Abstract
Purpose
To assess the validity and repeatability of saccadic reaction times (SRT) across the visual field up to 30° using eye movement perimetry (EMP).
Methods
Eighteen subjects (36 eyes) were shown a central stimulus on a flat monitor screen. Next, peripheral stimuli were shown using an overlap paradigm. Subjects were instructed to look at detected peripheral stimuli and then refixate the central stimulus again. In three repetitive measurement series, a total of 288 visual stimuli (3 series × 24 locations × 4 contrast levels) were presented. Levene's test for equality of variances was applied to test the effect of stimulus location and measurement series on SRT variance. A Wilcoxon signed ranks test was used to compare SRTs between measurement series.
Results
A total of 26 eyes were included in the study (72%). On average, 90.5% of the peripheral stimuli were labeled as ‘seen' based on eye movement responses. Between the series, the mean SD of SRT differences was approximately 100 ms. Significantly faster SRTs were only found at contrast level 0.8 in series III compared with series II. In series I, SRT variance was independent in 75% of all locations. Across the three series, SRT variance was independent in 87.5% of all locations.
Conclusions
The present study demonstrates low variability of SRT across the visual field up to 30° eccentricity and across measurement series.
Translational Relevance
SRT as a measure for visual field responsiveness may be a potential marker to detect risk areas in specific parts of the visual field.
Keywords: saccadic eye movements, automated perimetry, visual field, eye movements
Introduction
Standard automated perimetry (SAP) is currently the leading functional test to assess visual field deficits. To overcome some of the disadvantages of SAP (e.g., a high level of cooperation, attention, and effort from a subject) several studies reported on eye movement perimetry (EMP). This technique has been applied in adults1–3 and children.4,5 EMP is a method based on a prosaccade task to assess the extent of the visual field. Eye movements were either judged using observation1,5 or more objectively measured using eye tracking.2–4 So far, the outcome of these EMP-based visual field studies is “seen or not seen.” We have a focus on the timing aspects of the prosaccades that can be assessed in eye tracker based EMP (i.e., the saccadic reaction time [SRT]). For prosaccades, a direct sensory-to-motor correspondence exists between stimulus location and saccade endpoints.6 Since the impact of volitional planning is limited,6 prosaccades may reflect the effectiveness of processing visual information into an eye movement response. Preliminary data demonstrated a high correlation between visual field thresholds assessed with SAP and SRT values assessed with EMP (Pel JJ, et al. IOVS. 2012; ARVO E-Abstract 4812). As such, SRT may reflect responsiveness as an additional feature in visual field testing.
This feasibility study assesses the potential of SRT as a marker for responsiveness in visual field testing. In SAP, it was shown that the variance of visual field thresholds depends on eccentricity.7 To date, not much data is available about SRT variance across the complete visual field. In the past, SRT of prosaccades have been found to depend on many factors, such as size, contrast, movement and luminance of a stimulus,8,9 time of appearance,10,11 and natural ageing.12 Contradictory studies have found that SRT was either dependent13–16 or independent6,17,18 of eccentricity. Finally, it was shown that SRT changed with prior probability in the horizontal meridian, accounting for median latency differences up to 100 ms.9
The aim of the present study was to investigate (1) the effect of eccentricity on SRT, (2) the effect of location on the SRT variance in a large group of subjects, and (3) the variability and repeatability of SRT values assessed during a test–retest setting. In a series of measurements, peripheral stimuli were shown using an overlap paradigm within a radial visual field up to 30° eccentricity with four different contrast levels. This series was presented three times to healthy subjects with no known visual field deficits. Our hypotheses were that (1) SRT was dependent of eccentricity, (2) the SRT variance was equal in each tested location, and (3) the mean and variance of SRT values assessed at low- and high-contrast stimuli remained stable over time.
Materials and Methods
Participants
Healthy subjects (n = 18) between 17- and 44-years old and naïve to the purpose of the study were recruited at the Erasmus University Rotterdam. All subjects had normal- or corrected-to-normal vision. None of the subjects had (known) visual field deficits. Exclusion criteria were diabetes and a history of visual or neurological disorders. All participants were informed about the study and informed consent was obtained prior to testing. The experimental procedures were approved by the Medical Ethical Committee of Erasmus University Medical Centre, Rotterdam, The Netherlands (METC-2009-199). The study adhered to the Declaration of Helsinki for research involving human subjects.
Measurement Setup and Procedure
The EMP measurement setup consisted of a 32-inch flat monitor screen with a refresh rate of 60 Hz (ELO Intellitouch System; Elo Touch Solutions, Inc., Milpitas, CA) and an infrared-based video eye-tracker system with a sample frequency of 200 Hz (Chronos Vision, Berlin, Germany). Each subject placed the head on a chin rest 460 mm away from the monitor. The nontested eye was covered with an eye patch. Each measurement started with a calibration routine, showing blue colored dots with a 2° visual angle at 20° up, down, left, and right from the center position. Next, some practice stimuli were shown to explain the task. The stimuli (dots) were plotted at 24 grid locations against a 60% brightness background (140 cd/m2) (Fig. 1, top panel; 0% brightness is black and 100% brightness is white). Each stimulus was 0.6° in diameter. Corrected for observer distance, its surface was about 70% larger compared with a Goldmann size III stimulus. The peripheral stimuli were plotted in four different brightness levels: 70% brightness (150 cd/m2), 80% brightness (162 cd/m2), 90% brightness (175 cd/m2), and 100% brightness (190 cd/m2). These different grey levels are denoted as contrast levels 0.7, 0.8, 0.9, and 1.0. The peripheral stimuli with contrast level 0.7 and 0.8 were low contrast stimuli, while peripheral stimuli with contrast level 0.9 and 1.0 were high contrast stimuli. At the start of the test, a green stimulus (hue of 80) was shown in the center position of the monitor. Next, peripheral stimuli (hue of 160) were shown using an overlap paradigm, meaning that the central stimulus remained visible during the presentation of peripheral stimuli. Because the peripheral stimuli were shown on a flat monitor screen they were corrected in size for their eccentricity. Subjects were asked to fixate the central stimulus. After a random time between 1.5 and 2.5 seconds, a peripheral stimulus was shown for a fixed duration of 1.2 seconds. Each subject was instructed to look at a peripheral stimulus when noticed and to avoid searching for stimuli. After removal of the peripheral stimulus, a 0.2 second delay was programmed to reset the timing. Subjects were instructed to refixate the central stimulus within a random time between 1.5 and 2.5 seconds before the next peripheral stimulus was shown. This cycle was repeated until all 96 stimuli per series were shown. In three repetitive measurement series, with a short rest period in between, a total of 288 visual stimuli (3 series × 24 locations × 4 contrast levels) were shown. The order of stimulus projection (location and contrast) within each series was randomized. The total duration of the measurements (including task instructions and calibration) was approximately 45 minutes. The Chronos video images were stored on a hard disk for off line extraction of eye positions.
Figure 1. .
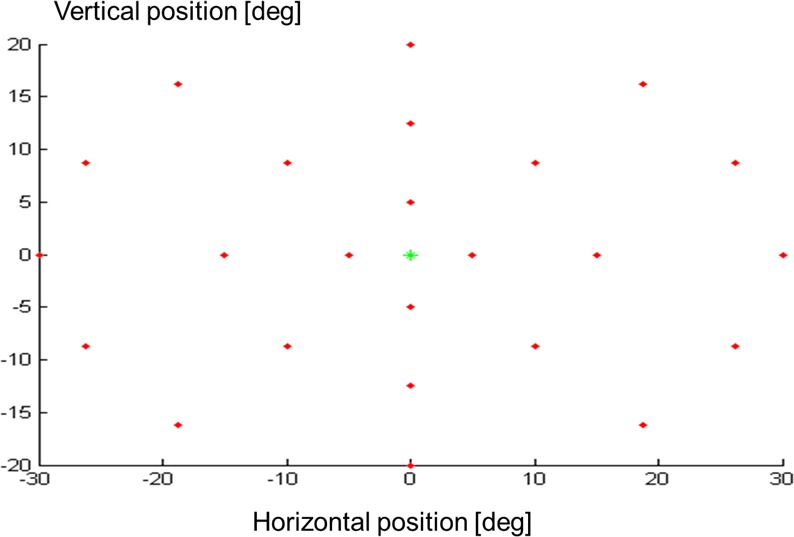
An illustration of the grid applied in the present study. A central stimulus was shown in the center position of the monitor. The peripheral stimuli were subsequently plotted at 24 grid locations (top panel). At each location, four different stimuli that varied in brightness levels were plotted. The bottom panel shows a schematic illustration of the central stimulus and superimposed some of the peripheral stimuli varying in contrast level.
Data Analysis
Eye movement data were transformed into gaze angle data using the Chronos Vision analysis programs (etd2.exe, version 3.4.0.0 and iris.exe, version 2.1.6.1, respectively, Chronos Vision). All 288 trials of each subject were visually inspected and analyzed using self written software in Matlab (MathWorks, Natick, MA). A decision algorithm to classify gaze angle data of each stimulus as ‘seen,' ‘not seen,' or ‘unknown.' This algorithm was based on previously reported structural eye movement analysis.2 First, it was checked if gaze was on the central stimulus. Next, a peripheral stimulus was labeled as ‘seen' when the primary saccade landed at the peripheral target location. A 2° fixation inaccuracy was taken into account for under- or overshoot corrections by means of correction saccades. A peripheral stimulus was labeled as ‘unseen' when no eye movement was made or when the first saccade did not land in the area of interest. Such an eye movement was denoted as ‘searching,' and it was removed for further analysis. A peripheral stimulus was labeled as ‘unknown' when no eye movement data were available due to poor eye tracking (e.g., blinking). Figure 2 presents an example of an eye movement from the central stimulus to a peripheral stimulus. The top panel illustrates gaze position relative to the peripheral stimulus location and the bottom panel shows the corresponding gaze velocity. Of each ‘seen' stimulus, the SRT was calculated as the time difference between stimulus presentation and the onset of the saccadic eye movement. This onset was defined as the moment that gaze velocity exceeded a 50°/s threshold value (Fig. 2, bottom panel).
Figure 2. .
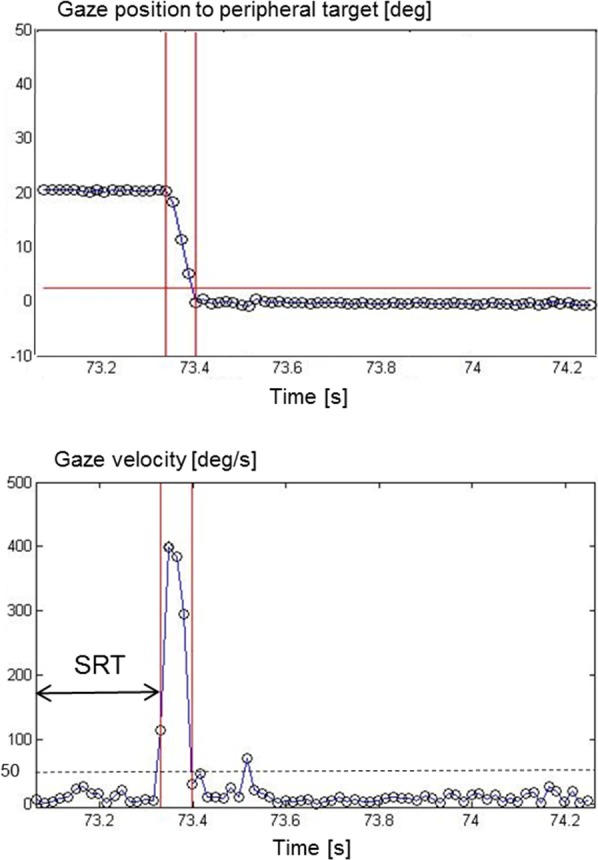
An example of an eye movement from the central to a peripheral stimulus. The top panel illustrates gaze position relative to the peripheral stimulus location and the bottom panel shows the corresponding gaze velocity. Of each ‘seen' stimulus, the saccadic reaction time (SRT) was calculated as the time difference between stimulus presentation and the onset of the saccadic eye movement. The onset of an eye movement was defined as the moment that gaze velocity exceeded a 50°/s threshold value.
Statistics
To test for SRT dependence on stimulus location, we clustered the peripheral stimulus locations at equal distances from the central stimulus. Three distance groups were formed, the first group at 5° (4 locations), the second group at 13.5° (8 locations), and the third group at 26° (12 locations). A linear mixed model was applied to the data collected during the first of three measurement series to test SRT dependence on stimulus contrast, distance, eye, and sex as fixed effects. Levene's test for equality of variances was applied to test the effect of stimulus location on SRT variance (1) within the first measurement series, and (2) across three measurement series. A Wilcoxon signed-rank test was applied to compare differences between two measurement series (series I and II, and series II and III). Significance level was set at P less than 0.05.
Results
Overall Performances
A total of 18 subjects (36 eyes) were asked to participate in the study. In one subject, one eye was excluded due to amblyopia and in nine eyes data collection failed during the calibration procedure (i.e., prior to testing). In three of these subjects, the pupils of both eyes were not sufficiently tracked by the Chronos eye tracker system (N = 6) and in three other subjects, this failed in one eye (N = 3). Thus, we included a total of 26 eyes from 15 subjects (an inclusion rate of 72%) for testing. None of these 15 subjects (mean age 23.8 ± 4.7 years) had any difficulties to perform the test. Table 1 presents the overall results of the total number of stimuli ‘seen' and ‘unseen' and the mean SRT values assessed during each measurement series as a function of contrast. From the total of 7488 presented peripheral stimuli in 26 eyes (13 left and 13 right eyes), 6775 were classified as ‘seen' (90.5%). In less than 1% of the stimuli ‘seen,' no SRT was calculated due to missing eye movement data (e.g., blinking). In the remaining approximately 9% of stimuli labeled as ‘unseen,' less than 0.5% of the stimuli were labeled as ‘searching.' Figure 3, top panel, shows the mean percentages and their SDs of the number of stimuli ‘seen' during each of three measurement series (mean value [μ] and μ per contrast). The number of stimuli seen decreased slightly from on average 92% in series I to 88% in series III. Figure 3, middle panel, shows the mean SRTs and their SDs of the peripheral stimuli seen during each measurement series (μ and μ per contrast). Overall, SRT (μ) was 329 (98) ms, SRT (0.7) was 372 (120) ms, SRT (0.8) was 323 (85) ms, SRT (0.9) was 315 (84), and SRT (1.0) was 307 (84) ms. Figure 3, bottom panel shows the differences in SRTs between series I and II, II and III, and I and III. This was done by subtracting SRT values at corresponding locations and contrast levels. On average, a difference of 4 (106) ms was found in SRTs. The differences were not significantly different from zero, indicating that there was no bias in SRT between the three measurement series.
Table. .
Total Number and Percentages of Stimuli ‘Seen' and ‘Unseen' (24 Locations × 26 Subjects = 624 Total Stimuli) Per Contrast Value Assessed During Three Measurements Series to Test Repeatability
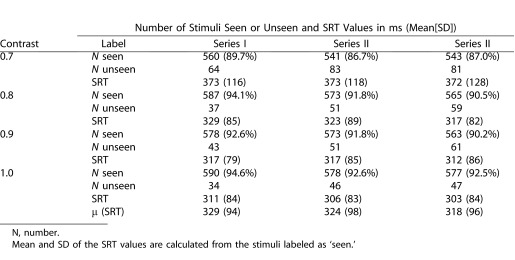
Figure 3. .
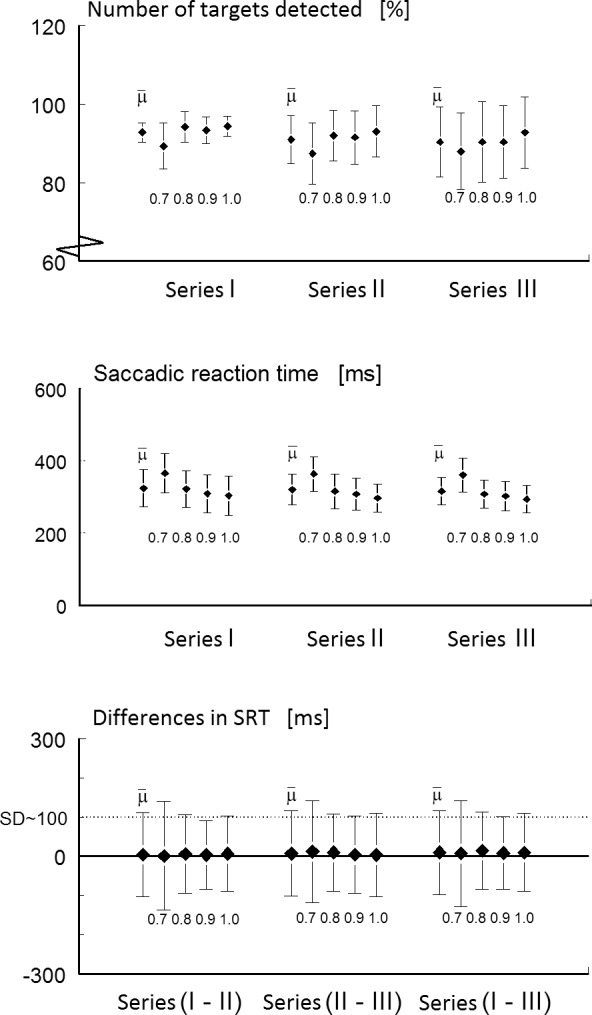
The top panel shows the mean percentages and their SDs of the number of stimuli ‘seen' during each of three measurement series (μ and μ per contrast). The middle panel shows the mean SRTs and their SDs of the peripheral stimuli seen during each measurement series (μ and μ per contrast). The bottom panel shows the differences in SRT values between series I and II, II and III, and I and III. On average, a difference of 4 (106) ms was found in SRT values.
Variability
Based on the first measurement series, the linear mixed model revealed a significant effect of contrast (F[3,1686.6] = 91.5, P < 0.001) and stimulus location (F[2,537.1] = 68.5, P < 0.001) on SRT, but no significant effects of eye (F[1,546.7] = 0.387, P = 0.534), and sex (F[1,13.2] = 2.2, P = 0.165). With increasing contrast levels SRT became significantly faster. Figure 4 illustrates the mean SRTs at contrast 0.7 (top panel) and at contrast 1.0 (bottom panel). Fast SRTs (∼250 ms [high contrast stimuli]) were depicted as light grey and slow SRTs (∼400 ms [low contrast stimuli]) as dark grey. Pairwise comparisons revealed that there was no significant difference in SRTs at all contrast levels, except between SRT (0.9) and SRT (1.0) (P = 0.185). The more peripheral a stimulus was shown, the higher the SRT, see Figure 5 for an overall illustration. Pairwise comparisons revealed that the SRTs significantly differed between the three distance groups. On average, SRT (5°) was 293 ms, SRT (13.5°) was 315 ms, and SRT (26°) was 351 ms. Levene's test for equality of variances revealed that in the majority of locations (18 out of 24 locations), the SRT variance was independent of location. In Figure 5, the six dependent locations were marked with a single asterisk (4 of 12 locations in the 26° group and 2 of 8 locations in the 13.5° group).
Figure 4. .
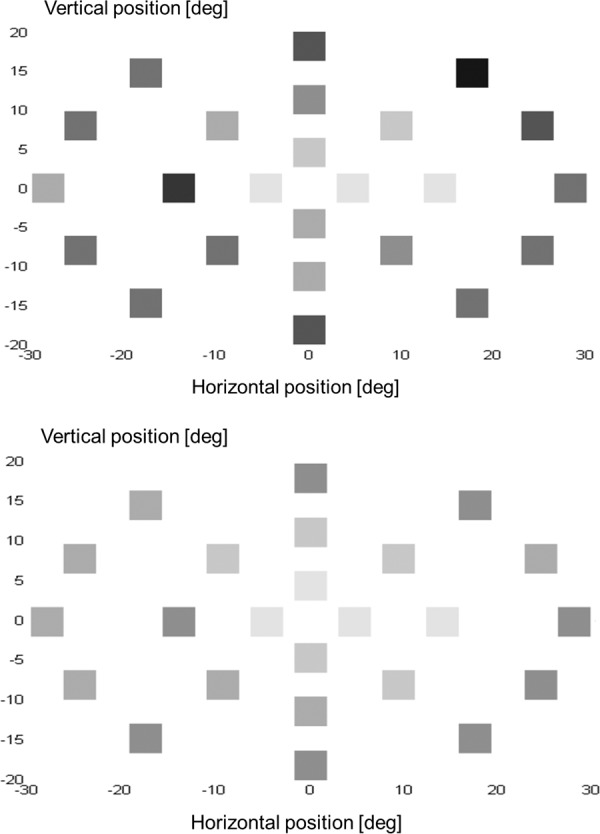
Illustration of the mean SRTs assessed at contrast level 0.7 (top panel) and at contrast level 1.0 (bottom panel).
Figure 5. .
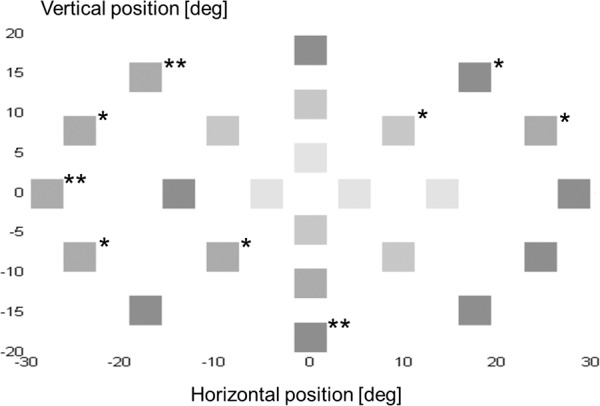
Illustration of the mean SRTs as a function of stimulus location. The more peripheral a stimulus was shown, the higher the SRT.
Repeatability
At each individual contrast level, the Wilcoxon signed ranks test showed no significant differences between SRT values of series I and II. This was also found between SRT values of series II and III, except for contrast level 0.8 (P < 0.05). At this contrast level, SRT values assessed in series III (median 310 ms) were significant faster than those assessed in series II (median 315 ms), z = −2.063, P < 0.05). Levene's test for equality of variances revealed that in a majority of locations (21 of 24 locations) the SRT variance was independent across the measurement series. In Figure 5, the locations at which SRT variance was dependent of measurement series are marked with a double asterisk (3 of 12 locations in the 26° group).
Discussion
To investigate the stability of SRT as a marker for responsiveness in visual field testing, we assessed its validity and repeatability during EMP. In the successfully tested eyes (inclusion rate of 72%), the number of stimuli seen slightly reduced from 92% to 88% over three measurement series. We found SRT dependence on stimulus contrast and location across the visual field up to 30° eccentricity. The SRT values at contrast level 0.9 were not significantly different from those measured at contrast level 1.0, suggesting that contrast level 0.9 could be omitted in future experiments. Most importantly, we found that in 75% of the tested locations, SRT variance was independent of stimulus location. In addition, its repeatability showed similar reliability. SRT variance was in 87.5% independent of the three measurements done at different time points. This suggests that variability of SRT as a measure for visual field responsiveness may be a reliable marker in visual field testing.
SRT Dependence
In our daily life, the state of visual attention, either engaged or disengaged,19 influences the saccadic reaction times toward a new visual stimulus.20 In eye movement studies, gap-and-overlap paradigms have been developed to trigger prosaccades. When a fixation target disappears upon peripheral stimulus projection (gap condition), mean SRT values are normally in the order of 100 to 140 ms. When fixation target remains visible (overlap condition), SRTs become prolonged to 250 ms.21–23 In the present study, the overlap condition was applied in combination with low and high contrast peripheral stimuli presented at 24 target locations. We found average SRTs varying from 280 ms (high contrast stimuli, 5° eccentricity) up to 400 ms (low contrast, 26° eccentricity). The fastest SRTs were a bit slower than the previously reported latencies in studies where controls were compared with patients with Parkinson's disease, for instance.24 In most of these studies, however, much more contrast was applied between background (black) and peripheral targets (white or red) as we did in the present study using a grey background and light grey to white peripheral stimuli. We think that this difference in contrast explains the differences in latencies. In addition, lowering the contrast and increasing the eccentricity up to 30° visual field significantly increased SRT. As stated, this result confirms the eccentricity effects that have been shown for prosaccades13–16 but it contradicts other studies.6,17,18 Darien et al.18 limited eccentricity to the horizontal meridian and they conducted their test using a white background in combination with a black fixation point and red peripheral targets. An explanation on a retinal level might be a process known as bleaching adaption.25 This is desensitization of the photoreceptors when exposed to bright background light. It might be that it influenced the responsiveness of the retinal layer to detect peripheral targets. Dafoe et al.6 examined the influence of eight radial directions and found SRT independence of eccentricity as well. They limited, however, eccentricity to an 8° visual field.
Variability and Repeatability
In SAP, the variability in threshold values generally increases with distance from the fixation point.7 EMP relies on a natural oculomotor response and not, as in SAP, on conflicting or contradicting verbal or motor responses.2 One of most crucial findings of the present study is that variability in SRT was equal across the majority of the tested locations. Note that, although the test grid in SAP was different to the test grid used in the present study, the ranges of the tested visual field are comparable. The differences in variability between SAP and EMP may be explained by differences between the methods applied. In SAP, a peripheral stimulus is shown for a short duration (∼80 ms). It requires a high level of attention not to miss such stimulus, especially when stimuli are presented in the periphery. In SAP, estimations for fixation losses and for false positive and negative errors are presented. The advantage of EMP is that it allows assessment of eye movements as an objective control measure of task performance. During data analysis, we checked fixation losses prior to a peripheral stimulus presentation. Subsequently, we checked if the primary saccade landed on the peripheral target, given an uncertainty of 2°. Saccades landing outside this area were characterized as ‘searching'. When only a saccadic vector is analyzed, as in saccadic vector analysis,4,5 and the end point of the saccade is not taken into account, then a ‘searching' response could erroneously be incorporating as a ‘seen' response. The chance that a false positive error occurs, is very small in EMP. A subject would have to make a goal directed eye movement to a peripheral target that she or he has not seen. False negative errors, however, can occur in EMP. Given a minimum contrast level of a peripheral target that was seen at a certain location, a false negative error would be a nonresponse to any of the higher contrast levels of peripheral targets at that location. On average, we classified about 5% of the ‘unseen' stimuli as ‘searching' and eliminated these responses for further analysis. We found that SRT values at each contrast level were not significantly affected by the measurement series, except for contrast level 0.8 between series II and series III. This difference was in the order of 5 ms, which is less than the overall differences found between contrasts 0.7 and 0.8 and between contrasts 0.8 and 0.9. We conclude that SRT variance was in 87.5% of the tested locations independent of measurement series.
Study Limitations
There are some study limitations that need to be addressed. A requirement for using EMP is sufficient tracking of the eyes during the test. In 9 out of the 36 eyes, tracking failed during the calibration procedure. This was an unexpected large number of eyes given that our subjects were young and fit individuals. This was due to problems with the rather old eye tracker equipment that we used. The video images of the eye tracker used in the present study made of the eyes were of acceptable quality, but still the software was not able to identify the pupils. We never experienced such a high dropout rate before. In three subjects eye tracking failed in both eyes. All three subjects were females and two of them had dark brown eyes. Prior to testing we asked the female participants to remove any eye makeup, but still we were not successful in tracking their pupils. In three others, measurement of one eye failed. In a group of 18 subjects (36 eyes), tracking problems in three eyes would have been a normal dropout rate (∼10%). The subjects that were included in the present study were healthy young volunteers without known visual field defects. We statistically confirmed that the SRTs of all subjects had the same distribution. However, we cannot rule out that some of our subjects did have some congenital asymptomatic visual field deficit (e.g., optic disc drusen or tilted discs) that could have been detected with SAP prior to EMP.
In the present study, we conducted three measurements series (even with a short break in between) to test SRT variability. For clinical application, one measurement series would be sufficient, but the testing time of approximately 15 minutes would still be long compared with SAP. We are developing an interactive algorithm that is able to decide during the test ‘on-line' whether a stimulus is seen. Such an approach would reduce the testing time to a total duration of 5 to 7 minutes, since contrast levels higher than the one seen at a given location can be removed from the list during the measurement.
Finally, we displayed the stimuli on a video graphics array-based monitor. A limiting factor is the small range of intensities that can be produced on such monitors (∼15 dB from the dimmest to the brightest intensities) compared with the intensities in SAP.4 In addition, luminance values can fluctuate between monitor corners. Recent commercially available light emitting diode monitors have uniform luminance and may be a good alternative for presenting the stimuli.
Clinical Applicability
EMP as well as SAP assess the quality and the extent of a subject's visual field. Feasibility studies in adults and children with and without visual field defects showed consistent visual fields ranging between 90% and 99%.1–5 With EMP, both groups reported alleviation of some of the stress and tediousness associated with SAP.3 Depending on the stimulus grid, EMP as well as SAP enables the detection of large visual field losses like in hemianopia, visual field narrowing, or even small field loss due to scotomas.5 In addition to general application in clinical visual field testing, EMP could open new possibilities of visual field testing in young children, people with motor impairments in the hand or arm, and patients with cognitive decline or mental retardation. Although EMP requires a minimum of active cooperation and is found less strenuous than SAP, this method excludes patients suffering from oculomotor apraxia. Patients with oculomotor problems such as strabismus (one eye calibration) or even nystagmus (analyzing the foveation periods only) can participate in EMP. The authors acknowledge that a commercially EMP is available that uses saccadic vector analysis and an observational EMP model.5 This webcam-based perimeter uses prosaccades in preferential looking responses to assess peripheral visual fields in young and developmentally delayed children. In the present paper, we investigated the variability of the responsiveness of the visual field as a new marker, which can only be detected using an eye tracker–based perimeter. Obviously, the infrared system incorporated in a helmet used in the present paper has clear limitations to be widely applied in clinical settings. Nowadays, remote infrared eye trackers are available, that we, as well as others, successfully apply in our lab in children and adults to assess eye movement responses.26 These eye trackers have sample rates of 60 Hz and higher and are accurate enough to detect the onset of eye movements to presented stimuli. By incorporating a SAP stimulus grid in EMP, comparisons in terms of responsiveness (EMP) and sensitivity (SAP) can be done in patients suspected of or diagnosed visual field loss (glaucoma) or reduced visual field contrast and sensitivity (cataracts). We suppose that prolonged SRT values in specific parts of the visual field compared with other parts are indicative for onset or progression in glaucoma. Indeed, preliminary data suggest that visual field sensitivity assessed with SAP correlates with visual field responsiveness assessed with EMP (Pel JJ, et al. IOVS. 2012; ARVO E-Abstract 4812). The question that still needs to be addressed is whether the magnitude of SRT variability assessed on a group level, is small enough to detect visual field defects at an individual level. In this respect, the present study showed that when SRTs to similar locations vary within the order of 100 ms. This finding confirms previous studies, in which prior probability was shown to account for latency differences in the horizontal meridian up to 100 ms.14 Thus, on a cognitive level, prior knowledge may explain some of the variation that exists in SRT. This suggest that SRT differences of 100 ms and higher, either compared with neighboring locations within the same subject or compared with SRTs from an age-matched control group, might indicate risk areas in specific parts of a subject's visual field.
Conclusions
We investigated the stability of SRT as a marker for responsiveness in visual field testing This study showed that in 75% of the tested locations, SRT variance was independent of stimulus location. In addition, SRT variance was in 87.5% independent of the three measurement done at different time points. These results suggests that variability of SRT as a measure for visual field responsiveness may be a reliable marker in visual field testing. As shown by others, eye movement perimetry opens possibilities to test the quality and the extent of visual fields in groups that are currently not tested due to their age, physical movement restrictions of arm and/or hand, or cognitive decline and mental retardation. We suggest that by incorporating saccadic response time as a measure for visual field responsiveness, application of this method might be extended to detect risk areas in specific parts of the visual field.
Acknowledgments
Supported by grants from Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, Landelijke Stichting voor Blinden en Slechtzienden, Stichting Glaucoomfonds, and Odas Stichting, which contributed through UitZicht.
Disclosure: J.J.M. Pel, (P); M.C.M. van Beijsterveld, None; G. Thepass, None; J. van der Steen, UitZicht (F), (P)
References
- 1.Trope GE, Eizenman M, Coyle E. Eye movement perimetry in glaucoma. Can J Ophthalmol. 1989;24:197–199. [PubMed] [Google Scholar]
- 2.Jernigan ME. Structural analysis of eye movement responses to visual field stimuli. Comput Biol Med. 1980;10:11–22. doi: 10.1016/0010-4825(80)90003-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim DE, Eizenman M, Trope GE. Eye movement perimetry. IEEE-EMBC. 1995;2:1629–1630. [Google Scholar]
- 4.Murray IC, Fleck BW, Brash HM, MacRae ME, Tan LL, Minns RA. Feasibility of saccadic vector optokinetic perimetry. A method of automated static perimetry for children using eye tracking. Ophthalmol. 2009;116:2017–2026. doi: 10.1016/j.ophtha.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Allen LE, Slater ME, Proffitt RV, Quarton E, Pelah A. A new perimeter using the preferential looking response to assess peripheral visual fields in young and developmentally delayed children. J AAPOS. 2012;16:261–265. doi: 10.1016/j.jaapos.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Dafoe JM, Armstrong IT, Munoz DP. The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp Brain Res. 2007;179:563–570. doi: 10.1007/s00221-006-0817-8. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetry threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter RH. Contrast, probability, and saccadic latency: evidence for independence of detection and decision. Curr Biol. 2004;14:1576–1580. doi: 10.1016/j.cub.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter RH, Williams ML. Neural computations of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- 10.Bevan W, Hardesty DL, Avant LL. Response latency with constant and variable interval schedules. Percet Mot Skills. 1965;20:969–972. doi: 10.2466/pms.1965.20.3.969. [DOI] [PubMed] [Google Scholar]
- 11.Oswal A, Odgen M, Carpenter RH. The time course of stimulus expectation in a saccadic decision task. J Neurophysiol. 2007;97:2722–2730. doi: 10.1152/jn.01238.2006. [DOI] [PubMed] [Google Scholar]
- 12.Munoz DP, Broughton JR, Goldring JE, Amstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- 13.Weber H, Aiple F, Fischer B, Latanov A. Dead zone for express saccades. Exp Brain Res. 1992;89:214–222. doi: 10.1007/BF00229018. [DOI] [PubMed] [Google Scholar]
- 14.Kalesnykas RP, Hallett PE. Retinal eccentricity and the latency of eye saccades. Vision Res. 1994; 34:517–531. doi: 10.1016/0042-6989(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 15.Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol. 1996;76:2822–2833. doi: 10.1152/jn.1996.76.5.2822. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JH. Eye position and target amplitude effects on human visual latencies. Exp Brain Res. 1996;109:457–466. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- 17.Frost D, Poppel E. Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: indications of a functional subdivision of the visual field. Biol Cybern. 1976;23:39–48. doi: 10.1007/BF00344150. [DOI] [PubMed] [Google Scholar]
- 18.Darrien JH, Herd K, Starling LJ, Rosenberg JR, Morrison JD. An analysis of the dependence of saccadic latency on target position and target characteristics in human subjects. BMC Neurosci. 2001;2:13. doi: 10.1186/1471-2202-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci. 1993;16:553–610. [Google Scholar]
- 21.Saslow MG. Latency for saccadic eye movement. J Opt Soc Am. 1967;57:1030–1033. doi: 10.1364/josa.57.001030. [DOI] [PubMed] [Google Scholar]
- 22.Ross LE, Ross SM. Saccade latency and warning signals: stimulus onset, offset, and change as warning events. Percept Psychophys. 1980;27:251–257. doi: 10.3758/bf03204262. [DOI] [PubMed] [Google Scholar]
- 23.Kalesnykas RP, Hallett PE. The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp Brain Res. 1987;68:115–121. doi: 10.1007/BF00255238. [DOI] [PubMed] [Google Scholar]
- 24.Terao Y, Fukuda H, Yugeta A, Hikosaka O, Nomura Y, Segawa M, Hanajima R, Tsuji S, Ugawa Y. Parkinson eye movements Initiation and inhibitory control of saccades with the progression of Parkinson's disease—changes in three major drives converging on the superior colliculus. Neuropsychologia. 2011;49:1794–1806. doi: 10.1016/j.neuropsychologia.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 26.Pel JJ, Manders JC, van der Steen J. Assessment of visual orienting behaviour in young children using remote eye tracking: methodology and reliability. J Neurosci Methods. 2010;189:252–256. doi: 10.1016/j.jneumeth.2010.04.005. [DOI] [PubMed] [Google Scholar]


