Abstract
Older, obese, and sedentary individuals are at high risk of developing diabetes and cardiovascular disease. Exercise training improves metabolic anomalies associated with such diseases, but the effects of caloric restriction in addition to exercise in such a high risk group are not known. Changes in body composition and metabolism during a lifestyle intervention were investigated in twenty three older, obese men and women (aged 66 ± 1 years, BMI 33.2 ± 1.4 kg.m−2) with impaired glucose tolerance. All volunteers undertook twelve weeks of aerobic exercise training (5 days per week for 60 min @ 75% VO2max) with either normal caloric intake (eucaloric group, 1901 ± 277 kcal.day−1, n = 12) or a reduced-calorie diet (hypocaloric group, 1307 ± 70 kcal.day−1, n = 11), as dictated by nutritional counseling. Body composition (decreased fat mass; maintained fat-free mass), aerobic fitness (VO2max), leptinemia, insulin sensitivity, and intramyocellular lipid accumulation (IMCL) in skeletal muscle improved in both groups (P < 0.05). Improvements in body composition, leptin and basal fat oxidation were greater in the hypocaloric group. Following the intervention there was a correlation between the increase in basal fat oxidation and the decrease in IMCL (r = −0.53, P = 0.04). In addition, basal fat oxidation was associated with circulating leptin after (r = 0.65, P = 0.0007), but not before the intervention (r = 0.05, P = 0.84). In conclusion, these data show that exercise training improves resting substrate oxidation and creates a metabolic milieu that appears to promote lipid utilization in skeletal muscle, thus facilitating a reversal of insulin resistance. We also demonstrate that leptin sensitivity is improved, but that such a trend may rely on reducing caloric intake in addition to exercise training.
Keywords: obesity, leptin, substrate oxidation, insulin sensitivity
Introduction
Skeletal muscle oxidative capacity is impaired in obese individuals, predisposing them to further weight gain and insulin resistance (16), and increasing the risk of developing type 2 diabetes (T2DM) and associated macrovascular complications (24, 35). Aging is also associated with impaired substrate utilization and insulin resistance, probably due to a sedentary lifestyle and elevated body fat causing impaired mitochondrial function (13, 22, 26, 30). Therefore older, obese, impaired glucose tolerant (IGT) cohorts are at high risk of developing metabolic diseases.
Aerobic exercise training improves oxidative capacity and insulin sensitivity in younger and older obese and/or IGT/T2DM individuals (8, 14, 15, 20, 36). Caloric restriction interventions also exhibit similar improvements in insulin sensitivity; yet such findings are inconsistent, and the impact on substrate metabolism is less clear (27, 32). The main drawback of hypocaloric weight-loss interventions is that fat-free mass, a major determinant of substrate utilization, in particular basal fat oxidation, is also often reduced (8, 15). Aging is associated with negative nitrogen balance and therefore older adults are at increased risk for developing sarcopenia (25). Exercise promotes protein synthesis rates which may help maintain fat-free mass in older individuals. Therefore, a weight loss program that includes exercise may be favorable to optimally improve insulin sensitivity and substrate metabolism in an older, obese, IGT population, thus reducing such a group's already high risk of developing macrovascular complications.
Impaired insulin action mainly occurs in obesity due to accumulation of intracellular fatty acid derivatives. However, adipocytokines are also documented to play a role. Circulating concentrations of leptin have been shown to be positively related to fat mass and inversely related to insulin sensitivity (33). Leptin is involved in satiety, via a hypothalamic signaling pathway (10), and in substrate metabolism, via direct peripheral tissue action (23). However, it has been demonstrated that leptin can increase fat oxidation (23), illustrating a paradox: it is possible that, in obesity, leptin resistance may arise (34). The relationship between leptin and fat oxidation was investigated in this study. In addition to leptin, another adipocytokine, adiponectin, has also been shown to be related to insulin sensitivity and substrate metabolism (37, 41). Therefore the relationship between adiponectin and fat oxidation was also measured in this study.
Currently, in older obese IGT individuals, it is unknown whether the combination of exercise training with a hypocaloric diet is more effective at improving factors associated with metabolic disease when compared to a similar exercise regime with a eucaloric macronutrient intake. This study serves to investigate such differences. It was hypothesized that exercise plus caloric restriction would improve insulin sensitivity and substrate metabolism to a greater extent than exercise training alone as a result of greater weight loss, particularly fat mass.
Experimental Procedures
Subjects
Twenty three older, obese, IGT (fasting plasma glucose > 5.6 mmol.l−1 and 2 h oral glucose tolerance test (OGTT) glucose concentration between 7.8 and 11.1 mmol.l−1) volunteers were recruited from the general population to take part in a twelve week exercise training intervention, whilst consuming either a eucaloric or a hypocaloric diet. Medical screening excluded individuals with heart, kidney, liver, intestinal, and pulmonary diseases, or those taking medications for hypertension, diabetes, or other obesity-related conditions. All volunteers were sedentary, and had been weight stable for six months prior to the study. The study was approved by the Institutional Review Board, and all subjects provided written informed consent in accordance with our guidelines for the protection of human subjects.
Intervention
Volunteers were divided into two BMI-matched groups: a eucaloric group (n = 12: four male, eight female; age = 66 ± 1 years; BMI = 34.7 ± 1.6 kg.m−2) and a hypocaloric group (n = 11: three male, eight female; age = 67 ± 1 years; BMI = 33.6 ± 1.3 kg.m−2). Both groups undertook 60 min of supervised moderate-intensity aerobic exercise (treadmill walking/cycle ergometry/stationary rowing) at 75% of maximal oxygen uptake capacity five days a week (Monday to Friday) for twelve weeks. Dietary records were collected for three days prior to the study, and individual nutritional counseling was provided weekly to monitor caloric intake. The eucaloric group were instructed to continue their typical dietary intake throughout the study, whilst the hypocaloric group were instructed to reduce their daily energy intake by ∼500 kcal. Several anthropometric and metabolic measures were taken pre- and post-intervention as described below during a three day inpatient stay at the General Clinical Research Centre.
Body composition
Height was measured to the nearest 1.0 cm without shoes. Body weight was measured to the nearest 0.1 kg with the subject wearing their underclothing and a hospital gown. Waist circumference (WC) was measured midway between the lower rib margin and iliac crest to the nearest 1.0 cm. Body density was determined by hydrostatic weighing and body fat mass was calculated using Siri equations as previously described (29).
Aerobic fitness
Each subject performed an incremental treadmill exercise test to determine their maximal oxygen consumption (VO2max). The speed was set between 2-4 mph and the incline of the treadmill was increased 2.5% every two minutes until fatigue. Exhaled air was collected and concentrations of O2 and CO2 were measured using an electrochemical O2 analyzer (Applied Electrochemistry, S-3A) and infrared CO2 analyzer (Beckman LB-2) respectively. Maximum aerobic capacity was achieved when at least two of the following criteria were achieved: (i) a plateau in VO2, (ii) a heart rate within 10 beats.min−1 of age-predicted maximum, and/or (iii) a respiratory exchange ratio > 1.0. Due to the acute effects of exercise on insulin sensitivity, the time period between the VO2max test and the euglycemic clamp was always at least 48 h. Post-intervention VO2max testing was performed the morning following the clamp procedure.
Basal substrate metabolism
On day 2 of the inpatient stay, following a twelve hour overnight fast, subjects were awakened at 0600 hours and taken by wheelchair to void and to be weighed, and then reclined in a semi-darkened, thermoneutral (22 ± 1°C) environment under a clear plastic hood (Brooks Instruments, Hatfield, PA) for 30 min for indirect calorimetry measurements, as previously described (40). The molar ratio of O2 consumed to CO2 produced was used to derive a measure of the relative amounts of substrate that were being oxidized (respiratory quotient, RQ). The Weir equation was used to calculate resting energy expenditure (39), and substrate oxidation rates were calculated according to Frayn (7). Timed urinary nitrogen excretion measurements were also made for estimates of protein oxidation rates (7). In brief, urine was collected from 0600 hours until completion of the calorimetry measures. Total volume and time of collection was recorded and aliquots were analyzed for urea nitrogen (Roche Modular Diagnostics, Indianapolis, IN).
Insulin sensitivity
On the final morning of the inpatient stay a hyperinsulinemic euglycemic clamp was performed as previously described (5, 19). Following an overnight fast, subjects voided morning urine and were weighed. An 18 gauge polyethylene catheter was inserted into an antecubital vein for the infusion of glucose (20% dextrose) and insulin. A second 20 gauge polyethylene catheter was inserted in retrograde fashion into a dorsal hand vein, and the hand was warmed in a heated box (∼65°C) for sampling of arterialized venous blood. After collection of baseline blood samples (5 ml), a percutaneous biopsy of the vastus lateralis muscle was obtained using the Bergstrom needle technique, as previously described (19). A primed 40 mU.m−2.min−1 insulin infusion commenced at t = 0 min followed by a two hour variable glucose infusion intended to maintain plasma glucose concentrations at 5 mmol.l−1. Arterialized blood samples (0.5 ml) were collected every 5 min for plasma glucose determination (Beckman Instruments, Fullerton, CA) so as to make continuous adjustments to the glucose infusion rate in order to maintain euglycemia. Insulin sensitivity was calculated as the mean glucose metabolized (clamp M) over the last 30 min period of the clamp, expressed as mg.kgFFM−1.min−1. A pre-clamp blood sample was also drawn for the measurement of plasma leptin by radioimmunoassay (Linco Research, St. Charles, MO), and plasma adiponectin by ELISA (R&D Systems, Minneapolis, MN).
Skeletal muscle histology
Muscle mounts (n = 14: 7 hypocaloric, 7 eucaloric) obtained from the vastus lateralis biopsy were stored at −70°C to be later analyzed for intramuscular lipid content (IMCL). IMCL was measured by a semi-quantitative method using the Oil-Red-O (ORO) technique as previously described by Goodpaster et al.(2000)(9). In brief, 10 μm cryosections were mounted on glass slides, air-dried, and immersed in an ORO (Sigma, St Louis, MO) solution (36 mg ORO, 12 ml 2-propanol, 8 ml dH2O) for 10 min. Slides were rinsed thoroughly in dH2O, allowed to dry, then fixed under a cover slip using Glycergel mounting medium (DAKO, Carpinteria, CA), and sealed using an acrylic polymer. Slides were viewed under a light microscope using 40× objective and bright field settings (Olympus BX61, Melville, NY), and digital images were captured (Hamamatsu Orca-ER, Bridgewater, NJ). Images were viewed and analyzed in grayscale format using NIH Image software (http://rsb.info.nih.gov/nih-image/). Quantification of IMCL was performed by identifying ORO-stained areas with staining intensity of <150 arbitrary grayscale units. This was repeated on five fields of view (750 × 550 pixels; 1 pixel = 0.18 μm2) per subject, and the mean cross-sectional muscle fiber area and mean lipid-stained area were calculated. Intramyocellular lipid accumulation was normalized for changes in fiber surface area: lipid surface area divided by fiber area multiplied by 100 (= lipid accumulation index [LAI, %]).
Statistics
Statistical analyses were carried out using Statview (SAS Institute, NC, USA), and all data are expressed as mean ± S.E.M. Normality of each variable was assessed using the Kolmorgorov-Smirnov test. Variables deviating from normal distributions (plasma leptin concentrations) were natural log-transformed (ln) prior to statistical analysis. Between-group (eucaloric vs. hypocaloric) comparisons for all variables were analyzed using two-way (group*time) repeated measures ANOVA. Tukey post hoc tests were applied to significant group*time interactions. Bivariate correlations were used to examine relationships between pre- to post-study changes (Δ) in basal fat oxidation, clamp M, and ln leptin with other variables. Additionally, separate bivariate analyses were also applied to variables at baseline, and to variables following the intervention (e.g. baseline leptin vs. baseline fat oxidation, and post-intervention leptin vs. post-intervention fat oxidation). This was carried out to identify changes in relationships as a result of the intervention. Statistical significance was accepted at P < 0.05.
Results
Dietary intake
During the twelve week intervention the eucaloric group consumed a similar daily caloric load (1901 ± 277 vs. 1866 ± 231 kcal.day−1; P = 0.87) and macronutrient composition to their existing diet. The hypocaloric group consumed approximately 33% fewer calories during the study compared to their existing diet (1307 ± 70 vs. 1964 ± 164 kcal.day−1; P = 0.008), during which there was also a shift toward reduced fat intake (30 ± 1 vs. 25 ± 1 %) and increased carbohydrate intake (51 ± 2 vs. 57 ± 1 %).
Body composition
Both study groups exhibited significant weight loss, improvements in BMI, and reductions in body fat mass and waist circumference between baseline and post-study measures (Table 1), but, as expected, weight, BMI, and fat mass showed greater changes in the hypocaloric group. Fat-free mass did not change in either group (Table 1; P > 0.05).
Table 1. Body composition, aerobic fitness, leptinemia, and insulin sensitivity.
| Variable | EUCALORIC GROUP | HYPOCALRIC GROUP | ||
|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | |
|
| ||||
| Age years | 66 ± 1 | 67 ±.1 | ||
| Weight kg | 96.2 ± 5.4 | 92.9 ± 5.1 ** | 95.3 ± 4.7 | 87.4 ± 4.2 *** |
| BMI kg.m−2 | 34.7 ± 1.6 | 33.5 ± 1.5 ** | 33.6 ± 1.3 | 30.8 ± 1.2 *** |
| FM kg | 42.3 ± 2.4 | 40.1 ± 2.6 * | 37.6 ± 2.8 | 31.6 ± 2.6 ** |
| FFM kg | 54.4 ± 3.5 | 53.0 ± 3.0 | 57.7 ± 3.4 | 55.8 ± 3.3 |
| WC cm | 117 ± 4 | 113 ± 3 * | 113 ± 3 | 107 ± 3 ** |
| V02max ml.kgFFM−1.min−1 | 34.6 ± 1.0 | 38.3 ± 1.4 ** | 36.3 ± 1.2 | 40.1 ± 1.2 * |
| Leptin ng.ml−1 | 25.8 ± 3.9 | 22.4 ± 3.5 * | 20.4 ± 4.5 | 12.6 ± 2 7 ** |
| Adiponectin μg.ml−1 | 7.66 ± 1.16 | 6.85 ± 1.65 | 7.60 ± 0.92 | 6.57 ± 0.93 |
| Clamp M mg.kgFFM−1.min−1 | 2.97 ± 0.43 | 4.21 ± 0.66 * | 2.92 ± 0.34 | 4.68 ± 0.56 ** |
Volunteers underwent a twelve week aerobic exercise training intervention with either a eucaloric (∼1900 kcal.day−1) or a hypocaloric (∼1300 kcal.day−1) diet. Significant differences from baseline are represented by
(P < 0.05),
(P < 0.01), and
(P < 0.001). Group*time interactions were found with weight, BMI, FM, and leptin (all P < 0.05). BMI is body mass index; FM is fat mass; FFM is fat-free mass; WC is waist circumference; VO2max is maximal oxygen uptake during exhaustive exercise; Clamp M is the mean glucose disposal rate during the last 30 min of a hyperinsulinemic euglycemic clamp. Data represents mean ± S.E.M.
Aerobic fitness
VO2max increased similarly in both groups (Table 1; eucaloric: 10.6 ± 3.0% increase, P = 0.003; hypocaloric: 11.8 ± 5.7% increase, P = 0.04).
Insulin sensitivity
Glucose metabolized during the hyperinsulinemic euglycemic clamp was improved by similar amounts in both groups (Table 1; eucaloric: 55.1 ± 19.0% increase, P = 0.03; hypocaloric: 65.1 ± 14.4% increase, P = 0.001 respectively).
Leptin and Adiponectin
Pre-intervention plasma leptin concentrations were similar between groups (Table 1). Leptin concentrations decreased in both groups following the intervention, with a larger reduction seen in the hypocaloric group (31.6 ± 6.0% decrease vs. 12.2 ± 3.8%. decrease). Analysis of plasma adiponectin concentrations did not reveal any effect of time or trial (Table 1; P > 0.05).
IMCL
Following the exercise training intervention, intramuscular lipids (LAI, %) decreased by 25.9 ± 12.4 and 34.3 ± 17.6% in the eu- and hypocaloric groups respectively (two-way ANOVA, effect of time P = 0.04; Table 2). No group differences were found (P = 0.66).
Table 2. Intramyocellular lipid.
| Variable | EUCALORIC GROUP | HYPOCALORIC GROUP | ||
|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | |
|
| ||||
| LAI % | 3.91 ± 0.58 | 2.98 ± 0.38 * | 3.85 ± 0.60 | 2.46 ± 0.30 * |
| IMCL area μm2 | 243.9 ± 29.5 | 176.3 ± 14.0 * | 229.8 ± 34.9 | 165.6 ± 17.8 * |
| Fiber area μm2 | 6586.0 ± 539.0 | 6144.0 ± 420.2 | 6116.0 ± 376.2 | 6855.0 ± 389.3 |
Study groups are described in Table 1. IMCL is intramuscular lipid. LAI is the lipid accumulation index, i.e. the mean percentage area of an individual skeletal muscle fiber that stains for lipid.
represents a statistically significant difference from the Pre-Intervention value (P < 0.05). Data represents mean ± S.E.M.
Basal substrate metabolism
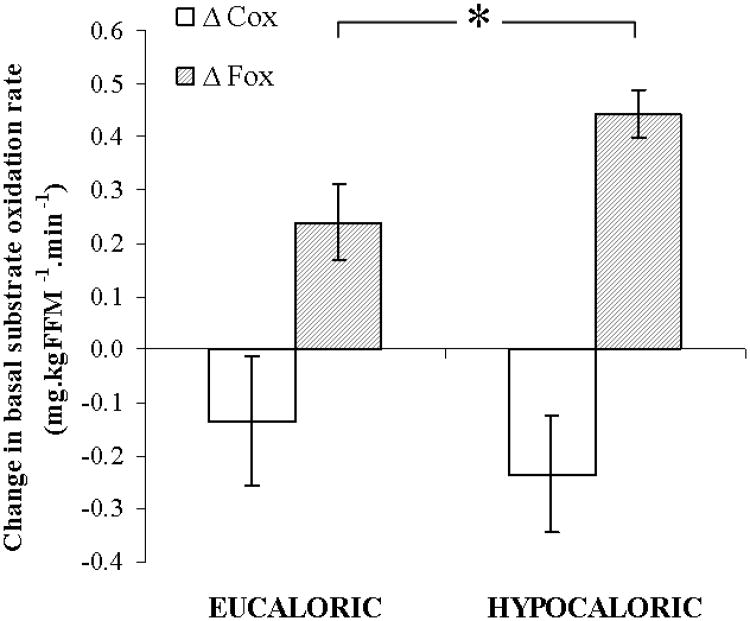
Table 3 indicates changes in basal substrate metabolism measured at rest following an overnight fast. The RQ was decreased equally in both groups (P = 0.03), whereas basal fat oxidation was significantly improved in the hypocaloric group only (38.4 ± 12.8% increase; P = 0.02). Figure 1 displays the change in basal carbohydrate and fat oxidation following the intervention in each group, illustrating a larger improvement in basal fat oxidation in the hypocaloric group (P = 0.03). Analysis of the change in basal fat oxidation between groups also revealed a statistically significant difference, showing a greater increase in the hypocaloric group (P = 0.05). No changes in energy expenditure, or carbohydrate and protein oxidation rates were found as a result of the interventions.
Table 3. Basal substrate metabolism.
| Variable | EUCALORIC GROUP | HYPOCALORIC GROUP | ||
|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | |
|
| ||||
| RQ | 0.81 ± 0.01 | 0.77 ± 0.02 * | 0.84 ± 0.02 | 0.81 ± 0.01 * |
| EE kcal. day−1 | 1565 ± 130 | 1460 ± 120 | 1633 ± 110 | 1621 ± 78 |
| Cox mg.kgFFM−1.min−1 | 1.68 ± 0.34 | 1.14 ± 0.27 | 2.19 ± 0.30 | 1.89 ± 0.15 |
| Fox mg.kgFFM−1.min−1 | 1.18 ± 0.13 | 1.29 ± 0.14 | 0.86 ± 0.11 | 1.19 ± 0.09 * |
| Pox mg.kgFFM−1.min−1 | 0.62 ± 0.13 | 0.88 ± 0.09 | 0.85 ± 0.19 | 0.64 ± 0.10 |
The study groups are as described in Table 1. Significant differences from baseline are represented by
(P < 0.05). No group*time interactions were found (P > 0.05). RQ is respiratory quotient; EE is energy expenditure; Cox is basal carbohydrate oxidation; Fox is basal fat oxidation; Pox is basal protein oxidation. Data represents mean ± S.E.M.
Figure 1. Changes in basal substrate metabolism.
Twenty three older, obese, and previously sedentary IGT individuals followed a twelve week aerobic exercise training intervention, combined with either a eucaloric (∼1900 kcal.day−1) or a hypocaloric (∼1300 kcal.day−1) diet. Basal fat oxidation showed a larger improvement in the hypocaloric group (Δ Fox: *, P = 0.03). No differences in the change in basal carbohydrate oxidation were found (Δ Cox: P = 0.87). Data represents mean ± S.E.M.
Correlation analyses
The majority of measured variables demonstrated no group*time interactions following analysis, therefore, because it appeared that changes with time were occurring independent of caloric consumption, data from the eucaloric and hypocaloric groups were pooled for correlation analyses. Table 4 indicates that changes in clamp M, leptin, and basal fat oxidation were mostly related to changes in body composition. Changes in clamp M were not found to be related to changes in leptin or basal fat oxidation, nor were changes in leptin found to be related to changes in basal fat oxidation. There was a significant association between the change in IMCL and the changes in basal fat oxidation (r = −0.53, P = 0.04; Table 4, Figure 2) and circulating leptin (r = 0.44, P = 0.05; Table 4). There was also a trend for a correlation between the change in circulating adiponectin and basal fat oxidation (r = 0.44, P = 0.05). In addition, Figure 3 illustrates that following the intervention a strong relationship developed between leptin and basal fat oxidation post-study (r = 0.65, P = 0.0007) that was not present at baseline (r = 0.046, P = 0.84). In a separate analysis of eu- and hypocaloric groups, this post-study relationship between leptin and basal fat oxidation was greatest in the hypocaloric group (hypocaloric vs. eucaloric: r = 0.79, P = 0.005 vs. r = 0.58, P = 0.08).
Table 4. Bivariate correlation analyses.
| Versus | Δ Clamp M | Δ ln Leptin | Δ F Ox |
|---|---|---|---|
|
| |||
| Δ Weight | −0.565 ** | 0.719 *** | −0.174 |
| Δ BMI | −0.481 * | 0.621 ** | −0.186 |
| Δ FM | −0.312 | 0.551 ** | −0.452 * |
| Δ FFM | −0.268 | 0.125 | 0.425 * |
| Δ WC | −0.065 | 0.235 | −0.300 * |
| Δ IMCL | 0.091 | 0.440 * | −0.529 * |
| Δ VO2max | 0.141 | −0.430 * | −0.079 |
| Δ ln Leptin | −0.381 | - | −0.258 |
| Δ Adiponectin | −0.336 | −0.045 | 0.440 |
| Δ Clamp M | - | −0.381 | −0.219 |
| Δ RQ | 0.357 | 0.108 | −0.813 *** |
| Δ EE | 0.017 | 0.144 | 0.409 * |
| Δ Cox | 0.323 | 0.229 | −0.415 |
| Δ Fox | −0.219 | −0.258 | - |
| Δ Pox | −0.195 | 0.114 | −0.198 |
Due to the lack of group*time interactions in the majority of variables, it was assumed that the exercise training was driving the changes independent of caloric intake; therefore, the intervention groups were pooled for correlation analyses. Values shown are Pearson's product moment correlation coefficients (r-values) for relationships between changes in variables (Δ) during the twelve week exercise training intervention. Significant correlations are represented by
(P < 0.05),
(P < 0.01), and
(P < 0.001). Raw plasma leptin concentration data were natural log transformed (ln leptin) for statistical analysis.
Figure 2. Relationship between intramyocellular lipid and basal fat oxidation.
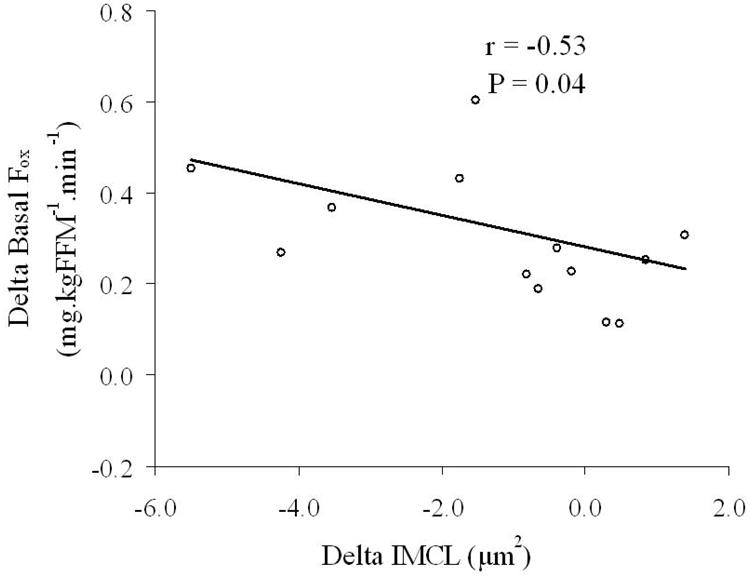
Data from the two study groups were pooled for correlation analyses. The data indicate that in older, obese, sedentary, IGT individuals the decrease in intramyocellular lipid (delta IMCL) and the increase in basal fat oxidation (delta basal Fox) as a result of the exercise training intervention were related (r = −0.53, P = 0.04).
Figure 3. Relationships between plasma leptin and basal fat oxidation.
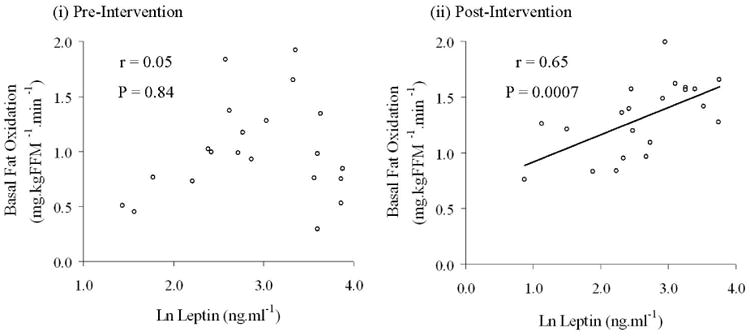
Data from the two study groups were pooled for correlation analyses. Raw plasma leptin concentration data deviated from a normal distribution and so was natural log (ln) transformed for statistical analysis. Panel (i) illustrates that in older, obese, sedentary, IGT individuals no relationship existed between plasma leptin and basal fat oxidation (r = 0.05, P = 0.84). Panel (ii) shows that following the twelve week exercise training regime, a significant relationship between plasma leptin and basal fat oxidation was present (r = 0.65, P = 0.0007).
Discussion
The main finding of this study is that a twelve week aerobic exercise training intervention improves body composition (weight, fat mass, waist circumference), aerobic fitness (VO2max), leptinemia, intramuscular lipid accumulation (IMCL), and insulin sensitivity (clamp M) in older, obese, previously sedentary, IGT individuals, independent of total daily caloric consumption; but that improvement in basal fat oxidation is dependent on caloric restriction in addition to exercise. In addition, the changes in IMCL were related to improvements in basal fat oxidation and leptinemia. Furthermore, although changes in basal fat oxidation were not found to be related to changes in insulin sensitivity, a positive relationship between basal fat oxidation and plasma leptin developed post-intervention, suggesting an improvement in leptin sensitivity. This trend was more pronounced when caloric restriction was undertaken in addition to exercise training. Similar findings in younger obese cohorts have been published by Goodpaster et al. (8) and He et al. (11) who found a relationship between fat utilization and insulin sensitivity, therefore our work suggests a possible age-related dissociation of these two variables.
Weight loss via reductions in fat mass lowers systemic free-fatty acid appearance into the circulation, thus reducing free-fatty acid availability to skeletal muscle tissue (2). In insulin resistant muscle, as found in older, obese, IGT individuals, where basal fat oxidation is impaired, this will limit the accumulation of intramuscular lipids and other fatty acid derivatives known to interfere with insulin signaling (21). IMCL content has been described to be elevated in insulin resistant patients (38), yet it appears that these two variables may disassociate following exercise training (4). In this study, we demonstrate reduced IMCL post-intervention, which, although not directly related to insulin sensitivity, was associated with changes in fat oxidation and circulating leptin. It has been suggested that decreased IMCL with increased rates of basal fat oxidation reduces reliance on plasma free-fatty acids and increases utilization of intramuscular lipid pools (31). Thus, from our data, we can speculate that reductions in IMCL in our subjects following exercise training may be due to an increase in IMCL utilization. These changes occur independently of caloric intake, suggesting that exercise is the key component of the intervention.
The addition of an exercise stimulus to caloric restriction regimes also upregulates skeletal muscle capillarization and mitochondrial density (12), and activates enzymes involved in fatty acid transport and oxidation (17, 18). Therefore it seems likely that exercise training plus caloric restriction would lead to greater improvements in substrate metabolism and insulin sensitivity than exercise training alone. However, this current study indicates that, despite larger changes in body composition and leptinemia following a hypocaloric exercise training regime, improvements in insulin sensitivity appear to be driven by exercise per se, independent of caloric intake. Alternatively, it is possible that insulin sensitivity reached a peak improvement in these individuals, one that can be achieved by caloric restriction or by exercise. To clarify a potential “ceiling effect” in these older obese subjects, the addition of a hypocaloric non-exercising cohort would be useful. Also, compared to a eucaloric exercise training regime, greater improvements in basal fat oxidation were seen in our hypocaloric exercise group, despite similar improvements in maximal aerobic capacity. To explain this finding, it is possible that the improvements in body composition, insulin sensitivity, and leptin seen in the eucaloric group were below a threshold that would extrapolate to an improvement in whole body substrate utilization. However, because insulin sensitivity changed equally between groups, it is unlikely that this contributes to the different changes seen in substrate oxidation. This concept therefore merits further attention in future studies.
Reduced fat mass leads to improvements in plasma adipocytokine concentrations. Such adipose-derived molecules have direct effects on insulin signaling and substrate utilization (23, 33). Here we demonstrate reduced post-intervention circulating leptin concentrations. Further to this, our data demonstrate that a state of leptin resistance existed in our older, obese, IGT cohort, shown by the lack of correlation between leptin and basal fat oxidation (Figure 3). However, as a result of the exercise training interventions, leptin sensitivity was improved, particularly in the hypocaloric group (hypocaloric vs. eucaloric: R = 0.790, P = 0.005 vs. R = 0.579, P = 0.08), suggesting that exercise plus caloric restriction is required to increase fat oxidation via a leptin-mediated pathway in older, obese, IGT individuals. Leptin has been shown to upregulate fat oxidation via an AMPK-related pathway (23). Other data also indicate that leptin fails to upregulate fat oxidation in obese humans when compared to age- and gender-matched lean individuals, providing further evidence of a defect in leptin signaling in obesity (34). To our knowledge, no data on the effects of exercise training upon leptin-induced fat oxidation exist in older obese IGT humans; however, it has been shown that an exercise training regime can upregulate ACC activity (a leptin-sensitive enzyme) and fat oxidation in lean, middle-aged individuals (31). These observations suggest a direction for future work. In this study, we demonstrate that the change in circulating leptin is related to the improvement in IMCL. This is of particular mechanistic interest, as the improvement in leptin sensitivity as a result of the lifestyle intervention may upregulate AMPK-related fat oxidation pathways, increasing intramuscular lipid utilization. Adiponectin is another adipocytokine implicated with insulin sensitivity and substrate oxidation. Analysis showed no effect of exercise or caloric consumption upon circulating plasma adiponectin. This finding strengthens the evidence that changes in plasma leptin concentrations as a result of diet and exercise are involved in the changes in levels of intramyocellular lipid and basal fat oxidation. However, the currently available data on exercise training, diet and adiponectin, whilst limited, are quite conflicting (1, 3, 28). This is an area that certainly warrants further investigation when considering adiponectin's potential effect on substrate utilization and insulin sensitivity (37, 41).
A limitation of the present study is that we did not study a hypocaloric non-exercising group. This would further control the study by isolating the effects of weight loss per se on the measured metabolic variables. Additionally, the majority of the volunteers were female, and the small number of males recruited precluded any gender-difference analyses. Although subjects were postmenopausal, which would rule out the effects of estrogen on body composition and substrate metabolism, gender differences have been reported with leptin responses and action (6). Future work should attempt to establish potential gender differences in aging to ascertain optimal sex-specific lifestyle interventions. Despite this, clear improvements in body composition, insulin sensitivity, and basal fat metabolism were seen, factors that are of great importance to an old, obese, IGT group in reducing their risk of developing cardiovascular disease. In addition, weight loss was not associated with loss of fat-free mass, suggesting that regular aerobic exercise may have a beneficial impact upon the state of sarcopenia and osteopenia that develops in such age groups. Maintaining metabolic function and mobility via the preservation of muscular and skeletal strength is of particular importance to an older, obese group, thus regular exercise prescription is sensible. In addition, it is important to note that eleven (three eucaloric, eight hypocaloric) of the twenty three volunteers were no longer classified as IGT (FPG > 5.6 mmol.l−1) following the intervention. FPG is a strong predictor of diabetes progression, and so, this study shows that just twelve weeks of lifestyle intervention is enough to greatly reduce disease risk in older, obese individuals, and to normalize glucose tolerance in approximately 50% of participants.
The major take home message of this study is that regular moderate-intensity aerobic exercise is the driving force behind improvements in insulin sensitivity when older, obese, and previously sedentary IGT individuals follow an exercise and diet regime. Despite greater changes in body composition following a calorie-restricted exercise intervention, such changes did not extrapolate to greater changes in insulin sensitivity when compared to a eucaloric exercise intervention. The loss of body fat mass in addition to the maintenance of fat-free mass is of importance to older cohorts. Such changes were demonstrated in this study. We also document that older, obese, IGT individuals exhibit leptin resistance with respect to basal fat oxidation, and that exercise training improves leptin sensitivity independent of caloric intake. This is a novel insight into the understanding of the onset of metabolic disease in obese sedentary groups. Further work in this area is warranted to identify the mechanistic pathways.
Acknowledgments
The authors wish to thank the volunteers for their outstanding dedication and effort. We wish to acknowledge the nursing/dietary staff of the General Clinical Research Center and the technical/engineering staff and students who helped with the implementation of the study and assisted with data collection. This research was supported by NIH grants AG12834 (JPK), and GCRC grants MO1 RR10732, RR00080, and RR018390.
References
- 1.Abbasi F, Chang SA, Chu JW, Ciaraldi TP, Lamendola C, McLaughlin T, Reaven GM, Reaven PD. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R139–144. doi: 10.1152/ajpregu.00287.2005. [DOI] [PubMed] [Google Scholar]
- 2.Blaak EE, Wolffenbuttel BHR, Saris WHM, Pelsers MMAL, Wagenmakers AJM. Weight Reduction and the Impaired Plasma-Derived Free Fatty Acid Oxidation in Type 2 Diabetic Subjects. J Clin Endocrinol Metab. 2001;86:1638–1644. doi: 10.1210/jcem.86.4.7397. [DOI] [PubMed] [Google Scholar]
- 3.Bluher M, Bullen JW, Jr, Lee JH, Kralisch S, Fasshauer M, Kloting N, Niebauer J, Schon MR, Williams CJ, Mantzoros CS. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 4.Bruce CR, Kriketos AD, Cooney GJ, Hawley JA. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia. 2004;47:23–30. doi: 10.1007/s00125-003-1265-7. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan DE, Vaile JC, Petley GW, Phillips DI, Godsland IF, Owens P, Moore VM, Cockington RA, Robinson JS. Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regulatory Peptides. 2007;140:37–42. doi: 10.1016/j.regpep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 10.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 11.He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res. 2004;12:761–769. doi: 10.1038/oby.2004.92. [DOI] [PubMed] [Google Scholar]
- 12.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 13.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 14.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. Jama. 1995;274:1915–1921. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Goodpaster BH, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 16.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 17.Kiens B. Effect of endurance training on fatty acid metabolism: local adaptations. Med Sci Sports Exerc. 1997;29:640–645. doi: 10.1097/00005768-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirwan JP, del Aguila LF, Hernandez JM, Williamson DL, O'Gorman DJ, Lewis R, Krishnan RK. Regular exercise enhances insulin activation of IRS-1 associated PI3-kinase in human skeletal muscle. J Appl Physiol. 2000;88:797–803. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- 20.Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year old men and women. J Gerontol. 1993;48:M84–90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- 21.Krebs M, Roden M. Molecular mechanisms of lipid-induced insulin resistance in muscle, liver and vasculature. Diabetes Obes Metab. 2005;7:621–632. doi: 10.1111/j.1463-1326.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 22.Melanson KJ, Saltzman E, Russell RR, Roberts SB. Fat oxidation in response to four graded energy challenges in younger and older women. Am J Clin Nutr. 1997;66:860–866. doi: 10.1093/ajcn/66.4.860. [DOI] [PubMed] [Google Scholar]
- 23.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 26.Nagy TR, Goran MI, Weinsier RL, Toth MJ, Schutz Y, Poehlman ET. Determinants of basal fat oxidation in healthy Caucasians. J Appl Physiol. 1996;80:1743–1748. doi: 10.1152/jappl.1996.80.5.1743. [DOI] [PubMed] [Google Scholar]
- 27.Nicklas BJ, Rogus EM, Goldberg AP. Exercise blunts declines in lipolysis and fat oxidation after dietary-induced weight loss in obese older women. Am J Physiol. 1997;273:E149–E155. doi: 10.1152/ajpendo.1997.273.1.E149. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary VB, Jorett AE, Marchetti CM, Gonzalez F, Phillips SA, Ciaraldi TP, Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Schrauwen P, van Aggel-Leijssen DPC, Hul G, Wagenmakers AJM, Vidal H, Saris WHM, van Baak MA. The effect of a 3-month low-intensity endurance training program on fat oxidation and acetyl-CoA carboxylase-2 expression. 2002;51:2220–2226. doi: 10.2337/diabetes.51.7.2220. [DOI] [PubMed] [Google Scholar]
- 32.Schutz Y, Tremblay A, Weinsier RL, Nelson KM. Role of fat oxidation in the long-term stabilization of body weight in obese women. Am J Clin Nutr. 1992;55:670–674. doi: 10.1093/ajcn/55.3.670. [DOI] [PubMed] [Google Scholar]
- 33.Segal KR, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes. 1996;45:988–991. doi: 10.2337/diab.45.7.988. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283:E187–192. doi: 10.1152/ajpendo.00542.2001. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000-2002. Diabetes Care. 2005;28:1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 36.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, Delany J, Kelley DE. Effects of Physical Activity and Weight Loss on Skeletal Muscle Mitochondria and Relationship to Glucose Control in Type 2 Diabetes Mellitus. Diabetes. 2007 doi: 10.2337/db07-0141. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 38.van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab. 2004;287:E558–565. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 39.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson DL, Kirwan JP. A single bout of concentric resistance exercise increases basal metabolic rate 48 hours after exercise in healthy 59-77-year-old men. J Gerontol A Biol Sci Med Sci. 1997;52:M352–355. doi: 10.1093/gerona/52a.6.m352. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]