 We, and others, have also shown that ocular demodicosis, verified by microscopic examination and the counting of mites on epilated eye lashes, is strongly correlated with ocular surface inflammation associated with blepharitis, conjunctivitis, meibomian gland dysfunction, and even sight-threatening keratitis,20–25 not to mention an overlooked risk factor of pterygium recurrence.26
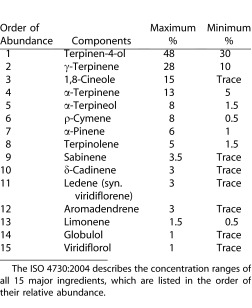
Tea tree oil (TTO) is a natural essential oil steam-distilled from the Australian native plant Melaleuca alternifolia and has long been used by the Aborigines for wounds and cutaneous infections. Its natural composition is well-characterized by approximately 100 constituents,27 but the commercial TTO is regulated by the International Organization of Standardization (ISO4730:2004)28 and must contain a specific range of 15 major ingredients as shown in Table 1. Previously, it has been reported that lid scrub with different concentrations of TTO is effective in reducing Demodex mite counts and ocular surface inflammation associated with blepharitis, conjunctivitis, and keratitis.20–25,29–33 Although lid scrub with 50% TTO is effective in killing mites and improving patients' signs and symptoms, it can cause ocular irritation.22,29,34 Thus, it is important to eliminate unwanted chemical ingredients in TTO, and/or reduce the effective concentration of TTO in order to reduce ocular irritation and promote overall safety. As a first step toward this objective, we sought to identify the most active pharmaceutical ingredient in TTO using an in vitro killing assay.
Table 1. .
Chromatographic Profile of Tea Tree Oil (From ISO 4730:2004)
Materials and Methods
Materials
TTO was obtained from the Essential Oil Company (Portland, OR). All TTO ingredients listed from Table 1 were purchased from Sigma-Aldrich (St. Louis, MO) except for δ-Cadinene and Viridiflorol, which were purchased from ISCA Technologies (Riverside, CA). Aseptically, each ingredient was diluted with mineral oil (Sigma-Aldrich) in 7.4-mL amber vials (Fisher-Scientific, Pittsburgh, PA) to the required concentrations of 100%, 50%, 25%, 10%, 5%, 2.5%, and 1%. The vials were then sealed after being vortexed for 1 minute. In order to study the potential synergistic effect, 50/50 mixtures of Terpinen-4-ol, α-Terpineol, Terpinolene, and/or γ-Terpinene were prepared in 50-mL conical tubes (BD Biosystems, Franklin Lakes, NJ), vortexed for 1 minute each to ensure adequate mixing, and then stored in 7.4-mL amber vials.
In Vitro Killing Assay
We employed the same in vitro killing assay to evaluate the above solutions as previously reported,34 following the tenets of the Declaration of Helsinki. After the patient had consented with the microscopic diagnosis, epilated eyelashes containing cylindrical dandruff were immediately evaluated under a light microscope using 40× and 100× magnifications to detect and count Demodex adult mites, larvae, and eggs. Movements of the legs were used to judge whether mites were alive or dead. Following addition of various solutions, each live adult mite (excluding eggs and larvae, as they are more vulnerable to killing) was monitored for up to 150 minutes. The survival time when the mite ceased any movement was recorded for comparison.
In Vivo Killing Assay
A patient with ocular demodicosis had consented and was instructed to use Cliradex (Bio-Tissue, Doral, FL), a lid cleanser containing simply water and Terpinen-4-ol (T40) with a few minor ingredients to help solubilize the T4O. To apply the lid cleanser, the patient would close their eyes and cleanse the eyelid with lateral side to side motions. The skin around the eye was then allowed to air dry for 1 minute before the opening of the eyes. This procedure was practiced twice a day as recommended. Demodex counts from eight epilated eyelashes per visit were recorded to monitor the lid cleanser's killing effect.
Statistical Analysis
All experiments were performed with a sample size of six unless otherwise specified. Data were reported as means ± SD and analyzed with Microsoft Excel (Microsoft, Redmont, WA). The data between groups were evaluated for statistical significance using Student's t-test and results were reported as P values, where P less than 0.05 were considered statistically significant.
Results
In Vitro Killing of Adult Mites by Individual TTO Ingredients
Previously, we have reported that TTO exhibits a dose-dependent killing effect on adult Demodex mites.34 To identify the active ingredients in TTO that are responsible for the above killing effect, we obtained all 15 major ingredients in TTO from their commercial sources. During the preparation of test solutions in serial concentrations, we noted that δ-Cadinene and Globulol were not able to be dissolved in mineral oil at any concentration. Because of their relative low abundance in TTO (i.e., ranked as #10 and #14 [Table 1]), we excluded them from the study. Out of the remaining 13 ingredients, we noted that Sabinene and Viridiflorol, which are ranked as #9 and #15 according to their relative abundance in TTO (Table 1), only dissolved at concentrations of 75% or less in mineral oil (Table 2).
Table 2. .
Survival Time of Adult Demodex Folliculorum When Exposed to TTO or Its Main Ingredients
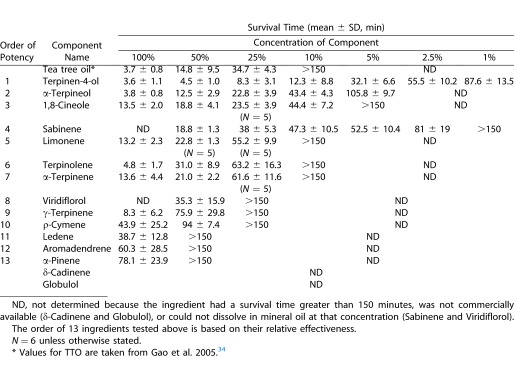
Consistent with our previous report,34 the control (i.e., 100% mineral oil) did not have any killing effect when observed for 150 minutes. Compared with mineral oil, the remaining 13 ingredients exhibited a dose-dependent killing effect (Table 2). We subdivided them into “effective” (<150 minutes) and “ineffective” (>150 minutes) based on the average survival time achieved by the solution with concentrations of 25%. Terpinen-4-ol, which is ranked as #1 based on relative abundance, was found to be the most effective and was the only ingredient in TTO that remained effective at a concentration of 1% (Table 2). Following Terpinen-4-ol, those exhibiting effective killing were ranked as follows: α-Terpineol, 1,8-Cineole, Sabinene, Limonene, Terpinolene, and α-Terpinene, which were ranked as #5, #3, #9, #13, #8, and #4, respectively, according to relative abundance in TTO. For the remaining six ingredients that were determined to be “ineffective” because they exhibited no killing effect at the concentration of 25%, their order of potency was determined by the average survival time at the concentrations of 50% and 75%, respectively. Their potency order was Viridiflorol, γ- Terpinene, ρ-Cymene, Ledene, Aromadendrene, and α-Pinene, which were ranked as #15, #2, #6, #11, #12, and #7, respectively, according to relative abundance in TTO (Table 2). These results concluded that Terpinen-4-ol was consistently ranked as #1 according to relative abundance in TTO and relative potency at the concentration of 25%. Except for Terpinen-4-ol, the order of relative potency of the remaining 12 ingredients did not match with that of relative abundance.
Interaction Between Ingredients
We then investigated whether there was any interaction between Terpinen-4-ol and other ingredients in TTO in the killing effect. This was achieved by comparing the average survival time achieved by Terpinen-4-ol alone at 100% compared with that achieved by an equal mixture of Terpinen-4-ol and the other ingredient. The survival times for T4O/Terpinolene and T4O/α-Terpineol were 2.2 ± 0.41 and 7.2 ± 0.79 minutes, respectively. Compared with the survival time shown in Table 2, Terpinen-4-ol exerted a positive synergistic effect with Terpinolene and an antagonistic effect with α-Terpineol in killing mites (both P < 0.05). Terpinen-4-ol did not exhibit any positive or negative effect with γ-Terpinene (4.3 ± 1.7 minutes, P > 0.05), which was determined to be ineffective.
In Vivo Killing Effect of T4O
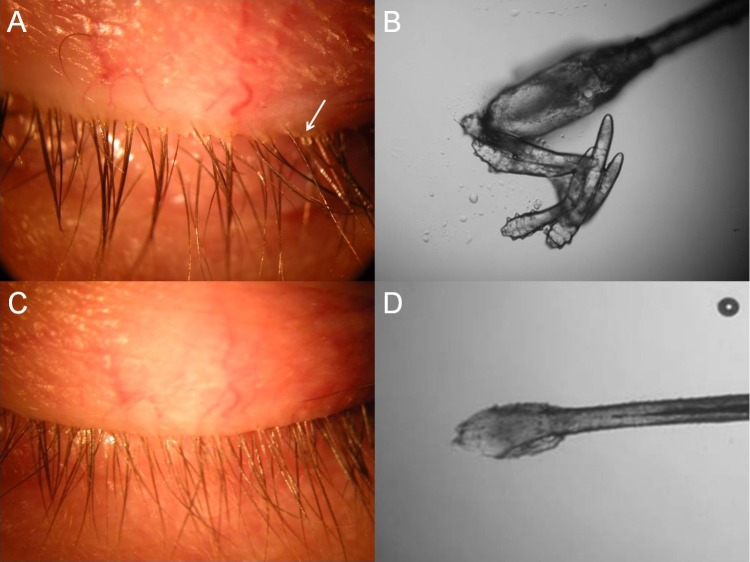
To establish the clinical and therapeutic value of our findings, we examined whether T4O could eradicate mites in a 61-year-old female presenting with bilateral persistent ocular burning pain and scratchy eyelids for 1 year. She was previously diagnosed with chronic blepharitis and dry eye syndrome. Conventional treatment including topical artificial tears and serum eye drops, hot compresses, insertion of punctual plugs, and oral doxycycline had not shown any significant improvement. Clinical examination revealed the presence of cylindrical dandruff in many lash roots (Fig. 1A, arrow) suggestive of Demodex blepharitis.20 Microscopic examination of a total of eight lashes, two epilated randomly from each eyelid, confirmed the presence of mites (Fig. 1B) and disclosed the total Demodex mite count of 22. After continuous use of Cliradex lid cleanser for 8 weeks, the patient noted marked resolution of symptoms. Repeated examination showed clearer lashes (Fig. 1C) and no mites (Fig. 1D).
Figure 1. .
In vivo effect of Terpinen-4-ol on eradication of Demodex mites. Before treatment, cylindrical dandruff was found in many lashes (A, arrow) and mites were detected under microscopic examination of the epilated lash (B). After treatment with the T4O lid cleanser, the lashes were clean (C) and no mite was detected in the epilated lash (D).
Discussion
Among a total of 15 major ingredients in TTO, we have identified Terpinen-4-ol as the most potent (i.e., most active) ingredient based on the dose-dependent survival time measured by an in vitro killing assay (Table 2). Terpinen-4-ol was the only ingredient that is ranked as #1 according to both relative abundance and relative potency, suggesting that it is primarily responsible for TTO's Demodex killing effect. Not surprisingly, Terpinen-4-ol was the only ingredient that exhibited a killing effect at a concentration as low as 1% in mineral oil. Furthermore, the potency of Terpinen-4-ol at a given concentration was greater than TTO at an equivalent concentration. For example, 5% Terpinen-4-ol exerted an average survival time of 32.1 ± 6.6 minutes similar to 34.7 ± 4.3 minutes of 25% TTO (P > 0.05, Table 2), which should contain 7.5% to 12% Terpinen-4-ol based on the calculated range of abundance in TTO (Table 1). Such an increasing potency by Terpinen-4-ol alone might be contributed by the exclusion of ingredients that do not exert comparable potency. Indeed, the order of relative potency did not match with that of relative abundance for all remaining 12 ingredients that could be dissolved in mineral oil. For example, γ-Terpinene, which is ranked as #2 in relative abundance, was found to be “ineffective.” Moreover, the potency of Terpinen-4-ol could be enhanced by a positive synergistic relationship with Terpinolene, but could also be mitigated by an antagonistic relationship with α-Terpineol (both P < 0.05). One potential mechanism to explain such an antagonistic relationship is a decreased solubility of T4O when mixed with other ingredients.35 This finding is important for future selection of excipients to formulate T4O-containing therapeutics.
Thus, it is highly plausible to promote the potency of killing Demodex mites by using Terpinen-4-ol alone without other ingredients in TTO. When TTO was used for treatments as topical formulations to the skin, allergic reactions were observed.36–44 These reactions have been linked to prolonged storage of TTO and the oxidation products formed within which generate ρ-cymene (an ineffective ingredient) and other compounds like peroxides, epoxides, and endoperoxides.45,46 These studies also suggested that the most important allergens could be terpinolene, α-terpinene, ascaridol and 1,2,4-trihydroxymethane, of which the first two were “effective” in killing mites as determined by our study. We, thus, strongly suspect that elimination of other active and ineffective ingredients could reduce irritation and allergic reactions that have been reported for TTO in previous studies.
In conclusion, our results showed that Terpinen-4-ol is the most active ingredient in TTO in exerting Demodex mite-killing effects. This finding is particularly exciting, as other studies have shown that Terpinen-4-ol is also the most active ingredient in TTO to exert antibacterial47–49 and antifungal50,51 effects. Such a wide range of antimicrobial effects of Terpinen-4-ol have been shown against several microbes responsible for hospital-acquired infections and ocular surface infections including Methicillin-resistant Staphylococcus aureus (MRSA), Methicillin-sensitive Staphylococcus aureus (MSSA), Coagulase-negative Staphylococci (CoNS), and Pseudomonas aeruginosa.47,52–56 Although the antimicrobial effect of Terpinen-4-ol has been attributed to the compromised cytoplasmic membrane,57 it remains unclear how it exerts its miticidal effect. Additionally, Terpinen-4-ol also possesses anti-inflammatory properties by suppressing superoxide production and pro-inflammatory cytokines,58,59 evidenced by a notable resolution of ocular irritation and inflammatory signs and vision improvement in some patients presumably due to reduction of corneal inflammation and neovascularization.20,29 Furthermore, the improvement was coupled with a stable lipid tear film (verified by tear interference images) presumably due to improvement of meibum lipids in 6 of 22 eyes.29 Collectively, these data support the inclusion of Terpinen-4-ol as the most active pharmaceutical ingredient in future formulations to treat a number of ocular and cutaneous diseases caused by demodicosis that may be associated with or without concomitant bacterial or fungal infections.
Acknowledgments
Supported by grants from the National Institutes of Health, National Eye Institute, Bethesda, MD R43 EY019586-01 (to SCGT), and TissueTech, Inc. and Ocular Surface Center, Miami, FL (contract grant sponsor).
Disclosure: S. Tighe, TissueTech, Inc. (E); Y.-Y. Gao, (P); S.C.G. Tseng, TissueTech, Inc. (I, E), National Eye Institute, National Institutes of Health (F), (P)
References
- 1.Post CF, Juhlin E. Demodex folliculorum and blepharitis. Arch Dermatol. 1963;88:298–302. doi: 10.1001/archderm.1963.01590210056008. [DOI] [PubMed] [Google Scholar]
- 2.Rufli T, Mumcuoglu Y, Cajacob A, Buchner S. Demodex folliculorum: aetiopathogenesis and therapy of rosacea and perioral dermatitis (author's trans) [in German] Dermatologica. 1981;162:12–26. [PubMed] [Google Scholar]
- 3.Forton F. Demodex and perifollicular inflammation in man: review and report of 69 biopsies [in French] Ann Dermatol Venereol. 1986;113:1047–1058. [PubMed] [Google Scholar]
- 4.Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol. 1993;128:650–659. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol. 1993;28:443–448. doi: 10.1016/0190-9622(93)70065-2. [DOI] [PubMed] [Google Scholar]
- 6.Georgala S, Katoulis AC, Kylafis GD, et al. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol. 2001;15:441–444. doi: 10.1046/j.1468-3083.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- 7.Basta-Juzbasic A, Subic JS, Ljubojevic S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin Dermatol. 2002;20:135–140. doi: 10.1016/s0738-081x(01)00244-9. [DOI] [PubMed] [Google Scholar]
- 8.Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3–6. doi: 10.1080/000155502753600795. [DOI] [PubMed] [Google Scholar]
- 9.Buechner SA. Rosacea: an update. Dermatology. 2005;210:100–108. doi: 10.1159/000082564. [DOI] [PubMed] [Google Scholar]
- 10.English FP, Nutting WB. Demodicosis of ophthalmic concern. Am J Ophthalmol. 1981;91:362–372. doi: 10.1016/0002-9394(81)90291-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheikhrouhou F, Makni F, Neji S, et al. Human demodicidosis in Sfax area (Tunisia) [in French] Bull Soc Pathol Exot. 2010;103:238–242. doi: 10.1007/s13149-010-0066-8. [DOI] [PubMed] [Google Scholar]
- 12.Garbacewicz A, Udziela M, Grytner-Ziecina B, et al. Demodex infections in general Polish population, in patients suffering from blepharitis, and among people who work with microscopes. Klin Oczna. 2010;112:307–310. [PubMed] [Google Scholar]
- 13.Turk M, Ozturk I, Sener AG, et al. Comparison of incidence of Demodex folliculorum on the eyelash follicule in normal people and blepharitis patients. Turkiye Parazitol Derg. 2007;31:296–297. [PubMed] [Google Scholar]
- 14.Czepita D, Kuzna-Grygiel W, Kosik-Bogacka D. Investigations on the occurrence as well as the role of Demodex follicuforum and Demodex brevis in the pathogensis of blepharitis [in polish] Klin Oczna. 2005;107:80–82. [PubMed] [Google Scholar]
- 15.Kemal M, Sumer Z, Toker MI, et al. The prevalence of Demodex folliculorum in blepharitis patients and the normal population. Ophthalmic Epidemiol. 2005;12:287–290. doi: 10.1080/092865805910057. [DOI] [PubMed] [Google Scholar]
- 16.Demmler M, de Kaspar HM, Mohring C, Klauss V. Blepharitis. Demodex folliculorum, associated pathogen spectrum and specific therapy [in German] Ophthalmologe. 1997;94:191–196. doi: 10.1007/s003470050100. [DOI] [PubMed] [Google Scholar]
- 17.Humiczewska M. Demodex folliculorum and Demodex brevis (Acarida) as the factors of chronic marginal blepharitis [in Polish] Wiad Parazytol. 1991;37:127–130. [PubMed] [Google Scholar]
- 18.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7:S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 19.Roth AM. Demodex folliculorum in hair follicles of eyelid skin. Ann Ophthalmol. 1979;11:37–40. [PubMed] [Google Scholar]
- 20.Gao YY, Di Pascuale M, Li W, et al. High prevalence of Demodex in eye lashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3094–3098. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10:505–510. doi: 10.1097/ACI.0b013e32833df9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirkhah A, Casas V, Li W, et al. Corneal manifestations of ocular Demodex infestation. Am J Ophthalmol. 2007;143:743–749. doi: 10.1016/j.ajo.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Chun Y, Clinical Kim J. and immunological responses in ocular demodecosis. J Korean Med. 2011;26:1231–1237. doi: 10.3346/jkms.2011.26.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SH, Chun YS, Kim JH, et al. The relationship between demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906–2911. doi: 10.1167/iovs.09-4850. [DOI] [PubMed] [Google Scholar]
- 25.Liang L, Safran S, Gao Y, et al. Ocular demodicosis as a potential cause of pediatric blepharoconjunctivitis. Cornea. 2010;29:1386–1391. doi: 10.1097/ICO.0b013e3181e2eac5. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, He H, Sheha H, Tseng SCG. Ocular demodicosis as a risk factor of pterygium recurrence. Ophthalmology. 2013;120:1341–1347. doi: 10.1016/j.ophtha.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Brophy JJ, Davies NW, Southwell IA, Stiff IA, Williams LR. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree) J Agric Food Chem. 1989;37:1330–1335. [Google Scholar]
- 28.International Organization for Standardization. Oil of Melaleuca, terpinen-4-ol type (tea tree oil) 2004 ISO-4730. [Google Scholar]
- 29.Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007;26:136–143. doi: 10.1097/01.ico.0000244870.62384.79. [DOI] [PubMed] [Google Scholar]
- 30.Gao YY, Xu DL, Huang LJ, et al. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea. 2011;31:14–17. doi: 10.1097/ICO.0b013e31820ce56c. [DOI] [PubMed] [Google Scholar]
- 31.Li J, O'Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117:870–877. doi: 10.1016/j.ophtha.2009.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo H, Kim T, Kim K, Wee S, Chun Y, Kim J. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. J Korean Med Sci. 2012;27:1574–1579. doi: 10.3346/jkms.2012.27.12.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima T, Ishida R, Sato EA, et al. In vivo evaluation of ocular demodicosis using laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:565–569. doi: 10.1167/iovs.10-5477. [DOI] [PubMed] [Google Scholar]
- 34.Gao YY, Di Pascuale M, Li W, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89:1468–1473. doi: 10.1136/bjo.2005.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox S, Mann C, Markham J. Interactions between components of the essential oil of Melaleuca alternofolia. J Appl Microbiol. 2001;9:492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 36.Fritz TM, Burg G, Krasovec M. Allergic contact dermatitis to cosmetics containing Melaleuca alternifolia (tea tree oil) [in French] Ann Dermatol Venereol. 2001;128:123–126. [PubMed] [Google Scholar]
- 37.de Groot AC. Airborne allergic contact dermatitis from tea tree oil. Contact Dermatitis. 1996;35:304–305. doi: 10.1111/j.1600-0536.1996.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 38.Aspres. N, Freeman S. Predictive testing for irritancy and allergenicity of tea tree oil in normal human subjects. Exogenous Dermatology. 2003;2:258–261. [Google Scholar]
- 39.Knight TE, Hausen BM. Melaleuca oil (tea tree oil) dermatitis. J Am Acad Dermatol. 1994;30:423–427. doi: 10.1016/s0190-9622(94)70050-8. [DOI] [PubMed] [Google Scholar]
- 40.Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. A multicenter study of the German Contact Dermatitis Group [in German] J Dtsch Dermatol Ges. 2003;1:629–634. doi: 10.1046/j.1610-0387.2003.03727.x. [DOI] [PubMed] [Google Scholar]
- 41.Rutherford T, Nixon R, Tam M, Tate B. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4.5 years. Australas J Dermatol. 2007;48:83–87. doi: 10.1111/j.1440-0960.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 42.Khanna M, Qasem K, Sasseville D. Allergic contact dermatitis to tea tree oil with erythema multiforme-like id reaction. Am J Contact Dermat. 2000;11:238–242. doi: 10.1053/ajcd.2000.7631. [DOI] [PubMed] [Google Scholar]
- 43.Varma S, Blackford S, Statham BN, Blackwell A. Combined contact allergy to tea tree oil and lavender oil complicating chronic vulvovaginitis. Contact Dermatitis. 2000;42:309–310. [PubMed] [Google Scholar]
- 44.Rubel DM, Freeman S, Southwell IA. Tea tree oil allergy: what is the offending agent? Report of three cases of tea tree oil allergy and review of the literature. Australas J Dermatol. 1998;39:244–247. doi: 10.1111/j.1440-0960.1998.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 45.Hausen BM, Reichling J, Harkenthal M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am J Contact Dermat. 1999;10:68–77. doi: 10.1016/s1046-199x(99)90002-7. [DOI] [PubMed] [Google Scholar]
- 46.Hausen BM. Evaluation of the main contact allergens in oxidized tea tree oil. Dermatitis. 2004;15:213–214. [PubMed] [Google Scholar]
- 47.Hammer KA, Carson CF, Riley TV. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother. 2012;56:909–915. doi: 10.1128/AAC.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loughlin R, Gilmore BF, McCarron PA, Tunney MM. Comparison of the cidal activity of tea tree oil and terpinen-4-ol against clinical bacterial skin isolates and human fibroblast cells. Lett Appl Microbiol. 2008;46:428–433. doi: 10.1111/j.1472-765X.2008.02334.x. [DOI] [PubMed] [Google Scholar]
- 50.Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 51.Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081–1085. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 52.Thomsen NA, Hammer KA, Riley TV, Van Belkum A, Carson CF. Effect of habituation to tea tree (Melaleuca alternifolia) oil on the subsequent susceptibility of Staphylococcus spp. to antimicrobials, triclosan, tea tree oil, terpinen-4-ol and carbacrol. Int J Antimicrob Agents. 2013;41:343–351. doi: 10.1016/j.ijantimicag.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Cox S, Mann C, Markham J. Interactions between components of the essential oil of Melaleuca alternofolia. J Appl Microbiol. 2001;9:492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 54.Sherwal BL, Verma AK. Epidemiology of ocular infection due to bacteria and fungus-a prospective study. JK Science. 2008;10:127–131. [Google Scholar]
- 55.Wong VW, Lai TY, Chi SC, Lam DS. Pediatric ocular surface infections: a 5-year review of demographics, clinical features, risk factors, microbiological results, and treatment. Cornea. 2011;30:995–1002. doi: 10.1097/ICO.0b013e31820770f4. [DOI] [PubMed] [Google Scholar]
- 56.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 57.Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tee) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother. 2002;46:1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hart PH, Brand C, Carson CF, et al. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000;49:619–626. doi: 10.1007/s000110050639. [DOI] [PubMed] [Google Scholar]
- 59.Brand C, Ferrante A, Prager RH, Riley T, Carson CF, Finlay-Jones JJ, Hart PH. The water soluble-components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm Res. 2001;50:213–219. doi: 10.1007/s000110050746. [DOI] [PubMed] [Google Scholar]