Abstract
Background
The role and the optimal measurement method of serum HER2 levels are not defined in patients with metastatic breast cancer (MBC). We prospectively assessed the prognostic value of serum HER2 levels in MBC using two methods, enzyme immunoassay (EIA) and chemiluminescence immunoassay (CLIA).
Methods
We collected blood samples from patients with MBC at baseline and at subsequent 3- to 4-week intervals up to 12 weeks. Samples were divided, and serum HER2 levels were determined using EIA and CLIA. We also determined whether serum HER2 levels had decreased by ≥20% at first follow-up. These results were evaluated against overall survival, progression-free survival, and tumor response.
Results
We obtained 196 samples from 52 patients. In 59 samples from patients who received trastuzumab, serum HER2 positivity rates were significantly lower for EIA (n = 22) than for CLIA (n = 33, P = 0.042); in 137 samples from patients who did not receive trastuzumab, there was no significant difference in rates of serum HER2 positivity for CLIA (n = 83) and EIA (n = 80). Serum HER2 level at baseline, the level at first follow-up, and a decrease of ≥20% between baseline and first follow-up were not associated with overall survival, progression-free survival, and tumor response.
Conclusions
Chemiluminescence immunoassay was a more sensitive method than EIA for measuring serum HER2 levels in patients who received trastuzumab. However, because serum HER2 levels did not correlate with patient outcome, we do not currently recommend measuring serum HER2 levels by either method for prognostic evaluation in patients with MBC.
Keywords: Breast neoplasm, HER2, Metastasis, Serum HER2, Trastuzumab
Introduction
Although HER2 positivity has been reported to correspond between serum samples and HER2-positive primary breast tumors, elevated serum HER2 levels have been detected in 8.5–34% of patients with HER2-negative primary tumors [1–5]. The prognostic value of serum HER2 expression in patients with metastatic breast cancer (MBC) has been controversial. Whereas several studies have shown that HER2-positive serum at the time of diagnosis of metastasis was related to poor prognosis [1, 3, 6, 7], other studies have indicated that the serum HER2 level has no prognostic value [8, 9]. However, the methods for measuring serum HER2 levels varied in these studies.
Full-length HER2 is a 185-kDa transmembrane receptor composed of an extracellular domain, transmembrane domain, and intracellular domain [10]. Serum HER2 level is measured by antibody binding to the extracellular domain shed from HER2 transmembrane receptor. There are two major methods of measuring serum HER2 level: enzyme immunoassay (EIA) and chemiluminescence immunoassay (CLIA). The EIA method has been reported to be clinically relevant as a predictive marker for monitoring tumor relapse [11]. However, the monoclonal antibodies 6G10 and SV-2-61 in the EIA kit may compete in the serum with trastuzumab, a HER2-targeting agent that is given to patients with HER2-overexpressing breast cancers. Therefore, the concentration of HER2 protein has been thought to be underestimated by EIA if trastuzumab coexists in the serum. On the other hand, two monoclonal antibodies in the CLIA kit, TA-1 and NB-3, specifically bind to independent epitopes of the extracellular domain of HER2 (p105 protein) which is the different site recognized by trastuzumab [12, 13]. Therefore, the concentration of HER2 protein measured by CLIA is not affected by trastuzumab when concentrations of the HER-2/neu extracellular domain were measured in serum samples from patients with breast cancer [10, 14]. Thus, we hypothesized that CLIA might have a higher positivity rate than EIA for measuring HER2 expression in patients receiving trastuzumab. However, this hypothesis has not been confirmed in a clinical study. The purposes of this prospective study were to compare the two methods of measuring serum HER2 level, CLIA and EIA, and to assess the prognostic value of serum HER2 status in patients with MBC.
Materials and methods
Patients and sample collection
This prospective study was performed at St. Luke’s International Hospital, Tokyo, Japan, between August 2007 and July 2008. Women with MBC who were newly diagnosed and started systemic therapy or who changed to a new line of therapy because of disease progression were eligible. The study protocol was approved by the institutional review board, and all patients gave informed consent.
Inclusion criteria were as follows: invasive breast carcinoma had been diagnosed by histopathological findings, distant metastatic disease had been detected radiologically and/or pathologically, and the HER2 status in the primary tumor had been confirmed. Patients with only local recurrences or only skin metastases were excluded. Patients with bilateral breast cancers were also excluded because of the potential difficulty in judging from which primary tumor the metastasis originated; the hormone receptor and HER2 status of metastatic tumors can differ from those of the primary tumor [15–21].
HER2 overexpression in the primary tumor was defined as a HercepTest score of 3+ by immunohistochemical analysis (IHC), or 2+ by IHC and HER2 gene amplification by fluorescence in situ hybridization (FISH) analysis. Blood specimens of 7.5 ml were collected at the initiation of the new line of therapy (baseline) and at 3- to 4-week intervals up to 12 weeks. Patients remained in this study until their disease progressed and therapy was changed or until they died.
CT scans were performed before the initiation of therapy and after 12 weeks to confirm patients’ radiological response to the therapy according to the Response Evaluation Criteria in Solid Tumors (RECIST) [22]; response was classified as complete response, partial response, stable disease, or progressive disease. Clinicians and patients were blinded to the results of serum HER2 level testing.
Evaluation of serum HER2 protein
Two kinds of kits were used to measure the concentration of HER2 protein in serum. The patients’ serum samples were each divided into two samples. Levels of HER2 protein were measured by the ErbB-2 EIA kit (Nichirei Biosciences, Tokyo, Japan) and the Centaur-HER2/neu CLIA kit (Bayer Diagnostics, NY, USA) in accordance with the manufacturers’ instructions. For the Centaur-HER2/neu kit, samples were automatically processed in an ADVIA Centaur System (Siemens Healthcare Diagnostics, IL, USA). The HER2 threshold values for healthy women were 5.4 ng/ml using the EIA kit and 15.2 ng/ml using the CLIA kit.
We assessed the serum HER2 level at baseline and at first follow-up (3–4 weeks after the initiation of therapy). We also documented how many cases had a decrease in serum HER2 level of ≥20% at first follow-up compared with the baseline level. This threshold value of 20% was defined based on a previous report [6].
Statistical analysis
For descriptive statistics, categorical and ordinal variables were analyzed using a Fisher’s exact test and trend test, respectively; continuous variables were analyzed using a Student’s t test or nonparametric test, where appropriate.
Survival analysis was performed with Kaplan–Meier curve analysis with a log-rank test for statistical significance. Cox proportional hazards models were fitted to determine the association of clinicopathological factors with the risk of progression and death after adjustment for other patient and disease characteristics. For overall survival from the date of initiation of a new line of therapy, the analyses were conditioned on the patients who were alive at the time point of last follow-up. For progression-free survival, the analyses were conditioned on the patients who had no progression at the time point of last follow-up. A two-tailed P value less than 0.05 was considered statistically significant. All statistical analyses were done using SPSS version 17 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 56 patients with MBC were originally enrolled in this prospective study. Four of the 56 patients were excluded from analysis: 1 patient underwent surgery to control local bleeding, 1 patient refused to undergo testing, and 2 patients identified a history of contralateral breast cancer after enrolling in the study. Table 1 shows the characteristics of the remaining 52 patients. The median age at diagnosis was 54 years (range 32–74 years), and the median follow-up time for determination of overall survival was 655 days (range 18–1275 days). One hundred and ninety-six samples from the 52 patients were used for the comparison of serum HER2 levels measured by CLIA and EIA. Because 3 patients’ blood samples were not examined at the first follow-up, we assessed 49 patients’ data to determine the prognostic value of serum HER2 levels at baseline and at first follow-up and of a decrease of ≥20% between baseline and first follow-up.
Table 1.
Patient characteristics
| Age (year) | |
| Median | 54.1 |
| Range | 32–74 |
| Follow-up (days) | |
| Median | 655.0 |
| Range | 18–1275 |
| Estrogen and progesterone receptor status | |
| Positive for either | 33 |
| Negative for both | 19 |
| HER2/neu status in primary tumor | |
| Positive (3+, 2+/FISH+) | 19 |
| Negative (0, 1+, 2+/FISH−) | 33 |
| Therapy given in this study | |
| 1st line | 20 |
| 2nd line | 6 |
| 3rd line or higher | 26 |
| Type of therapy initiated at the time of registration | |
| Hormone alone | 6 |
| Hormone and chemotherapy | 6 |
| Chemotherapy alone | 22 |
| Chemotherapy and HER2-targeting agent | 16 |
| Trastuzumab | 15 |
| Lapatinib | 1 |
| Trastuzumab alone | 1 |
| Sunitinib alone | 1 |
| History of operation | |
| Yes | 41 |
| No | 11 |
| Therapy response at 12 weeks | |
| Partial response | 21 |
| Stable disease | 10 |
| Progressive disease | 21 |
| Survival status at end of follow-up | |
| Alive | 36 |
| Dead | 16 |
In univariate analysis, the number of therapies that patients had received before this study was associated with progression-free survival (P = 0.017) and overall survival (P = 0.006). In Cox regression analysis, patient age, HER2 status, hormone receptor status, tumor size in the primary tumor, lymph node status, and whether trastuzumab was given during the study were not statistically associated with progression-free survival and overall survival.
Serum HER2 levels by CLIA versus EIA
Serum HER2 positivity was detected in 116 of the 196 samples from 52 patients (59.2%) by CLIA and in 102 of the 196 samples (52.0%) by EIA during the study period (Table 2). Of 71 samples from 19 patients with HER2-positive primary tumors, the serum HER2 level was positive in 45 samples (63.4%) by CLIA and in 34 samples (47.9%) by EIA. Of 125 samples from 33 patients with HER2-negative primary tumors, the serum HER2 level was positive in 71 samples (56.8%) by CLIA and in 68 patients (54.4%) by EIA. Serum HER2 positivity by either method was not related to HER2 positivity in the primary tumor (P = 0.426). However, for both methods, the median serum HER2 level was significantly higher in patients with HER2-positive primary tumors than in patients with HER2-negative primary tumors (CLIA: 63.6 vs. 18.2 ng/ml, respectively, P = 0.019; EIA: 8.27 vs. 7.26 ng/ml, P = 0.034; Fig. 1). Therefore, we assessed the correlation of HER2 positivity between sera and primary tumor in 137 samples from 36 patients who did not received trastuzumab. Of 16 samples from 4 patients with HER2-positive primary tumors, the serum HER2 level was positive in 14 samples (87.5%) by CLIA and in 12 samples (75.0%) by EIA. Of 121 samples from 32 patients with HER2-negative primary tumors, the serum HER2 level was positive in 69 samples (57.0%) by CLIA and in 68 patients (56.2%) by EIA. Rates of serum HER2 positivity determined by CLIA were significantly lower in patients with HER2-positive primary tumor than in patients with HER2-negative primary tumor (P = 0.027), but this association did not hold by EIA (P = 0.18).
Table 2.
Serum HER2 level in 196 samples from 52 patients by whether they received trastuzumab
| Trastuzumab (n = 59)
|
No trastuzumab (n = 137)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum HER2 positive
|
Serum HER2 negative
|
P | Serum HER2 positive
|
Serum HER2 negative
|
P | |||||
| n | % | n | % | n | % | n | % | |||
| CLIA | 33 | 56 | 26 | 44 | 0.042 | 83 | 61 | 54 | 39 | 0.711 |
| EIA | 22 | 37 | 37 | 63 | 80 | 58 | 57 | 42 | ||
CLIA chemiluminescence immunoassay, EIA enzyme immunoassay
Fig. 1.
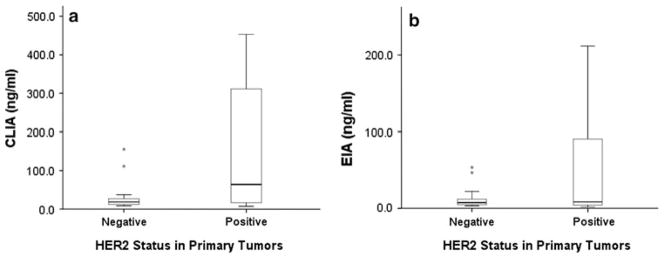
Box plots showing serum HER2 levels in patients with HER2-negative and -positive primary tumors. a By CLIA, median serum HER2 levels were significantly higher in patients with HER2-positive primary tumors than in patients with HER2-negative primary tumors (63.6 vs. 18.2 ng/ml, respectively; P = 0.019). b By EIA, median serum HER2 levels were also significantly higher in patients with HER2-positive primary tumors than in patients with HER2-negative primary tumors (8.27 vs. 7.26 ng/ml, respectively; P = 0.034)
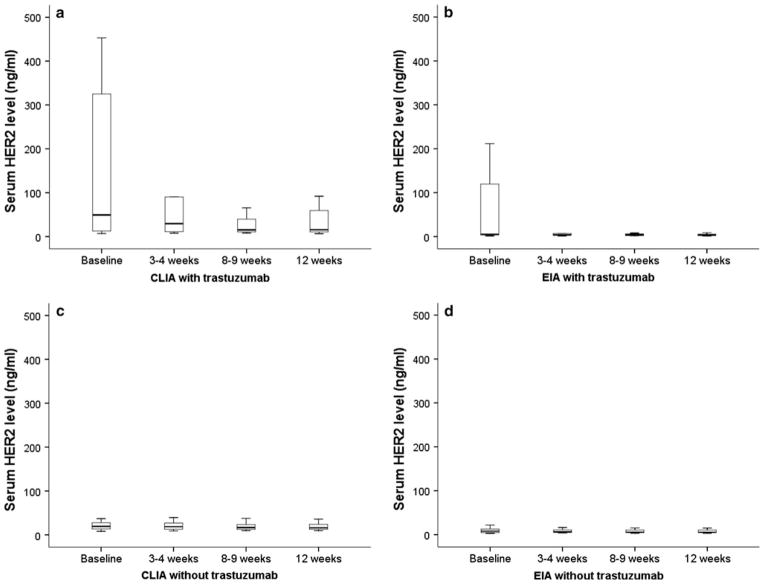
We assessed serum HER2 positivity in the presence of trastuzumab. In 59 samples from 16 patients who received trastuzumab, rates of serum HER2 positivity determined by EIA were significantly lower (n = 22, 37%, P = 0.042) than those determined by CLIA (n = 33, 56%, Table 2) during the study period (Fig. 2a, b). In contrast, in 137 samples from 36 patients who did not receive trastuzumab, the serum HER2 positivity rates were not significantly different between CLIA (n = 83, 61%) and EIA (n = 80, 58%) (Fig. 2c, d).
Fig. 2.
Box plots showing change of serum HER2 levels in patients who received trastuzumab or not during study period. a By CLIA, serum HER2 levels in patients who received trastuzumab. b By EIA, serum HER2 levels in patients who received trastuzumab. c By CLIA, serum HER2 levels in patients who did not receive trastuzumab. d By EIA, serum HER2 levels in patients who did not receive trastuzumab
Association of serum HER2 level with prognosis
Serum HER2 positivity was detected in 32 of 49 patients (65.3%) at baseline and in 30 patients (61.2%) at first follow-up by CLIA and in 28 patients (57.1%) at baseline and in 28 patients (57.1%) at first follow-up by EIA. A serum HER2 level decrease of ≥20% at first follow-up was detected in 13 patients (26.5%) by CLIA and in 10 patients (20.4%) by EIA. Serum HER2 level at baseline and at first follow-up and a decrease of ≥20% at first follow-up were not associated with overall survival (CLIA: P = 0.675, 0.866, and 0.471, respectively; EIA: P = 0.502, 0.734, and 0.644) and progression-free survival (CLIA: P = 0.372, 0.364, and 0.445, respectively; EIA: P = 0.185, 0.162, and 0.845) regardless of the assay method as determined by log-rank analysis. In the patients with HER2-positive primary tumors, serum HER2 levels (at baseline and at first follow-up and a decrease of ≥20% at first follow-up) were not associated with overall survival (CLIA: P = 0.845, 0.575, and 0.858, respectively; EIA: P = 0.875, 0.485, and 0.344) and progression-free survival (CLIA: P = 0.218, 0.327, and 0.887, respectively; EIA: P = 0.523, 0.952, and 0.658).
Receiver operating characteristic (ROC) curve analyses were used to determine whether the changes in serum HER2 level (estimated by comparing the serum HER2 levels at first follow-up to the levels at baseline) correlated with overall survival. The areas under the ROC curve for these analyses were 0.586 for CLIA and 0.565 for EIA. Thus, the changes in serum HER2 level did not correlate with overall survival for either assay method.
Correlation of serum HER2 level with tumor response
We further determined whether serum HER2 level could contribute to early prediction of tumor response (Table 3). Serum HER2 positivity at baseline and at first follow-up was compared to tumor response assessed at 12 weeks. The radiographic tumor assessment showed that at 12 weeks 19 patients had progressive disease (PD) and 30 patients had non-PD, including partial response or stable disease. At baseline, serum HER2 had been positive by CLIA in 13 of the 19 patients with PD (68.4%) and in 19 of the 30 patients with non-PD (63.3%), and had been positive by EIA in 13 of the 19 patients with PD (68.4%) and in 15 of the 30 patients with non-PD (50.0%). At first follow-up, serum HER2 had been positive by CLIA in 15 of the 19 patients with PD (78.9%) and in 16 of the 30 patients with non-PD (53.3%), and had been positive by EIA in 12 of the 19 patients with PD (63.2%) and in 16 of the 30 patients with non-PD (53.3%). There was no statistical correlation between serum HER2 positivity and tumor response using Fisher’s exact test (baseline: P = 0.767 by CLIA and 0.073 by EIA; first follow-up: P = 0.246 by CLIA and 1.000 by EIA).
Table 3.
Correlation between serum HER2 level and tumor response in 49 evaluable patients by assay method
| CLIA
|
EIA
|
||||||
|---|---|---|---|---|---|---|---|
| Baseline
|
First follow-up
|
Baseline
|
First follow-up
|
||||
| Serum HER2 | n | Serum HER2 | n | Serum HER2 | n | Serum HER2 | n |
| 19 Patients with PD | |||||||
| Negative | 6 | Negative | 4 | Negative | 6 | Negative | 5 |
| Positive | 2 | Positive | 1 | ||||
| Positive | 13 | Negative | 0 | Positive | 13 | Negative | 2 |
| Positive | 13 | Positive | 11 | ||||
| 30 Patients with non-PD | |||||||
| Negative | 11 | Negative | 11 | Negative | 15 | Negative | 10 |
| Positive | 0 | Positive | 1 | ||||
| Positive | 19 | Negative | 3 | Positive | 15 | Negative | 0 |
| Positive | 16 | Positive | 15 | ||||
CLIA chemiluminescence immunoassay, EIA enzyme immunoassay, PD progressive disease
Discussion
In this study, we compared two methods of measuring serum HER2 level and assessed the prognostic role of serum HER2 levels in patients with MBC. Our results demonstrated concordance of serum HER2 positivity using the two methods in patients who did not receive trastuzumab. However, this was not the case for patients who received trastuzumab. We prospectively confirmed that CLIA was a more sensitive laboratory test than EIA for patients who received trastuzumab. However, the serum HER2 levels at baseline and first follow-up and the change in serum HER2 level between baseline and first follow-up did not have a prognostic role with either assay method.
In this study, we first determined the HER2 status of patients’ serum samples using CLIA and EIA. In previous studies, positive serum HER2 levels have been observed [4, 6, 23–26]. Carney et al. [1] reviewed previous reports and showed that approximately 43% (23–80%) of patients had elevated serum HER2 levels at the time of first diagnosis of metastases. Several subsequent studies [9, 23] had findings that concurred with this result. However, the methods of measurement and cut-off levels for positivity of serum HER2 levels were not standardized in these studies. Furthermore, the methods of measurement of serum HER2 levels had not been directly compared. In our study, the positivity rates were 65.3% for CLIA and 57.1% for EIA at baseline.
We next demonstrated the concordance/discordance rates of HER2 status between serum samples and primary tumors. Seventy-four percent of patients with HER2-positive primary tumors and 76% of patients with HER2-negative primary tumors had HER2-positive serum during the study period when measured by CLIA. Several studies attributed the finding of HER2-positive serum in patients with HER2-negative primary tumors to different times and methods of assessment of the primary tumors and sera [1, 4, 27]. Molina et al. [3] reported that <10% of HER2-positive cells from primary tumors that had been assessed as HER2 negative could activate and cause metastasis. Some studies [20, 28, 29] reported 20% discordance between HER2 status in primary tumors and metastatic lesions. The relationship between serum HER2 level and tumor spread was reported [3, 26, 30]. Increased serum HER2 levels were found in patients with advanced disease more often than in patients with localized metastases; in a similar vein, in our study the high rate of serum HER2 positivity in patients with HER2-negative primary tumors might have been observed because 26 of 52 patients (50%) received third- or higher-line therapy. However, we also found that serum HER2 levels were significantly higher in patients with HER2-positive primary tumors than in patients with HER2-negative primary tumors when considering the influence of trastuzumab.
We also assessed the prognostic role of serum HER2 levels in patients with MBC considering the effects of therapy at 12 weeks. In our study, a serum HER2 level at baseline or first follow-up and a decrease of ≥20% at first follow-up were not associated with prognosis. Furthermore, we assessed the prognostic role of serum HER2 in patients with HER2-positive primary tumors because the serum HER2 level at baseline was low in patients with HER2-negative primary tumors. However, no significant role was found in this patient group. Therefore, we concluded that the use of serum HER2 levels for predicting prognosis is not appropriate.
The optimal degree of change in serum HER2 levels for predicting prognosis has not been established. Therefore, we wanted to develop an optimal cut-off point for the changes in serum HER2 levels associated with survival using an ROC curve. However, the rather low area under the ROC curve showed no correlation between the change of serum HER2 level and survival. Furthermore, serum HER2 levels at baseline and first follow-up did not correlate with tumor response at 12 weeks in our study. We concluded that serum HER2 level does not predict tumor response to treatment earlier than the currently used method, a CT scan at 12 weeks.
Recently, the clinical relevance of the genotype of IgG Fragment C receptor (FcγR) as predictive and prognostic markers in HER2-positive MBC treated with trastuzumab-based therapy has been reported [31]. The antibodies bind to cancer cells via IgG receptors. Musolino et al. demonstrated that the combination of FcγRIIIa-158 V/V and −131 H/H genotypes was significantly correlated with objective response rate and progression-free survival and with a high rate of trastuzumab-mediated cytotoxicity. This marker is promising for predicting the clinical effect of trastuzumab, although further prospective study is needed to confirm the role. Compared with FcγR, the interesting aspect of serum HER2 level is its potential for a clinical role in patients with HER2-negative breast cancer. However, considering previous reports and our results, we do not think that serum HER2 shows promise as a clinical indicator compared with the genotype of FcγR.
In summary, we prospectively confirmed that CLIA was a more sensitive method than EIA for measuring serum HER2 levels in patients who received trastuzumab. However, because serum HER2 levels did not correlate with patient outcome, we do not currently recommend measuring serum HER2 levels by either method for prognostic evaluation in patients with MBC. Further investigation of the prognostic role of serum HER2 is warranted in a definitive well-powered prospective study.
Acknowledgments
The authors thank Bibari Nakamura and Keiko Shimizu from St. Luke’s International Hospital for help in collecting clinical data; Yuji Shimoda, Masayuki Shimada, Takeshi Watanabe, and Yuki Matsuo from SRL Inc. for analysis of serum HER2 levels in the blood samples; and Sunita Patterson, Department of Scientific Publications, MD Anderson Cancer Center, for editorial review. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Conflict of interest SRL Inc. provided analysis of serum HER2 levels in the blood samples at St. Luke’s International Hospital (N. Hayashi, S. Nakamura, A. Yoshida, and H. Yagata). G. N. Hortobagyi is a consultant to Allergan, Genentech, Merck, and SanofiAventis, and has received research funding from Novartis. All other coauthors have no conflict of interest.
Contributor Information
Naoki Hayashi, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1354, Houston, TX 77030, USA. Department of Breast Surgical Oncology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan. Second Department of Pathology, The Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan.
Seigo Nakamura, Department of Breast Surgical Oncology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan. Department of Breast Surgical Oncology, The Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan.
Yasuharu Tokuda, Institute of Clinical Medicine, Graduate School of Comprehensive Human Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8577, Japan.
Hiroshi Yagata, Department of Breast Surgical Oncology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan.
Atsushi Yoshida, Department of Breast Surgical Oncology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan.
Hidekazu Ota, Second Department of Pathology, The Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan.
Gabriel N. Hortobagyi, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1354, Houston, TX 77030, USA
Massimo Cristofanilli, Department of Medical Oncology, Fox Chase Cancer Center Philadelphia, 333 Cottman Avenue, Philadelphia, PA 19111-2497, USA.
Naoto T. Ueno, Email: nueno@mdanderson.org, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1354, Houston, TX 77030, USA
References
- 1.Carney WP, Neumann R, Lipton A, et al. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem. 2003;49:1579–1598. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 2.Fehm T, Jager W, Kraemer S, et al. Changes of serum HER2 status during clinical course of metastatic breast cancer patients. Anticancer Res. 2004;24:4205–4210. [PubMed] [Google Scholar]
- 3.Molina R, Jo J, Filella X, et al. C-erbB-2 oncoprotein in the sera and tissue of patients with breast cancer. Utility in prognosis. Anticancer Res. 1996;16:2295–2300. [PubMed] [Google Scholar]
- 4.Kandl H, Seymour L, Bezwoda WR. Soluble c-erbB-2 fragment in serum correlates with disease stage and predicts for shortened survival in patients with early-stage and advanced breast cancer. Br J Cancer. 1994;70:739–742. doi: 10.1038/bjc.1994.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krainer M, Brodowicz T, Zeillinger R, et al. Tissue expression and serum levels of HER-2/neu in patients with breast cancer. Oncology. 1997;54:475–481. doi: 10.1159/000227606. [DOI] [PubMed] [Google Scholar]
- 6.Ali SM, Carney WP, Esteva FJ, et al. Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113:1294–1301. doi: 10.1002/cncr.23689. [DOI] [PubMed] [Google Scholar]
- 7.Ludovini V, Gori S, Colozza M, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–890. doi: 10.1093/annonc/mdm585. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Gagnon R, Di Leo A, et al. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized phase III study. J Clin Oncol. 2009;27:5552–5558. doi: 10.1200/JCO.2008.21.1763. [DOI] [PubMed] [Google Scholar]
- 9.Lennon S, Barton C, Banken L, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in meta-static breast cancer. J Clin Oncol. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 10.Leary AF, Hanna WM, van de Vijver MJ, et al. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009;27:1694–1705. doi: 10.1200/JCO.2008.17.3989. [DOI] [PubMed] [Google Scholar]
- 11.Sugano K, Ushiama M, Fukutomi T, et al. Combined measurement of the c-erbB-2 protein in breast carcinoma tissues and sera is useful as a sensitive tumor marker for monitoring tumor relapse. Int J Cancer. 2000;89:329–336. doi: 10.1002/1097-0215(20000720)89:4<329::aid-ijc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Cook GB, Neaman IE, Goldblatt JL, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001;21:1465–1470. [PubMed] [Google Scholar]
- 13.Luftner D, Cheli C, Mickelson K, et al. ADVIA Centaur HER-2/neu shows value in monitoring patients with metastatic breast cancer. Int J Biol Markers. 2004;19:175–182. doi: 10.1177/172460080401900301. [DOI] [PubMed] [Google Scholar]
- 14.Payne RC, Allard JW, Anderson-Mauser L, et al. Automated assay for HER-2/neu in serum. Clin Chem. 2000;46:175–182. [PubMed] [Google Scholar]
- 15.Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 16.Lower EE, Glass E, Blau R, et al. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009;113:301–306. doi: 10.1007/s10549-008-9931-6. [DOI] [PubMed] [Google Scholar]
- 17.Regitnig P, Schippinger W, Lindbauer M, et al. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203:918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 18.Santinelli A, Pisa E, Stramazzotti D. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122:999–1004. doi: 10.1002/ijc.23051. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu C, Fukutomi T, Tsuda H, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. doi: 10.1002/(sici)1096-9098(200001)73:1<17::aid-jso5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Zidan J, Dashkovsky I, Stayerman C, et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Y, Booser DJ, Sneige N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer. 2005;103:1763–1769. doi: 10.1002/cncr.20987. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–R443. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostler WJ, Schwab B, Singer CF, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:1618–1624. doi: 10.1158/1078-0432.ccr-0385-3. [DOI] [PubMed] [Google Scholar]
- 25.Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 26.Andersen TI, Paus E, Nesland JM, et al. Detection of c-erbB-2 related protein in sera from breast cancer patients. Relationship to ERBB2 gene amplification and c-erbB-2 protein overexpression in tumour. Acta Oncol. 1995;34:499–504. doi: 10.3109/02841869509094014. [DOI] [PubMed] [Google Scholar]
- 27.Fehm T, Becker S, Duerr-Stoerzer S, et al. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res. 2007;9:R74. doi: 10.1186/bcr1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z, Caduff R, Pestalozzi B. Stability of the HER2 gene after primary chemotherapy in advanced breast cancer. Virchows Arch. 2005;446:136–141. doi: 10.1007/s00428-004-1164-4. [DOI] [PubMed] [Google Scholar]
- 29.Edgerton SM, Moore D, 2nd, Merkel D. erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol. 2003;11:214–221. doi: 10.1097/00129039-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]