Abstract
Purpose.
To determine the abundance of extracellular DNA (eDNA) in tear fluid of patients with dry eye disease (DED) and to report clinical outcomes after DNase I eyedrops use to reduce excessive tear fluid eDNA.
Methods.
Tear fluid was collected from healthy control subjects and patients with DED. The eDNA abundance was determined with the PicoGreen dye assay. The DED symptoms and clinical signs were recorded and correlated with eDNA abundance. Two patients with DED having excessive eDNA in tear fluid were treated with DNase I eyedrops.
Results.
The PicoGreen dye assay measures tear fluid eDNA abundance after a 2-minute incubation time. With longer incubations, admixed cells also contribute to eDNA measurements. The mean (SE) eDNA abundance in healthy control subjects' tear fluid was 1.4 (0.2) μg/mL. The mean (SE) eDNA abundance in tear fluid of patients with nonautoimmune DED, autoimmune DED, and graft versus host disease was significantly higher: the values were 2.9 (0.6), 5.2 (1.2), and 9.1 (2.3) μg/mL, respectively (P < 0.05). In most of these patients, the PicoGreen dye kinetic assay of tear fluid showed an increase in fluorescence signal due to the presence of viable cells in tear fluid. Tear fluid eDNA had the best correlation with corneal Rose Bengal staining (r = 0.55). Treatment of patients having DED with DNase I eyedrops reduced eDNA abundance, abrogated signal increase, and improved comfort.
Conclusions.
Excessive eDNA is present in tear fluid of patients with dry eyes. A novel therapeutic approach for managing DED may be to measure eDNA abundance in tear fluid with the PicoGreen dye assay and reduce excessive amounts with DNase I eyedrops.
Keywords: dry eye, tear fluid, extracellular DNA, DNase I, PicoGreen dye assay
Patients with dry eyes have excessive extracellular DNA and admixed cells in tear fluid. DNase I eyedrops clear excessive eDNA and neutrophil extracellular traps from tear fluid and improve signs and symptoms associated with dry eyes.
Introduction
Dry eye disease (DED) is a multifactorial disease of tears and the ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability that can potentially damage the ocular surface. It is accompanied by increased tear film osmolarity and ocular surface inflammation.1 Although the pathogenesis of DED is not fully understood, it is well recognized that inflammation has a prominent role in DED symptom development and amplification.2 The current paradigm suggests that ocular surface inflammation is triggered by surface epithelium stress caused by tear hyperosmolarity. Inflammation is sustained by activated antigen-presenting cells and T cells via the afferent and efferent limbs of the adaptive immune system.3,4 The immunopathological events that sustain the systemic adaptive immune response in DED have been characterized using animal models.2–4 However, the mechanisms that activate the adaptive immune response are poorly understood. This has hindered the development of novel treatments: cyclosporine ophthalmic emulsion (RESTASIS; Allergan, Inc., Irvine, CA) remains the only Food and Drug Administration (FDA)–approved dry eye pharmaceutical treatment in the United States.
Neutrophils are terminally differentiated cells that are vital to both the innate and acquired immune systems.5 When challenged by certain signals, including interleukin 8 and lipopolysaccharide, neutrophils undergo a programmed sequence of events that leads to the active release of their entire nuclear chromatin complex (DNA, associated histone-rich protein backbone, and embedded cathelicidin antimicrobial peptides) as a type of biologic spiderweb into the extracellular space or tissue.6 These extracellular DNA (eDNA) webs, termed neutrophil extracellular traps (NETs), were first described in 2004 by Brinkmann et al.7 NET release is a powerful method of neutrophil-mediated microbial killing and host defense. Although these eDNA traps are part of the innate immune response and an efficient mechanism of host defense, they may also contribute to immunopathology in chronic inflammatory diseases.8,9 NETs connect innate immune response to autoimmunity.10 Therefore, under conditions of chronic inflammatory responses, it might make sense to reduce the abundance of NETs and eDNA to interrupt the inflammatory cascade.9 We recently reported that NETs and eDNA may be a possible source of inflammation in DED.11,12 We showed increased ocular surface eDNA and molecular components of NETs in a population of patients with severe dry eyes, which is a likely consequence of reduced tear fluid nucleases (e.g., DNase I). Our data provide the rationale for using DNase I eyedrops to clear NETs and eDNA from the ocular surface of patients with DED to reduce inflammation. Furthermore, because it is well established that excessive eDNA increases sputum viscosity in patients with cystic fibrosis, it may also increase tear fluid viscosity in patients with dry eyes, leading to ocular surface desiccation.13,14 A second mechanism by which DNase I may benefit patients with dry eyes is by clearing eDNA to reduce tear fluid viscosity.
Herein, we describe the use of the PicoGreen dye assay to determine eDNA abundance in tear fluid of patients with DED. The PicoGreen dye assay for detecting doublestranded DNA (dsDNA) in solution was first described by Singer et al.15 We also present the clinical outcomes of two patients with dry eyes having excessive eDNA in their tear fluid who were treated with DNase I eyedrops. To the best of our knowledge, this is the first report of topical DNase I use as a therapeutic agent in the eye. Our results may lead to novel diagnostic and therapeutic approaches based on detecting and clearing excess eDNA from tear fluid of patients with DED.
Methods
Study Population
Study approval was obtained from the Institutional Review Board of the University of Illinois at Chicago. Symptomatic patients with DED and asymptomatic healthy control subjects were enrolled, and informed consent was obtained from all participants after the nature and possible consequences of the research were explained. The research was conducted in accord with the requirements of the Health Insurance Portability and Accountability Act and the tenets of the Declaration of Helsinki. Patients were included if they had symptoms of ocular discomfort and the presence of one or more signs of DED such as a Schirmer I test result of less than 10 mm in 5 minutes or Rose Bengal staining of the cornea.16 Patients were divided into the following three groups: (1) nonautoimmune DED group (19 eyes of 13 patients), (2) autoimmune DED (e.g., Sjögren's syndrome) group (18 eyes of 11 patients), and (3) graft versus host disease group (19 eyes of 12 patients). The control group (17 eyes of 11 healthy control subjects) included individuals with no ocular symptoms, absent surface staining by Rose Bengal dye, and a Schirmer I test result exceeding 15 mm at 5 minutes.
Clinical Examination
Symptom burden analysis was performed with the Ocular Surface Disease Index (OSDI) questionnaire, which assesses symptoms, functional limitations, and effect of environmental triggers on symptoms due to dry eyes in a 12-item survey.17 Tear production was measured by Schirmer I test (without anesthesia) at 5 minutes using a Whatman filter strip number 41 (Haag-Streit, Essex, UK). The severity of ocular surface disease was assessed using Rose Bengal dye. Saline-moistened 1% Rose Bengal impregnated strips were used to instill the dye on the inferior palpebral conjunctiva, and scoring of corneal and conjunctival staining was performed by a slitlamp examination after 15 seconds using a modification of the grading system described by a 1995 National Eye Institute workshop.18 Corneal staining was graded in five zones, and conjunctival staining was graded in four zones. Each zone was graded from 0 to 3 based on the density of punctate staining.
DNA Abundance Measurement Using the PicoGreen Dye Fluorescence Assay
The PicoGreen dye fluorescence assay was performed using the Quant-iT PicoGreen dsDNA Assay Kit (catalog number P7589; Invitrogen, Carlsbad, CA) per the manufacturer's instructions. Fluorescence signal intensity was measured (excitation at 480 nm and emission at 520 nm) using a microplate reader (Synergy H1; BioTek, Winooski, VT). After 5-minute incubation, fluorescence signal intensity was measured every 15 seconds over 20 minutes. Values at 0 minutes (the initial value after the incubation time) and 20 minutes were subtracted from each other, calculated as a ratio to the initial value, and expressed as percentage change in fluorescence signal.
Tear Fluid eDNA Abundance Measurement
A 5-μL volume of tear fluid was collected from the lower lid margin and inferior fornix of all patients with DED and healthy control subjects using a blunt glass microcapillary tube (5-μL Drummond Microcaps, catalog number 21-170E; Thermo Fisher Scientific, Waltham, MA). To minimize reflex tearing, tears were collected from unanesthetized eyes before any clinical tests or examinations. A slitlamp was used with the broad beam at minimum intensity. For patients with very severe tear deficiency (Schirmer I test result of ≤1), a drop of preservative-free artificial tears (Refresh Optive Sensitive; Allergan, Inc.) was instilled in the eye, and conjunctival washings were collected after a 2-minute period. During this time, patients were instructed to gently close their eyelids and perform duction movements in all directions along with digital punctual occlusion. The collected tear fluid samples were transferred to DNase-free 200-μL microcentrifuge tubes (catalog number C-3310-1; BioExpress, Kaysville, UT), given a brief spin in a minicentrifuge (GeneMate Minifuge; BioExpress) to drive tear samples to the bottom of the tube, and processed for the PicoGreen dye assay within 1 to 4 hours of collection. Tear fluid samples were constantly kept on ice from the time of sample collection in the clinic to the addition of the PicoGreen dye. The eDNA abundance in tear fluid (corrected for the dilution factor) was determined using the trendline equation of the standards scatterplot shown in Figure 1A. To demonstrate that DNA is present in the assay mixture, DNase I (6 IU, catalog number EN0521; Thermo Fisher Scientific) was added along with excess metal ions (2.0 mM calcium chloride and 2.0 mM magnesium chloride) to the DNA-PicoGreen complex, and fluorescence signal intensity was measured over 20 minutes.19 Exponential decay was expected based on published data.20
Figure 1.
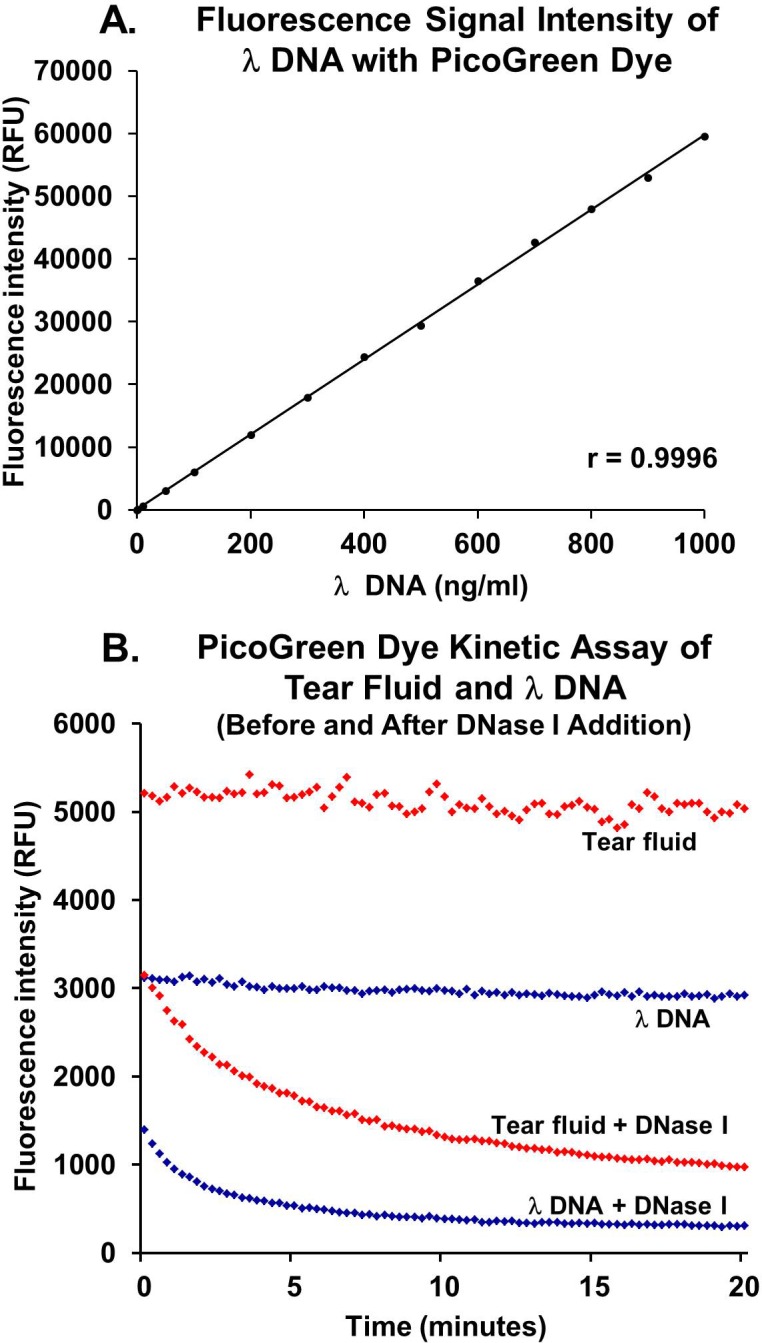
The PicoGreen dye assay to measure DNA abundance. (A) Fluorescence signal intensity measurement of λDNA with the PicoGreen dye (1:200 dilution) shows a linear relationship (r = 0.996). (B) The PicoGreen dye kinetic assay of λDNA and tear fluid from a patient having DED with and without DNase I addition. Stable emission of fluorescence is observed from the λDNA-PicoGreen dye complex. The addition of exogenous DNase I to the λDNA-PicoGreen dye complex leads to an exponential decay in fluorescence signal due to DNA degradation. Similarly, on the addition of DNase I to tear fluid eDNA-PicoGreen dye complex, fluorescence signal intensity decreases exponentially over time, indicating that the eDNA present in tear fluid is degraded by DNase I. RFU, relative fluorescence units.
Presence of Cells in Tear Fluid
Staining of live and dead nucleated cells in tear fluid of healthy control subjects (10 eyes of 5 controls) and patients with dry eyes (19 eyes of 11 patients) was performed using acridine orange/propidium iodide staining solution (catalog number CS2-0106-5ML; Nexcelom Bioscience, Lawrence, MA). Analysis was performed with an automated cell counter (Cellometer 2000; Nexcelom Bioscience).
Measurement of eDNA or Intracellular DNA Abundance in the PicoGreen Dye Assay
To investigate the relative contributions of eDNA and intracellular DNA (iDNA) to fluorescence signal intensity at 5 minutes and the reason for increased fluorescence signal in kinetic assays, we conducted the following experiments. Human corneolimbal epithelial (HCLE) cells, a kind gift from Dr. Ilene Gipson, were cultured in keratinocyte serum-free medium (KSFM; Invitrogen) containing bovine pituitary extract, epidermal growth factor, and an antibiotic-antimycotic solution at 37°C and 5% carbon dioxide. Cells were trypsinized after 70% confluence, resuspended in KSFM, and counted using a Neubauer chamber.
Lambda DNA (λDNA) (50 ng/mL) was mixed with varying numbers of HCLE cells (2-μL cell suspensions containing 0, 100, 500, or 1000 cells), and fluorescence signal intensity was measured. The λDNA served as the source for eDNA, and HCLE cells served as the source for iDNA. The assay procedure was similar to that described for tear fluid. To determine if the incubation period influenced measured fluorescence signal intensity, signal intensity was measured at 2-minute, 5-minute, and 25-minute incubation periods, and the results were expressed in terms of DNA abundance. For the above experiments, wells were prepared in triplicate, and fluorescence signal measurements from three separate runs were averaged for analysis. To determine whether the increase in fluorescence signal was due to iDNA release or iDNA staining, HCLE cells were cultured on coverslips, and live cells were stained with the PicoGreen dye (1:200 dilution) in either 1× Tris-EDTA (TE) buffer or KSFM for 2 or 25 minutes. After the respective incubation periods, the PicoGreen dye–containing buffer solutions were collected for fluorescence signal measurement of eDNA that may have been released from the cells into the buffer during incubation. The coverslips were washed twice with 1× PBS at the end of the respective incubation periods and examined directly using an inverted fluorescence microscope (Axio Observer; Carl Zeiss Meditec GmbH, Hamburg, Germany). The coverslips with 25-minute incubation were imaged first to optimize the fluorescence measurement settings; thereafter, the 2-minute incubation samples were imaged using identical settings.
Statistical Analysis
Following compilation of data using Microsoft Excel office statistics software (Redmund, WA), the arithmetic means (SEs of means) were calculated for all quantitative parameters. Quantitative variables were compared using Student's t-tests or ANOVA with post hoc tests (SPSS Statistics, version 22; IBM Corporation, Armonk, NY). χ2 Tests were performed to determine the presence of significant differences between categorical variables. Pearson's correlation coefficient was used to assess the correlation of eDNA abundance with clinical signs and symptoms. P < 0.05 was considered statistically significant.
Results
The PicoGreen Dye Assay Measures eDNA Abundance in Tear Fluid
We used the PicoGreen dye assay to measure tear fluid eDNA abundance. The PicoGreen dye at 1:200 dilution yielded a linear relationship (r = 0.9996) (Fig. 1A). This is in agreement with previously reported data.15,20 We measured fluorescence signal intensity from λDNA in solution and in tear fluid from patients with DED over a 20-minute kinetic assay (Fig. 1B). The fluorescence signal intensity from the λDNA-PicoGreen complex was stable over 20 minutes. With the addition of exogenous DNase I, an exponential decay in signal intensity was noted, confirming the presence of undigested dsDNA in the solution. Similarly, on the addition of DNase I, an exponential decay in fluorescence signal was observed in tear fluid from patients with severe DED, indicating that undigested eDNA was present in tear fluid. Data for λDNA and the tear fluid sample were analyzed as single-exponential decays and double-exponential decays. The fits for double-exponential decay were essentially perfect, whereas the exponential terms in the single-exponential decay functions had errors of less than 3%.
eDNA Abundance in Tear Fluid of Patients With Dry Eyes Correlates With Symptoms and Signs
We measured eDNA abundance in tear fluid using the PicoGreen dye assay and correlated these findings with symptom severity and clinical signs in patients with dry eyes. Symptom analysis was performed using the OSDI questionnaire. The OSDI scores were 35.8 (5.2) for patients with nonautoimmune DED, 34.5 (4.1) for patients with autoimmune DED (e.g., Sjögren's syndrome), and 54.0 (5.2) for patients with GVHD. The OSDI score for all patients with dry eyes was significantly higher than the 3.4 (0.7) for healthy control subjects (P < 0.05). The average aqueous tear production quantities in patients with nonautoimmune DED, autoimmune DED, and GVHD were 6.6 (1.1), 1.6 (0.8), and 0.5 (0.2) mm, respectively. Tear production in all patients with dry eyes was significantly lower than the 23.1 (1.7) mm seen in healthy control subjects (P < 0.05). The severity of ocular surface disease was assessed using Rose Bengal dye staining. The Rose Bengal ocular surface staining scores were 2.8 (0.6) for patients with nonautoimmune DED, 4.6 (0.7) for patients with autoimmune DED, and 5.5 (0.7) for patients with GVHD. Healthy control subjects had no ocular surface staining with Rose Bengal dye. The staining score was significantly higher in all the dry eye groups compared with that in healthy control subjects (P < 0.05). The corneal and conjunctival Rose Bengal staining scores were also analyzed independently. Corneal Rose Bengal staining scores in patients with nonautoimmune DED, autoimmune DED, and GVHD were 1.6 (0.5), 2.4 (0.5), and 3.1 (0.6), respectively. Conjunctival Rose Bengal staining scores in patients with nonautoimmune DED, autoimmune DED, and GVHD were 1.3 (0.3), 2.2 (0.4), and 2.4 (0.5), respectively. Both corneal and conjunctival staining scores were significantly higher for all patients with dry eyes compared with those for healthy control subjects (P < 0.05). The PicoGreen dye assay was used to measure eDNA abundance in tear fluid after a 5-minute incubation. Tears of patients with nonautoimmune DED had a mean eDNA abundance of 2.9 (0.6) μg/mL. Tears of patients with autoimmune DED had a mean eDNA abundance of 5.2 (1.2) μg/mL. Tears of patients with GVHD had a mean eDNA abundance of 9.1 (2.3) μg/mL. The mean eDNA abundance in all tear fluid samples of patients with dry eyes was higher than the 1.4 (0.2) μg/mL in healthy control subjects (P < 0.05). Significant differences were noted between the GVHD and nonautoimmune DED groups for eDNA abundance (P = 0.02), OSDI score (P = 0.02), aqueous tear production (P < 0.001), and Rose Bengal ocular surface staining score (P = 0.03). Between the GVHD and autoimmune DED groups, a significant difference was only noted for OSDI score (P = 0.05). Correlation analysis was performed to evaluate the association of tear fluid eDNA abundance with ocular signs and symptoms (Table). Tear fluid eDNA abundance correlated best with corneal Rose Bengal staining (r = 0.55). Tear fluid eDNA abundance correlated weakly with the Schirmer I test result (r = −0.39) and OSDI score (r = 0.35) (Table).
Table.
Correlation Matrix Between eDNA Abundance, Clinical Signs, and Symptom Analysis
|
Variable |
eDNA Abundance |
OSDI Score |
Schirmer I Test Result |
Corneal RB Dye Staining |
Conjunctival RB Dye Staining |
| eDNA abundance | 1 | ||||
| OSDI score | 0.37 | 1 | |||
| Schirmer I test result | −0.34 | −0.54 | 1 | ||
| Corneal RB dye staining | 0.55 | 0.42 | −0.54 | 1 | |
| Conjunctival RB dye staining | 0.19 | 0.39 | −0.43 | 0.27 | 1 |
RB, Rose Bengal.
Measurement of eDNA or iDNA Abundance in the PicoGreen Dye Assay
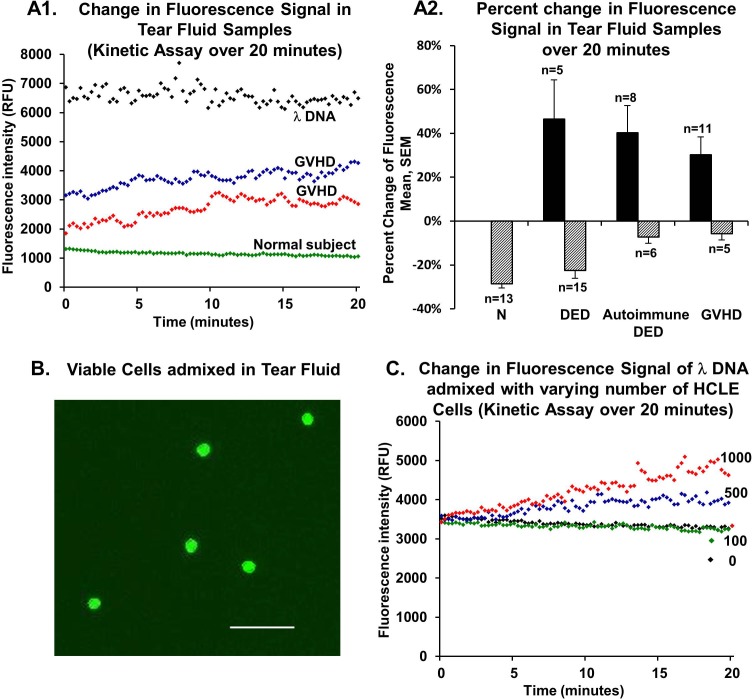
In 48% of tear fluid samples from patients with dry eyes, we observed an increase in fluorescence signal over 20 minutes with the PicoGreen dye kinetic assay (Fig. 2A1). In contrast, all healthy control subjects showed fluorescence decay, averaging 28.6% (1.9%). Fluorescence signal from λDNA also showed a small decay of 7.9% (1.1%) (n = 12). In the nonautoimmune DED group, tear fluid from 25% of eyes exhibited an increase in fluorescence signal of 46.6% (17.8%), whereas 75% had signal decay of 22.5% (3.5%). In contrast to these groups, tear fluid from most eyes in the autoimmune DED (57.1%) and GVHD (68.8%) groups showed an increase in signal over 20 minutes (40.3% [12.5%] and 30.2% [8.2%], respectively). The remaining eyes in these groups showed a minor decay in signal ranging from 5% to 7% (Fig. 2A2). When comparing eyes that showed increased fluorescence over time with those that did not, no significant difference in tear production was found (P = 0.08 for GVHD and P = 0.11 for autoimmune DED). When comparing eyes that had conjunctival washings with those that had surface tear fluid collection, the number of eyes showing fluorescence increase was not significantly different (P = 0.26).
Figure 2.
Fluorescence signal intensity change of tear fluid eDNA or λDNA measured with the PicoGreen dye kinetic assay. (A1) Representative curves of tear fluid from a healthy control subject showing decreasing signal and two patients with GVHD showing increasing signal intensity. The λDNA (100 ng/mL) is shown for comparison. (A2) Percentage change in fluorescence signal in healthy control subjects and patients with nonautoimmune DED, autoimmune DED, or GVHD over 20 minutes. (B) Staining of tear fluid cells with AO/PI dye shows viable cells (green). (C) Kinetic assays of mixtures of λDNA (50 ng/mL) and varying numbers of HCLE cells (0, 100, 500, or 1000) show changes in fluorescence signal dependent on the number of HCLE cells. Increased signal is seen with the addition of 500 and 1000 cells. AO/PI, acridine orange/propidium iodide; DED, nonautoimmune DED; N, normal healthy control subjects; RFU, relative fluorescence units. Scale bar: 50 μm.
We investigated the rationale for and interpretation of the observed increase in fluorescence signal in patients with DED. It is possible that some ocular surface cells (corneal or conjunctival) or bone marrow–derived cells (neutrophils) are also admixed in tear fluid samples that we collected using microcapillary tubes. We performed tear fluid staining in healthy control subjects and patients with dry eyes to determine the presence of live or dead nucleated cells (Fig. 2B). Approximately 95% of tear fluid samples from patients with dry eyes showed the presence of cells. In contrast, only 20% of tear fluid samples from healthy control subjects contained cells. Almost all cells were viable (97% in patients with dry eyes and 100% in healthy control subjects). Tear fluid from patients with dry eyes contained significantly more admixed viable cells (1.1 × 105 [0.4 × 105] cells/mL) compared with that from healthy control subjects (2.4 × 103 [1.6 × 103] cells/mL) (P < 0.05). The average diameter of viable cells was 10.1 (0.6) μm.
Next, we performed experiments to determine whether the presence of cells in tear fluid led to the observed increase in fluorescence signal. The PicoGreen dye kinetic assays were performed over 20 minutes after mixing λDNA (50 ng/mL) and HCLE cells (0, 100, 500, or 1000) (Fig. 2C). The increase in fluorescence signal over 20 minutes was more than 30% after adding 500 or 1000 cells (equivalent to 2.5 × 105 or 5.0 × 105 cells/mL), whereas the addition of 100 cells (equivalent to 0.5 × 105 cells/mL) or no cells resulted in a minor decay in signal intensity ranging from 5% to 7%. The results of these experiments suggest that the presence of admixed cells in quantities that are seen in patients with dry eyes (average of 1.1 × 105 cells/mL) can cause an increase in fluorescence signal intensity during a kinetic assay.
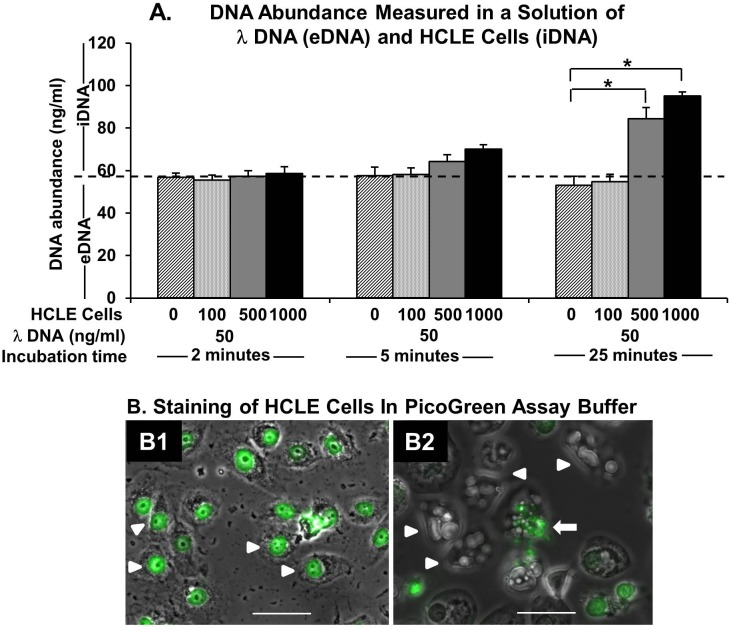
Next, we determined how cells in tear fluid increase the fluorescence signal in the PicoGreen dye kinetic assay. Specifically, is the signal increase due to iDNA staining or release of iDNA into the medium? We added HCLE cells (0, 100, 500, or 1000) to λDNA (50 ng/mL) and measured DNA abundance at 2, 5, and 25 minutes. At the 2-minute time point, DNA abundance with and without the addition of HCLE cells (100, 500, or 1000) were within ±5% of each other (Fig. 3A). At 5 minutes, DNA abundance measured with the addition of 500 or 1000 cells was greater than that measured without HCLE cell addition. It was even higher at 25 minutes. The DNA abundance was the highest with the addition of 1000 cells at 25 minutes. These results confirmed that at 2 minutes the PicoGreen assay measures only eDNA but that at 5 minutes or longer iDNA also contributes to the measured DNA abundance. Next, we determined whether degeneration of tear fluid cells due to the PicoGreen assay buffer and consequent iDNA release into the medium can explain the increase in fluorescence signal. We cultured HCLE cells on coverslips and stained them with the PicoGreen dye in assay buffer (1× TE buffer) without permeabilization. We observed staining of HCLE cell nuclei with the PicoGreen dye at 2 minutes (Fig. 3B1). At 25 minutes, HCLE cells appeared swollen, with numerous vacuoles. There was nuclear fluorescence loss, and we observed fluorescent nuclear fragments (Fig. 3B2). The PicoGreen dye assay buffer used to stain the cells was collected, and DNA abundance was determined. The eDNA abundance in the 25-minute sample was 4.2 times the amount of eDNA in the 2-minute sample (P < 0.001). There was no increase in fluorescence signal in either sample on kinetic assay. Taken together, these data suggest that the PicoGreen dye assay buffer induced cellular degeneration and release of iDNA into the medium, resulting in increased fluorescence signal over time.
Figure 3.
The DNA abundance measurement (eDNA or iDNA) in the PicoGreen dye assay. (A) The DNA abundance measured from a mixture of λDNA (50 ng/mL) and HCLE cells (0, 100, 500, or 1000) at different incubation durations (2, 5, or 25 minutes). At 2 minutes, the change in DNA abundance with the addition of HCLE cells is minimal. Thus, the measured DNA abundance at 2 minutes is due to eDNA. A significant increase in DNA abundance is seen with the addition of 500 or more cells at 25-minute incubation. Thus, the measured DNA abundance at 25 minutes is due to both eDNA and iDNA. (B) The HCLE cells stained with the PicoGreen dye in assay buffer (1× TE) at different incubation durations (2 and 25 minutes). (B1) Overlay of brightfield and fluorescent images at 2 minutes shows healthy HCLE cells with bright nuclear staining. Arrowheads indicate stained nuclei. (B2) Overlay of brightfield and fluorescent images at 25 minutes shows evidence of cell degeneration with an absence of nuclear staining. Arrowheads indicate swollen cells with vacuoles. The arrow points to a nucleus showing fragmentation. *P < 0.05. Scale bars: 50 μm.
Report of Cases Using DNase I Eyedrops in Patients With Dry Eyes Having Excessive Tear Fluid eDNA
We present the clinical outcomes of two patients having dry eyes with excessive tear fluid eDNA who were treated with recombinant human DNase (rhDNase) I eyedrops 0.1% four times a day (Pulmozyme; Genentech, San Francisco, CA). To the best of our knowledge, this is the first report of the use of DNase I as eyedrops for treating ocular surface disease.
Case 1.
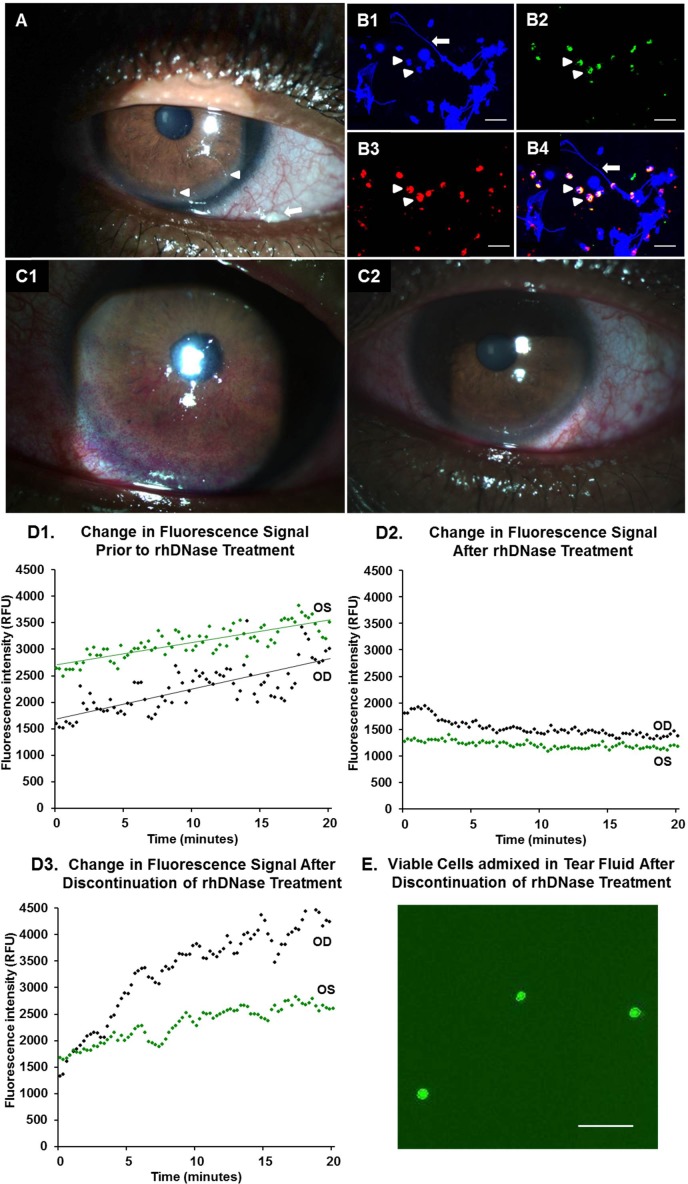
A 54-year-old African American man with a long-standing history of dry eyes was seen at our clinic for the management of severe ocular discomfort, despite treatment with cyclosporine 0.05% (RESTASIS; Allergan, Inc.) and loteprednol 0.5% (Lotemax; Bausch & Lomb, Incorporated, Rochester, NY) eyedrops and aggressive lubrication. He was unable to tolerate bandage contact lenses. Best-corrected visual acuity (BCVA) was 20/200 OD because of amblyopia and 20/25 OS. Symptom analysis showed moderate discomfort (OSDI score of 46.9). Examination demonstrated severe aqueous tear deficiency (Schirmer I test result of 1 mm OU). Slitlamp examination revealed the presence of numerous corneal filaments and mucoid films on the conjunctival surface (Fig. 4A). Staining of mucoid films with 4′,6-diamidino-2-phenylindole (DAPI) nuclear dye revealed the presence of extensive eDNA strands admixed with numerous neutrophils (Figs. 4B1–4). Rose Bengal dye staining revealed scores of 3 and 6 in the right and left corneas, respectively. The PicoGreen dye testing showed 2.5 and 4.3 μg/mL eDNA in the right and left eyes, respectively. Kinetic assays over 20 minutes demonstrated increases in fluorescence signal of 89.2% and 33.0% in the right and left eyes, respectively (Fig. 4D1). We elected to treat him with rhDNase 0.1% eyedrops four times a day in both eyes. The patient reported no complaints of burning, stinging, or other adverse effects on applying the eyedrops. After 2 months of treatment, the symptom analysis score had improved (OSDI score of 12.5, which was mild). Corneas did not stain with Rose Bengal dye, in contrast to the extensive staining seen before rhDNase eyedrops use (Figs. 4C1, 4C2). The eDNA abundance in tear fluid was reduced in the left eye (1.9 μg/mL) and unchanged in the right eye. Kinetic assay of tear fluid samples from both eyes showed decays in fluorescence signal over 20 minutes (24.14% and 6.96% in the right and left eyes, respectively) (Fig. 4D2). Corneal filaments resolved, and mucoid films were greatly reduced. The rhDNase treatment was discontinued after 2 months. Kinetic assays of tear fluid samples were performed again 1 month after discontinuation of rhDNase treatment (Fig. 4D3). Tear fluid from both eyes showed an increase in fluorescence signal over 20 minutes (248.9% and 56.1% in the right and left eyes, respectively) and the presence of viable cells (Fig. 4E). The eDNA abundance had increased in the left eye but not in the right eye.
Figure 4.
Case 1: Ocular surface findings of a patient with severe DED and excessive eDNA in tear fluid treated with rhDNase (Pulmozyme) 0.1% eyedrops. (A) Clinical photograph showing mucoid debris (arrow) and corneal filaments (arrowheads) on the ocular surface. (B1–4) Confocal immunofluorescence staining of the mucoid films was performed as described previously.5 (B1) The eDNA strands (arrow) stain with DAPI (blue). Arrowheads point to multilobed neutrophil nuclei. (B2) Cathelicidin staining within neutrophils (green, arrowheads) with goat polyclonal anti-cathelicidin (clone C-14; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). (B3) Neutrophil elastase staining within neutrophils (red, arrowheads) with mouse monoclonal anti-human neutrophil elastase (clone NP57; Dako Denmark A/S, Glostrup, Denmark). (B4) Overlay. The secondary antibodies were Dylight 488 anti-goat IgG for cathelicidin (1:1000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Dylight 594 conjugated anti-mouse IgG for neutrophil elastase. (C1) Clinical photograph taken before initiating rhDNase treatment shows extensive ocular surface staining with Rose Bengal dye. (C2) Clinical photograph taken after rhDNase treatment (0.1% four times a day) for 2 months shows resolution of corneal filaments and surface staining. (D1) The PicoGreen dye kinetic assay of the patient's tear fluid before initiating rhDNase treatment shows increasing signal over 20 minutes. (D2) Kinetic assay after rhDNase treatment for 2 months shows signal decay. (D3) Kinetic assay performed 1 month after discontinuation of rhDNase treatment shows an increase in signal. (E) Staining of tear fluid cells with AO/PI dye shows viable cells (green) after discontinuation of rhDNase treatment for 1 month. AO/PI, acridine orange/propidium iodide; RFU, relative fluorescence units. Scale bars: 50 μm.
Case 2.
A 49-year-old Caucasian woman with a history of dry eyes due to GVHD was seen at our clinic for the management of severe ocular surface discomfort and disease. The patient had received a bone marrow transplant in May 2010 for Sezary's syndrome. She was being treated with systemic prednisone (5 mg/d) and topical fluorometholone 0.1% (FML; Allergan, Inc.), cyclosporine 0.05% (RESTASIS; Allergan, Inc.), and aggressive lubrication with preservative-free artificial tear eyedrops and gels. Symptom analysis showed severe discomfort (OSDI score of 81.25). The BCVA was 20/20 OU. Examination showed severe aqueous tear deficiency (Schirmer I test result of 0 mm OU). Slitlamp examination revealed the presence of corneal filaments and mucoid films in the inferior conjunctival fornix. Rose Bengal dye staining revealed scores of 2 and 3 in the right and left corneas, respectively. Schirmer I test strip impressions were made on glass slides and stained with DAPI nuclear dye as described previously.11 Numerous eDNA strands were seen (Fig. 5A). Staining of mucoid films with DAPI also showed extensive eDNA strands (Fig. 5B). The PicoGreen dye tests of conjunctival washings demonstrated the presence of 3.9 and 3.3 μg/mL eDNA in the right and left eyes, respectively. Increases in fluorescence signal on kinetic assay over 15 minutes were 53.8% and 17.1% in the right and left eyes, respectively (Fig. 5C1). We elected to treat her with rhDNase 0.1% eyedrops four times a day in both eyes. The patient reported no complaints of burning, stinging, or other adverse effects on applying the eyedrops. After 1 month of treatment, the symptom analysis score had improved (OSDI score of 50, which was moderate). Corneal Rose Bengal staining decreased to a score of 1 in both eyes. The eDNA abundance in tear fluid was reduced (2.6 and 1.1 μg/mL in the right and left eyes, respectively). Kinetic assays over 15 minutes showed 6.2% and 6.3% decays in fluorescence signal in the right and left eyes, respectively (Fig. 5C2). Corneal filaments had resolved, and mucoid films were reduced.
Figure 5.
Case 2: Ocular surface findings of a patient with severe DED and excessive eDNA in tear fluid treated with rhDNase 0.1% eyedrops. Widefield fluorescence image after DAPI staining of Schirmer I test impressions (A) and mucoid film (B) processed as described previously.5 The eDNA strands (arrows) and abundant exfoliated cells (arrowheads) are seen. (C1) The PicoGreen dye kinetic assay of the patient's tear fluid before initiating rhDNase treatment shows an increase in signal over 20 minutes. (C2) Kinetic assay after rhDNase treatment for 1 month shows signal decay. RFU, relative fluorescence units. Scale bars: 50 μm.
Discussion
We recently demonstrated the presence of excessive amounts of eDNA and NETs on the ocular surface of patients with DED along with a deficiency of tear fluid nucleases.11 We proposed that the accumulation of eDNA in the tear film could be contributing to the ocular surface inflammation. Herein, we show that eDNA abundance can be measured in a small volume of tear fluid (2 μL) with a simple biochemical assay using the PicoGreen dye. Using this assay, we determined that patients with dry eyes have significantly higher eDNA abundance in their tear fluid compared with that in healthy control subjects. We also determined that tear fluid eDNA abundance has the strongest correlation with Rose Bengal dye corneal staining. Finally, in patients with severe dry eyes and excessive tear fluid eDNA, we showed that treatment with rhDNase I eyedrops (0.1%) four times a day reduced the abundance of eDNA and resolved corneal Rose Bengal staining.
The PicoGreen dye assay of tear fluid from patients with dry eyes showed two important results. The first finding is that fluorescence signal measurements at short incubation times (2–5 minutes) measure eDNA levels in tear fluid. The second finding is that the increase in fluorescence signal on kinetic assay over 20 minutes occurs due to the PicoGreen dye assay buffer–induced degeneration of admixed cells and consequent release of iDNA into the medium. Therefore, fluorescence measurement at longer incubation times (>5 minutes) assesses the amount of eDNA, as well as released iDNA, in tear fluid. In most patients with autoimmune DED and GVHD, we observed a high fluorescence signal at 5 minutes (implying a high abundance of eDNA), as well as a considerable increase in signal over 20 minutes (implying the presence of admixed cells). Vital dye staining of tear fluid from these patients confirmed the presence of viable cells. These cells were primarily neutrophils (data not shown), which are short-lived cells.5,21 However, inflammatory signals are capable of prolonging their life span by several days, during which they release inflammatory mediators and NETs.22 Because tear fluid of patients with DED contains several inflammatory cytokines,23 neutrophils in tear fluid of these patients may also have a prolonged life span, and they may participate in NET formation. We have previously shown the presence of neutrophils and exfoliated ocular surface cells within mucoid films in patients with DED.11 It is well known that the ocular surface epithelium undergoes continuous, dynamic turnover, which is increased in patients with DED; therefore, it would not be surprising to also find nucleated conjunctival or corneal cells in tear fluid.24–29 However, in contrast to neutrophils, it is not clear how long conjunctival or corneal cells in the tear film remain viable (because desquamated cells usually die soon thereafter) or whether eDNA released from these cells participates in NET formation. Our data show that an increase in fluorescence signal is sufficient to indicate the presence of cells in tear fluid. However, the change in fluorescence signal is not sufficient to estimate the number of cells in tear fluid, which requires appropriate cytological examination of tear fluid.
Our data demonstrate that patients with GVHD and autoimmune DED have the greatest abundance of eDNA in their tear fluid. These patients were markedly symptomatic and had severe tear deficiency with extensive ocular surface disease. Tear fluid eDNA abundance in patients with dry eyes did not correlate strongly with symptom severity. This finding is in agreement with studies30,31 documenting the disconnect between symptoms and signs in patients with DED. The method used to collect tear fluid may impact the measured eDNA abundance. In this study, tear fluid was collected directly from the ocular surface in healthy control subjects and the DED group, whereas conjunctival washings were performed for most patients with autoimmune DED (78% of eyes) and GVHD (68% of eyes). The washing-out tear collection method is a viable alternative for detecting many low-abundance biomarkers; however, substantially elevated biomarker levels may be underestimated.32 In the GVHD group, eDNA abundance obtained from eyes that had conjunctival washings was not significantly different compared with eyes where surface fluid was collected (P = 0.26). In the autoimmune DED group, statistical analysis was not performed because of the small number of eyes that had surface fluid collection. Conjunctival washings may have underestimated the eDNA abundance in the tears of patients with GVHD and autoimmune DED. Even so, patients with GVHD and autoimmune DED had significantly greater eDNA abundance compared with that in healthy control subjects.
We used a 0.8 μM PicoGreen dye concentration to maximize the dynamic range of DNA detection and detection sensitivity as has been reported previously.15 However, this concentration of the PicoGreen dye inhibits nuclease activity; therefore, we were unable to determine nuclease activity in tear fluid as has been described using lower PicoGreen dye concentrations (real-time DNase assay).20 Our data show that when the same λDNA sample was measured repeatedly the reproducibility of the fluorescence signal was ±2%. In addition, when λDNA samples were measured sequentially, the fluorescence signal was ±3% of baseline, irrespective of whether the solution was stored at room temperature or on ice (data not shown). Studies33,34 have shown that it is possible for the PicoGreen dye to be absorbed into a living cell and stain intracellular nuclear and mitochondrial DNA, with staining intensity increasing over time. Therefore, based on published data,33,34 one would expect that an increase in signal is due to increasing staining of iDNA within admixed cells, a result that is contrary to our findings. In the published studies,33,34 cells were incubated in cell culture medium (containing the PicoGreen dye), which may have resulted in less toxicity. In our experiments, HCLE cells were incubated with the PicoGreen dye assay buffer (1× TE), which induces cell and nuclear degeneration. When we performed the same experiments with cell culture medium instead of the PicoGreen dye assay buffer, we also observed increasing intensity of intracellular mitochondrial staining with longer incubation times. Therefore, iDNA release into the medium occurs due to the toxic effects of the PicoGreen dye assay buffer on HCLE cells. We used a 5-minute incubation based on the manufacturer's recommendation (Quant-iT PicoGreen dsDNA Assay Kit, catalog number P7589; Invitrogen); however, our data show that it would be preferable to shorten the incubation time to 2 minutes to detect eDNA abundance with negligible iDNA contribution. There are clinical implications of our findings that viable cells are present in tear fluid of patients with dry eyes and that toxic effects on cultured corneal cells cause iDNA release into the medium. It is well known that preservatives in eyedrops such as benzalkonium chloride cause conjunctival and corneal cell toxicity.35–37 The use of preserved eyedrops in patients with DED may induce toxicity in viable cells that are admixed in tear fluid, leading to iDNA release into tear fluid, thus increasing inflammatory stress (eDNA) on the ocular surface.
The addition of exogenous DNase I, an enzyme that selectively cleaves DNA,38 to tear fluid samples that showed high eDNA abundance resulted in exponential decay of fluorescence signal intensity, confirming the presence of eDNA in tear fluid (Fig. 1B). This finding also provides the rationale for using DNase I eyedrops in patients with DED to clear eDNA from their tear fluid. Our finding that exogenous DNase I application results in exponential signal decay also provides indirect evidence for the absolute or relative deficiency of nucleases in tears; had active nucleases been present in sufficient amounts, eDNA would have been digested. Based on this rationale, and recognizing that eDNA is a possible source of ocular surface inflammation,11,12 we used topical DNase I to treat two patients with severe recalcitrant DED who had excessive eDNA in their tear fluid. We used a nonpreserved, sterile, colorless, highly purified solution of rhDNase, which is available as Pulmozyme (Genentech). The rhDNase is used as an inhaled solution (nebulizer) in the management of patients with cystic fibrosis to improve pulmonary function.39 The rhDNase (0.1%) eyedrops were applied four times a day. After 2 months of treatment with rhDNase, corneal Rose Bengal staining and mucoid strands decreased, and the patients reported increased ocular comfort. The eDNA abundance in tear fluid was reduced, and the pretreatment increase in fluorescence signal over a kinetic analysis reversed to a signal decay. That this decay was observed after DNase I treatment, as opposed to increasing signal before treatment, suggests that the DNase I may have reduced the number of cells admixed in tear fluid. Similar signal decay is seen in healthy control subjects and patients with dry eyes who do not have cells in their tear fluid. DNase I can diffuse across the cell membranes of stressed or dying cells and induce apoptosis.40,41 Therefore, it is possible that DNase I may have promoted degeneration of cells in tear fluid.
Because eDNA is a possible source of inflammation in DED,11,12 reducing its abundance on the ocular surface with DNase I may have contributed to the observed clinical benefits. The benefits may also be due to reduced tear fluid viscosity because of DNase I–mediated clearing of excessive eDNA. The viscosity of human tears is of some importance because they have to be viscous enough to protect and lubricate the eye surface yet not so viscous that the high-shear forces applied during a blink cause drag and damage the ocular epithelium.42 In disease states such as dry eyes, tears show degradation of many physical properties, including surface tension and osmolarity, and slight increases in tear viscosity have been reported.14,42 To the best of our knowledge, this is the first report of the use of DNase I eyedrops for treating ocular surface disease. The clinical outcomes of the two case reports described above point to the potential of DNase I as a novel treatment for DED and provide the rationale for conducting clinical trials to investigate the beneficial effects of rhDNase eyedrops. We have initiated clinical trials using rhDNase 0.1% eyedrops four times a day under an Investigational New Drug Application assigned by the FDA. The use of rhDNase for dry eye treatment should be regarded as investigational: the safety and efficacy of rhDNase eyedrops use in humans have not yet been established. Adverse effects are possible due to systemic absorption of the drug, and patients may develop anti–DNase I antibodies that may cross-react with endogenous DNase I. Because NETs have an important role in the innate defense against microbes, treatment with DNase I to clear NETs may increase susceptibility to ocular surface infections. Symptom relief is an important goal in patients having dry eyes, with the proviso that treatment does not interfere with pathogen defense. Therefore, we do not recommend DNase I eyedrops use in clinical practice outside of controlled trials. DNase I requires divalent metal ions for enzymatic action,19 and this point has clinical implications. There are several contact lens solutions and artificial tear eyedrops that contain EDTA as an inactive ingredient. These EDTA-containing eyedrops, especially if used frequently, may chelate calcium and magnesium metal ions to reduce the enzymatic action of nucleases in tear fluid, leading to the accumulation of undigested eDNA on the ocular surface.
In conclusion, we have described the use of a nucleic acid assay to determine tear fluid DNA abundance. Based on our data, we recommend collecting 2-μL tear fluid samples and performing the PicoGreen dye kinetic assays over 20 minutes. Fluorescence signal at 2-minute incubation will measure eDNA abundance in tear fluid. Increase in fluorescence signal over the next 20 minutes will indicate the presence of viable admixed cells in tear fluid. The use of DNase I eyedrops to clear excessive eDNA from tears may abrogate a previously unrecognized source of ocular surface inflammation and lead to improvement in DED-associated signs and symptoms.
Acknowledgments
Human corneolimbal epithelial cells were a kind gift from Ilene Gipson.
Supported by Grants EY018874 and R01EY023656 from the National Eye Institute (SJ), by Core Grant EY001792 from the National Eye Institute, by Midwest Eye Banks (SJ), and by Research to Prevent Blindness.
Disclosure: S. Tibrewal, None; J. Sarkar, None; S.H. Jassim, None; S. Gandhi, None; S. Sonawane, None; S. Chaudhary, None; Y.-S. Byun, None; Y. Ivanir, None; J. Hallak, None; J.H. Horner, None; M. Newcomb, None; S. Jain, P
References
- 1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 2. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012; 130: 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial–immune cell interaction in dry eye. Cornea. 2008; 27 (suppl 1); S9–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012; 31: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013; 210: 1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper PR, Palmer LJ, Chapple IL. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol. 2013; 63: 165–197 [DOI] [PubMed] [Google Scholar]
- 7. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004; 303: 1532–1535 [DOI] [PubMed] [Google Scholar]
- 8. Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012; 189: 2689–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon D, Simon HU, Yousefi S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy. 2013; 68: 409–416 [DOI] [PubMed] [Google Scholar]
- 10. Radic M, Marion TN. Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. Semin Immunopathol. 2013; 35: 465–480 [DOI] [PubMed] [Google Scholar]
- 11. Sonawane S, Khanolkar V, Namavari A, et al. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012; 53: 8253–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDermott AM. New insight into dry eye inflammation. Invest Ophthalmol Vis Sci. 2012; 53: 8264 [DOI] [PubMed] [Google Scholar]
- 13. Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011; 6: e28526 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3235130/. Accessed November 15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiffany JM. The viscosity of human tears. Int Ophthalmol. 1991; 15: 371–376 [DOI] [PubMed] [Google Scholar]
- 15. Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997; 249: 228–238 [DOI] [PubMed] [Google Scholar]
- 16. Bron AJ, Smith JA, Calonge M. 2007 Report of the International Dry Eye Workshop (DEWS): methodologies to diagnose and monitor dry eye disease. Ocul Surf. 2007; 5: 108–152 [DOI] [PubMed] [Google Scholar]
- 17. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118: 615–621 [DOI] [PubMed] [Google Scholar]
- 18. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21: 221–232 [PubMed] [Google Scholar]
- 19. Wiberg JS. On the mechanism of metal activation of deoxyribobuclease I. Arch Biochem Biophys. 1958; 73: 337–358 [DOI] [PubMed] [Google Scholar]
- 20. Tolun G, Myers RS. A real-time DNase assay (ReDA) based on PicoGreen fluorescence. Nucleic Acids Res. 2003; 31: e111 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC203337/. Accessed November 15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011; 18: 1457–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011; 11: 519–531 [DOI] [PubMed] [Google Scholar]
- 23. Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012; 18: 797–802 [PMC free article] [PubMed] [Google Scholar]
- 24. Hanna C, O'Brien JE. Cell production and migration in the epithelial layer of the cornea. Arch Ophthalmol. 1960; 64: 536–539 [DOI] [PubMed] [Google Scholar]
- 25. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442–1443 [PubMed] [Google Scholar]
- 26. Cenedella RJ, Fleschner CR. Kinetics of corneal epithelium turnover in vivo: studies of lovastatin. Invest Ophthalmol Vis Sci. 1990; 31: 1957–1962 [PubMed] [Google Scholar]
- 27. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120: 330–337 [DOI] [PubMed] [Google Scholar]
- 28. Ladage PM, Jester JV, Petroll WM, et al. Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Invest Ophthalmol Vis Sci. 2003; 44: 1056–1063 [DOI] [PubMed] [Google Scholar]
- 29. Ren H, Wilson G. Apoptosis in the corneal epithelium. Invest Ophthalmol Vis Sci. 1996; 37: 1017–1025 [PubMed] [Google Scholar]
- 30. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004; 23: 762–770 [DOI] [PubMed] [Google Scholar]
- 31. McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012; 57: 293–316 [DOI] [PubMed] [Google Scholar]
- 32. Guyette N, Williams L, Tran MT, et al. Comparison of low-abundance biomarker levels in capillary-collected nonstimulated tears and washout tears of aqueous-deficient and normal patients. Invest Ophthalmol Vis Sci. 2013; 54: 3729–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bereiter-Hahn J, Vöth M. Distribution and dynamics of mitochondrial nucleoids in animal cells in culture. Exp Biol Online. 1997; 1: 1–17 [Google Scholar]
- 34. Ashley N, Harris D, Poulton J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp Cell Res. 2005; 303: 432–446 [DOI] [PubMed] [Google Scholar]
- 35. Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29: 312–334 [DOI] [PubMed] [Google Scholar]
- 36. Uematsu M, Kumagami T, Shimoda K, et al. Influence of alkyl chain length of benzalkonium chloride on acute corneal epithelial toxicity. Cornea. 2010; 29: 1296–1301 [DOI] [PubMed] [Google Scholar]
- 37. Sarkar J, Chaudhary S, Namavari A, et al. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012; 53: 1792–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujihara J, Yasuda T, Ueki M, et al. Comparative biochemical properties of vertebrate deoxyribonuclease I. Comp Biochem Physiol B Biochem Mol Biol. 2012; 163: 263–273 [DOI] [PubMed] [Google Scholar]
- 39. Wagener JS, Kupfer O. Dornase alfa (Pulmozyme). Curr Opin Pulm Med. 2012; 18: 609–614 [DOI] [PubMed] [Google Scholar]
- 40. Eulitz D, Mannherz HG. Inhibition of deoxyribonuclease I by actin is to protect cells from premature cell death. Apoptosis. 2007; 12: 1511–1521 [DOI] [PubMed] [Google Scholar]
- 41. Tinazzi E, Puccetti A, Gerli R, et al. Serum DNase I, soluble Fas/FasL levels and cell surface Fas expression in patients with SLE: a possible explanation for the lack of efficacy of hrDNase I treatment. Int Immunol. 2009; 21: 237–243 [DOI] [PubMed] [Google Scholar]
- 42. Gouveia SM, Tiffany JM. Human tear viscosity: an interactive role for proteins and lipids. Biochim Biophys Acta. 2005; 1753: 155–163 [DOI] [PubMed] [Google Scholar]