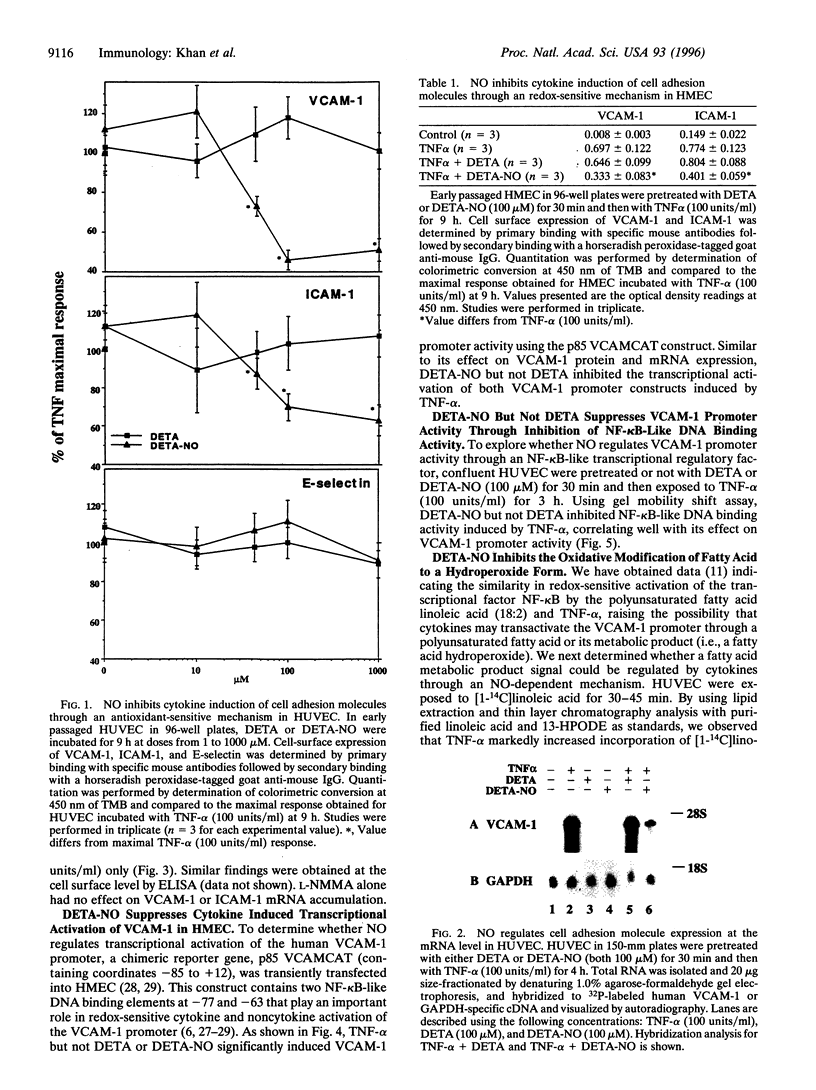
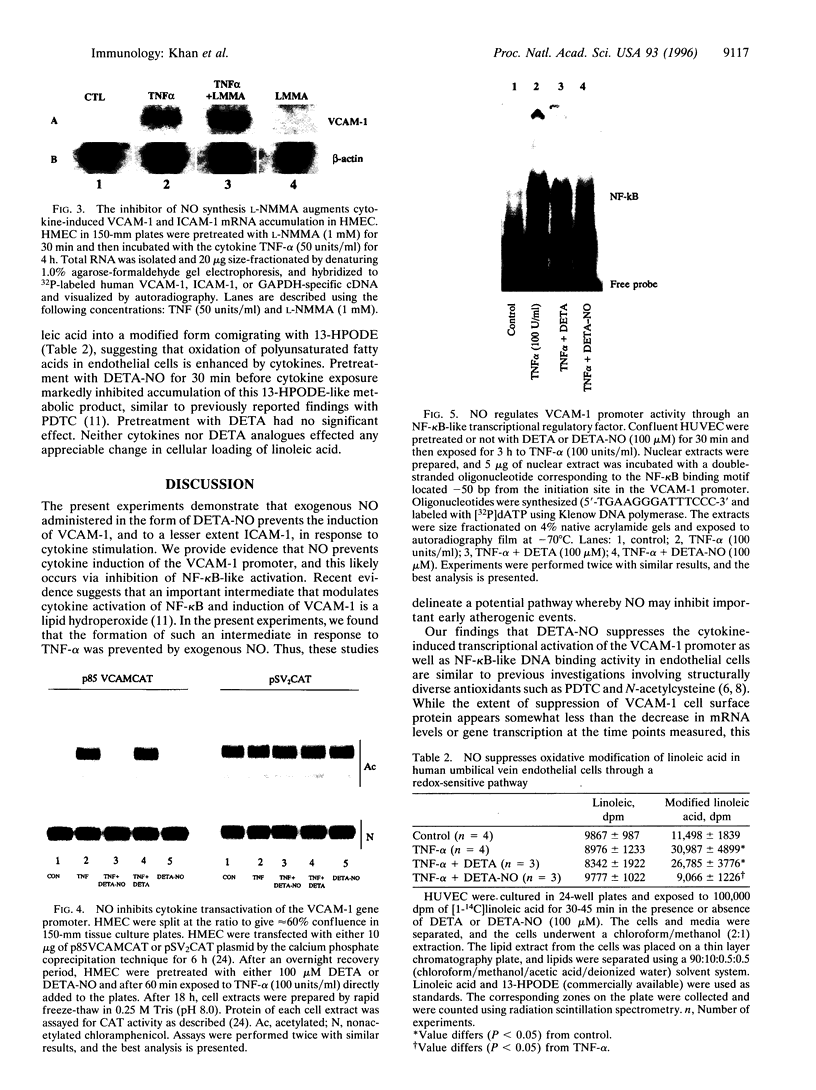
Abstract
Decreased nitric oxide (NO) activity, the formation of reactive oxygen species, and increased endothelial expression of the redox-sensitive vascular cell adhesion molecule 1 (VCAM-1) gene in the vessel wall are early and characteristic features of atherosclerosis. To explore whether these phenomena are functionally interrelated, we tested the hypothesis that redox-sensitive VCAM-1 gene expression is regulated by a NO-sensitive mechanism. In early passaged human umbilical vein endothelial cells and human dermal microvascular endothelial cells, the NO donor diethylamine-NO (DETA-NO, 100 microM) reduced VCAM-1 gene expression induced by the cytokine tumor necrosis factor alpha (TNF-alpha, 100 units/ml) at the cell surface level by 65% and intracellular adhesion molecule 1 (ICAM-1) gene expression by 35%. E-selectin gene expression was not affected. No effect on expression of cell adhesion molecules was observed with DETA alone. Moreover, DETA-NO suppressed TNF-alpha-induced mRNA accumulation of VCAM-1 and TNF-alpha-mediated transcriptional activation of the human VCAM-1 promoter. Conversely, treatment with NG-monomethyl-L-arginine (L-NMMA, 1 mM), an inhibitor of NO synthesis, augmented cytokine induction of VCAM-1 and ICAM-1 mRNA accumulation. By gel mobility shift analysis, DETA-NO inhibited TNF-alpha activation of DNA binding protein activity to the VCAM-1 NF-kappa B like binding sites. Peroxy-fatty acids such as 13-hydroperoxydodecanoeic acid (linoleyl hydroperoxide) may serve as an intracellular signal for NF-kappa B activation. Using thin layer chromatography, DETA-NO (100 microM) suppressed formation of this metabolite, suggesting that DETA-NO modifies the reactivity of oxygen intermediates in the vascular endothelium. Through this mechanism, NO may function as an immunomodulator of the vessel wall and thus mediate inflammatory events involved in the pathogenesis of atherosclerosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989 Mar 15;142(6):1963–1969. [PubMed] [Google Scholar]
- Cohen R. A., Zitnay K. M., Haudenschild C. C., Cunningham L. D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988 Nov;63(5):903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Singer A. H., Tsao P., Zera P., Rowan R. A., Billingham M. E. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992 Sep;90(3):1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee G., Rice-Evans C., Obeyesekera S., Meraji S., Jacobs M., Bruckdorfer K. R. The modulation of ferryl myoglobin formation and its oxidative effects on low density lipoproteins by nitric oxide. FEBS Lett. 1991 Dec 2;294(1-2):38–42. doi: 10.1016/0014-5793(91)81338-9. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hart C. M., Tolson J. K., Block E. R. Fatty acid supplementation protects pulmonary artery endothelial cells from oxidant injury. Am J Respir Cell Mol Biol. 1990 Nov;3(5):479–489. doi: 10.1165/ajrcmb/3.5.479. [DOI] [PubMed] [Google Scholar]
- Hart C. M., Tolson J. K., Block E. R. Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. Am J Physiol. 1991 Jun;260(6 Pt 1):L481–L488. doi: 10.1152/ajplung.1991.260.6.L481. [DOI] [PubMed] [Google Scholar]
- Iademarco M. F., McQuillan J. J., Rosen G. D., Dean D. C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. 1992 Aug 15;267(23):16323–16329. [PubMed] [Google Scholar]
- Khan B. V., Parthasarathy S. S., Alexander R. W., Medford R. M. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995 Mar;95(3):1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Liao F., Berliner J. A., Mehrabian M., Navab M., Demer L. L., Lusis A. J., Fogelman A. M. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991 Jun;87(6):2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991 Aug;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morley D., Maragos C. M., Zhang X. Y., Boignon M., Wink D. A., Keefer L. K. Mechanism of vascular relaxation induced by the nitric oxide (NO)/nucleophile complexes, a new class of NO-based vasodilators. J Cardiovasc Pharmacol. 1993 Apr;21(4):670–676. doi: 10.1097/00005344-199304000-00023. [DOI] [PubMed] [Google Scholar]
- Neish A. S., Williams A. J., Palmer H. J., Whitley M. Z., Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med. 1992 Dec 1;176(6):1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X. F., Smith C. W., Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res. 1994 Jun;74(6):1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Zheng B., Kuo J. F., Harrison D. G. Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arterioscler Thromb. 1994 Jun;14(6):1007–1013. doi: 10.1161/01.atv.14.6.1007. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Slowik M. R., De Luca L. G., Ritchie A. J. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993 Jun 1;150(11):5114–5123. [PubMed] [Google Scholar]
- Rubanyi G. M. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991 May;46(1):27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Vascular effects of oxygen-derived free radicals. Free Radic Biol Med. 1988;4(2):107–120. doi: 10.1016/0891-5849(88)90071-8. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. B., Agranoff A. B., Nabel E. G., Leung K., Duckett C. S., Neish A. S., Collins T., Nabel G. J. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993 Oct;13(10):6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Li L. J., Sepp N. T., Caughman S. W., Lawley T. J. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992 Jul 15;149(2):698–705. [PubMed] [Google Scholar]
- Thomson L., Trujillo M., Telleri R., Radi R. Kinetics of cytochrome c2+ oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch Biochem Biophys. 1995 Jun 1;319(2):491–497. doi: 10.1006/abbi.1995.1321. [DOI] [PubMed] [Google Scholar]
- Weber C., Erl W., Pietsch A., Ströbel M., Ziegler-Heitbrock H. W., Weber P. C. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb. 1994 Oct;14(10):1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- Wertheimer S. J., Myers C. L., Wallace R. W., Parks T. P. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J Biol Chem. 1992 Jun 15;267(17):12030–12035. [PubMed] [Google Scholar]
- Whelan J., Ghersa P., Hooft van Huijsduijnen R., Gray J., Chandra G., Talabot F., DeLamarter J. F. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. 1991 May 25;19(10):2645–2653. doi: 10.1093/nar/19.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. R., Brock T. A., Chang L. Y., Crapo J., Briscoe P., Ku D., Bradley W. A., Gianturco S. H., Gore J., Freeman B. A. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]







