Abstract
Purpose
Oncolytic viruses (OV) based on herpes simplex virus type 1 (HSV1) are being utilized in clinical trials for a variety of cancers. The OV, rQNestin34.5, utilizes a nestin promoter/enhancer to selectively drive robust viral replication in malignant glioma cells. We have discovered that this promoter becomes extensively methylated in infected glioma cells, reducing OV efficacy.
Experimental Design
We utilized demethylating drugs (5-azacytidine), Decitabine or Valproic Acid (VPA) in both in vitro and in vivo malignant glioma models to determine if they improved the efficacy of rQNestin34.5 therapy.
Results
Utilization of demethylating agents, such as 5-azacytidine (5-Aza), improved OV replication and tumor cell lysis in vitro and, in fact, synergized pharmacologically by Chou-Talalay analysis. In vivo the combination of the demethylating agents, 5-Aza or Decitabine, with rQNestin34.5 significantly prolonged the survivorship of athymic mice harboring intracranial human glioma xenografts over single agent alone.
Conclusion
These results thus provide further justification for the exploration of demethylating agents when combined with the OV, rQNestin34.5, in preclinical therapeutics and possibly clinical trials for malignant glioma.
Keywords: Glioblastoma multiforme, experimental therapeutics, virotherapy, decitabine, rQNestin34.5
INTRODUCTION
Oncolytic viruses (OV) are either natural mutants or genetically engineered strains of viruses that replicate and lyse tumor cells in a relatively selective fashion (1). Although there are several types of OVs, those based on herpes simplex virus type 1 (HSV1) have been widely studied in animal models and in human clinical trials (2, 3). Subjects have been reported to tolerate the treatment well, although evidence for efficacy awaits testing in phase 3 trials. Efficacy is predicated on sufficient replication and OV bio-distribution to permit direct cytotoxicity and/or antitumor immune responses to occur. In this context, evidence from clinical trials appears to show that replication needs to be improved for a sufficient therapeutic effect to occur (4, 5).
In order to improve replication, efforts to understand how the OV and the host interact are needed. One of the intracellular mechanisms of defense against a viral infection lies in epigenetic silencing of viral genes. While for some viruses, such as EBV, methylation of CpG islands in genes and promoters is one mechanism for gene silencing (6), for HSV1 the evidence for CpG methylation playing a major role has been controversial, with recent data showing that the HSV1 genome is not extensively methylated at CpG sites (7–9). Notwithstanding this, it is not known if oncolytic HSV1s (oHSV1), engineered with heterologous (such as tumor-specific) promoters, would be different. This is a significant question to explore since effective demethylating agents exist that could be utilized in a clinical trial with oHSV.
rQNestin34.5 is a genetically engineered oHSV1 where a heterologous cellular Nestin promoter-enhancer element drives expression of a single copy of the late viral gene, ICP34.5, whose function results in increasing the ability of HSV1 to replicate (10, 11). This oHSV is under preclinical development for an eventual clinical trial (E.A. Chiocca, unpublished). We hypothesized that this promoter element could be a potential site for silencing of ICP34.5 gene expression by methylation. In this report, we show that infected glioma cells extensively methylate the Nestin promoter and demethylating agents, such as 5′-azacytidine or Decitabine, synergize with rQNestin34.5 replication and glioma cytotoxicity in vitro as well as leading to enhanced therapeutic effects in vivo. Therefore, demethylating agents could be combined with rQNestin34.5 in the treatment of gliomas.
Materials and Methods
Reagents
Dulbecco’s modified minimal essential medium (DMEM), Neurobasal medium, Hank’s Balanced Salt Solution (HBSS), penicillin and streptomycin, GlutaMax, B27 supplement were purchased from Invitrogen (Carlsbard, CA, USA). Human basic fibroblast growth factor (hEGF) and epidermal growth factor (hFGF) were purchased from R&D Systems, Inc (Minneapolis, MN, USA). VPA, 5-Azacytidine, and Decitabine (5-aza-2′-dexoxycytidine) were purchased from SIGMA Aldrich (St Louis, MO, USA). Cell proliferation Kit I (MTT) was purchased from Rosh (Indianapolis, IN, USA)
Cells and Medium
Human U87ΔEGFR glioma cells were maintained in DMEM supplemented with 2% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Human primary OG02 and G169 gliomas were established from surgical specimens resected from glioblastoma patients and were grown as tumor spheres in medium consisting of Neurobasal medium with x1 GlutaMax, with B27 supplement (1Å~; Invitrogen), hFGF (20 ng/ml), hEGF (20 ng/ml), penicillin (100 U/ml), streptomycin (100 μg/ml). All cells were cultured at 37 °C in an atmosphere containing 5% carbon dioxide.
Virus replication assay
The oHSV, rQNestin34.5, was previously described (10) and replication assay were conducted as per previous publications (12). Briefly, after incubation with VPA for 20h or 5-Aza for 2 days, cells were seeded onto 24-well plates at 2 × 104 cells/well in 500 μl of media and infected with oHSV at a multiplicity of infection (MOI) of 0.05. GFP expression was used as an indicator for the presence of oHSV. After 3 days, cells were harvested with supernatants at indicated times in triplicate. After three freeze/thaw cycles and sonication, titers of infectious progeny virus were determined by plaque assay on Vero cells.
Methylation status analysis
Genomic DNA and virus DNA from rQNestin34.5 infected cells were obtained using PureLink Genomic DNA mini kit (Invitrogen). The DNA was treated using bisulfite-modification with the EZ DNA Methylation Kit purchased from Zymo Research Corp (Irvine, CA, USA). The Nestin promoter/enhancer region (10) of rQNestin34.5 was amplified using the following methylation-specific and non-methylation specific primer pairs: methylation-specific F agtagtagcgaataagaag and R ttattagacgttgatagtta; non-methylation-specific F gtggatttgggaatgtggag and R tcctcaaccaaaaccaacct. PCR products were extracted and cloned using TOPO TA Cloning kit (Invitrogen). Each clone was subjected to sequence analysis using the James Comprehensive Cancer Center Nucleic Acid Shared Resource.
Quantitative real time PCR
Total RNA was isolated using Quick-RNA miniprep kit (Zymo Reserch Inc.) and reverse transcribed using the ImProm-II Reverse Transcriptase (Promega, Madison, WI), Quantitative real-time PCR was performed using a Replex2 Master Cycler (Eppendorf, Hauppauge, NY), and Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbard, CA, USA). The following sequences of PCR primers were used for the analysis: gC: F ccttgccgtggtcctgtgga and R gttggggtttggggtcgatg; γ1 34.5: F acagtcccaggtaacctcca and R agtcgtcgtcatcgtcgtc; ICP4: F cgacacggatccacgaccc and R gatccccctcccgcgcttcgtccg; CTUS1: F ggtgatcgcctgtctcc and R cattgccaatcgaaccc; CTRS1/2: F caacgctactgcaaaac and R gacggggtgctgtaac; CTRS3: F cacgaacgacgggagcg and R cacccaaggtgcttacc; ICP27: F acccagccagcgtatccacc and R acaccataagtacgtggcatgt; GAPDH: F ggagtcaacggatttggtcg and R ggaatcattggaacatgtaaacc.
Cell viability assay by MTT exclusion assay
Cell were treated with 5-Aza for 2 days before infection with rQNestin34.5. Three days after the infection, cell viability was determined by MTT assay as previously described. Triplicate wells were counted each time. The results were computed and the combination index for pharmacologic synergy analysis was utilized with the software program, CompuSyn (ComboSyn, Inc., Paramus, NJ, USA).
Mouse in vivo survival experiments
Female athymic mice (6 to 8 weeks of age) were obtained from the National Cancer Institute. To generate intracerebral xenograft models, 2.5 × 104 G169 cells in 4 μl or 2 x 105 human U87ΔEGFR established glioma cells were stereotactically implanted into the brains of athymic mice. After implantation, mice were randomly assigned to each experimental group. On day 7, mice were treated by intratumoral injection of 1 × 105 PFU of rQNestin34.5 alone, 1 × 105 PFU of rQNestin34.5 in 4 μl of 10μM 5-Aza, 10μM 5-Aza alone, or HBSS alone. Mice were then observed until they became moribund, at which point they were sacrificed, and the presence of intracranial tumors was confirmed. All in vivo procedures were approved by the Subcommittee on Research Animal Care at Ohio State University Medical Center.
Statistical analyses
The log rank tests were used to compare Kaplan-Meier survival curves followed by the procedures of Holm-Sidak method for multiple comparisons. For the experiment of Figure 4, due to crossing survival curves the Fleming and Harrington weight function with p=0.5, q=2 was utilized in order to place greater weight on events that occurred at later time points (13).
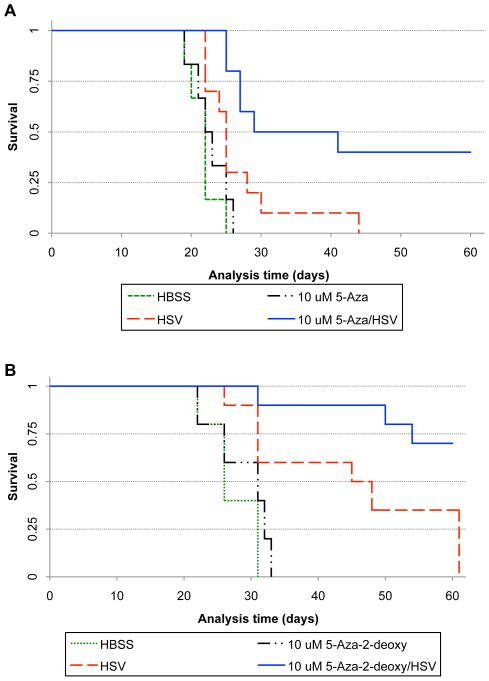
Figure 4. Kaplan-Meier survival curves of athymic mice bearing intracerebral G169 primary human GBM cells for the combination of 5-Aza (panel A, C) or Decitabine (Panel B) and oHSV in vivo.
A) The oHSV (HSV), rQNestin34.5, (1×105 pfu/mouse) with or without 10 μM 5-Aza or HBSS were intracerebrally inoculated on day 7 post-implantation of G169 primary glioma cells (HBSS, n = 5; 5-Aza, n = 4; oHSV, n = 10; oHSV and 5-Aza, n = 10, p=0.0227, log-rank test). B) oHSV (1×105 pfu/mouse) with or without 10 μM Decitabine (5-Aza-2D), or HBSS were intracerebrally inoculated on day 7 post-implantation of G169 primary glioma cells (HBSS, n = 6; Decitabine, n = 6; oHSV, n = 10; oHSV and Decitabine, n = 10, p=0.0057, log-rank test). C) oHSV (1 × 105 pfu/mouse) with or without 5-Aza (10 μM) were intracerebrally inoculated on day 7 post-implantation of human U87ΔEGFR glioma cells (HBSS, n=6; oHSV, n=10; oHSV and 5-Aza, n=10, p=0.0027 for overall comparison and p = 0.064 for oHSV vs. oHSV and 5-Aza comparison, Fleming and Harrington test).
Results
The oHSV, rQNestin34.5, possesses a CpG rich island in the Nestin promoter/enhancer region upstream of ICP34.5
rQNestin34.5 (10, 11) is an oHSV that is currently in preclinical development for a possible clinical trial in humans with malignant glioma (Chiocca, E.A., unpublished). The engineered virus encompasses a Nestin promoter-enhancer sequence upstream of one copy of the γ1 34.5 gene that encodes for ICP34.5. This allows for robust replication and lysis in Nestin-expressing cells. Malignant gliomas express high levels of Nestin, likely in the glioma “stem like” cell subpopulation (14–17). rQNestin34.5 possesses other deletions: both endogenous copies of γ1 34.5 have been removed and the viral gene, encoding for ICP6 has also been partially deleted and its remaining sequences have been fused with an active copy of GFP. This defect in ICP6 restricts virus replication to cells with p16 defects (i.e. tumor cells) (4). When we analyzed this nestin promoter sequence, we realized that several CpG rich islands were present (Supplemental Figure 1). This information thus may mean that expression of γ1 34.5 could be limited by methylation of its promoter shortly after infection.
Demethylation of rQNestin34.5’s ICP34.5 promoter
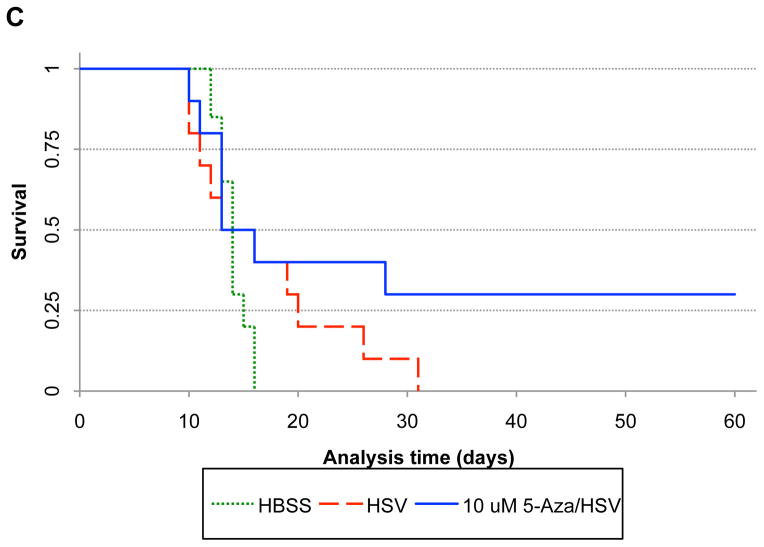
Based on the above finding, we hypothesized that this promoter would be methylated upon tumor cell infection and that treatment with demethylating agents could increase oHSV gene expression and replication. In fact, analysis of this promoter region after infection confirmed that it is hypermethylated (Figure 1A). There were 45 methylated cytidines (Supplemental Figure 1). Treatment with the demethylating agent 5-Azacytidine (5-Aza) led to promoter demethylation, as expected (Figure 1A). Treatment of infected cells with valproic acid (VPA), an HDAC inhibitor reported to lead to DNA demethylation (18–22), also led to Nestin promoter demethylation (Figure 1A) and both VPA and 5-Aza resulted in demethylation of almost all previously methylated cytidines (Figure 1B). Therefore, demethylation of the Nestin promoter in infected cells was possible with either 5-Aza or VPA.
Figure 1. Demethylation of CpG in the Nestin promoter after treatment with 5-Azacytidine (5-Aza) or Valproic Acid (VPA).
A) Methylation profile of viral DNA harvested from OG02 cells exposed to VPA (3 mM) for 20 hours or 5-Aza (5 μM) for 48 hours. After bisulfite modification, the bisulfite-modified DNA was analyzed by methylation specific PCR using the nestin promoter primers to detect methylated (left) and unmethylated (right) CpG (primers listed in supplemental figure 1). B) The number of methylated cytidines assayed after using the methylated (left) and unmethylated (right) primers is shown after infected OG02 cells were incubated with vehicle (−), VPA or 5-Aza. Each dot represents one assayed clone.
Demethylating agents enhance oHSV gene expression and replication
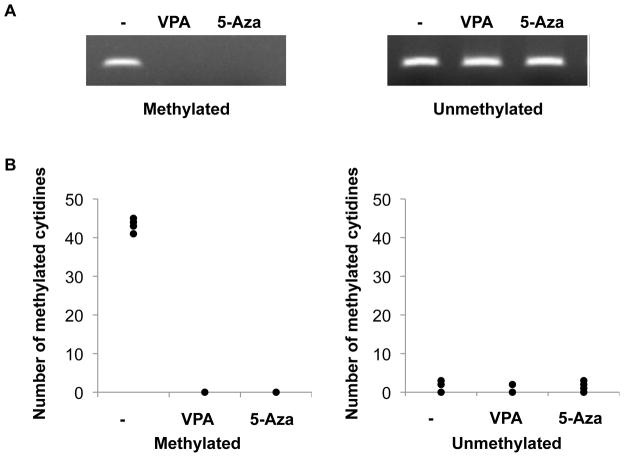
We then proceeded to determine if 5-Aza or VPA enhanced rQNestin34.5 gene expression and viral replication. Figure 2A shows that treatment of rQNestin34.5-infected cells with either drug led to a significant several fold increase in the expression of the viral genes γ1 34.5, ICP4, CTUS1, gC, CTRS1/2, CTRS3 and ICP27. rQNestin34.5 also expresses a GFP transgene. To test if 5-Aza also increased virus replication, we visualized GFP fluorescence from oHSV in glioma cells. There was a visible increase in the number and size of GFP-positive infected glioma cells in both U87ΔEGFR cells and primary GBM derived OG02 neurosphere cultured spheroids. In OG02 primary glioma neurospheres, 5-Aza doses were increased up to 6.2 μM (Figure 2B). At higher doses, 5-Aza led to cell death and reduced viral plaque formation. Similarly, there was a visible increase in the number and size of GFP-positive plaques, as 5-Aza doses were increased to 2.1 μM in the established human U87ΔEGFR glioma cell line (Figure 2C), with reduction at higher doses due to 5-Aza mediated cell death. Quantification of viral titers confirmed a significant increase in viral replication mediated by 5-Aza in both OG02 (Figure 2D) and U87ΔEGFR glioma cells (Figure 2E). Therefore, these results show that demethylating agents lead to an increase in oHSV gene expression and enhanced replication in established and primary glioma cells.
Figure 2. Demethylating agents increase oHSV replication.
A) Relative fold increase in the expression level of several oHSV genes was measured by quantitative RT-PCR 8h following oHSV infection (MOI 1.0) of primary human OG02 cells treated with VPA (3 mM) or 5-Aza (5 μM) vs. vehicle. B and C) 5-Aza treatment (number over each box represents concentration in 5-Aza) increases the intensity and number of GFP-expressing OG02 (panel B) and U87ΔEGFR (panel C) cells, one day following rQNestin34.5 infection (MOI 0.05). D and E) Relative titers of rQNestin34.5 were assayed, 3 day following virus infection (MOI 0.05) of OG02 (panel D) or U87ΔEGFR (panel E) cells, in the presence of increasing concentrations of 5-Aza. Each data point shown represents the mean ± standard deviation (SD) of three replicates for each sample.
rQNestin34.5 and 5-Azacytidine act synergistically in glioma cell killing
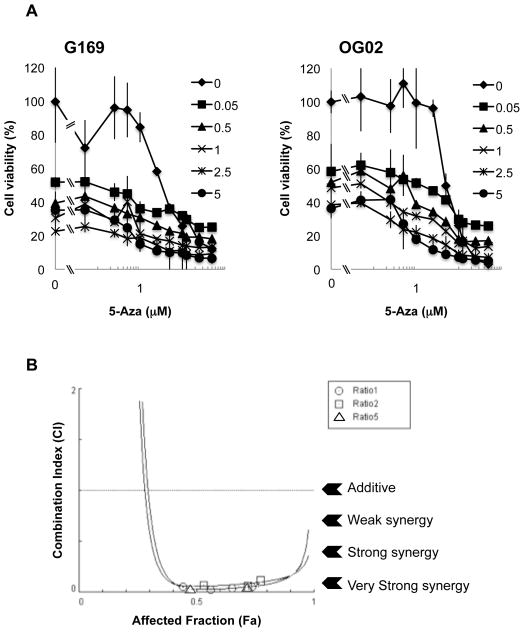
We sought to determine if there was evidence of pharmacologic synergy between oHSV and 5-Aza in glioma cell killing. First, we determined dose-effect combinations of 5-Aza and oHSV on two primary glioma cells, G169 and OG02. Figure 3A shows that oHSV alone led to 50% cell killing at a dose of 0.05 pfus in G169 and 0.5 pfus in OG02 primary glioma cells. 5-Aza alone led to a median effect on cell survival at a dose of approximately 4 μM for either cell (not shown). Chou-Talalay analyses were then performed (23, 24). Figure 3B shows that there was very strong synergy for the majority of affected fractions after combination treatment with 5-Aza and oHSV. These results thus provided confirmation that oHSV and 5-Aza act in a synergistic fashion in mediating cytotoxic effects against glioma cells.
Figure 3. Pharmacologic Synergy between 5-Aza and rQNestin34.5.
A) Cell viability (measured by MTT) of primary G169 and OG02 glioma cells was assayed 2 days after infection with rQNestin34.5 at different MOIs (0–5) in the presence of different doses of 5-Aza and oHSV. Data shown represents the mean ± SD of three replicates for each sample. B) The combination indexes of 5-Aza and oHSV were calculated using Chou-Talalay analyses. An IC 50 was selected to determine outcome. Each ratio group included MOIs of 0.05, 0.5, 1.0, 2.5, and 5.0 for oHSV. Ratio group 1 included 0.05, 0.5, 1.0, 2.5, or 5.0 μM of 5-Aza combined with each of the above MOIs for oHSV. Ratio group 2 included 0.1, 1.0, 2.0, 5.0, or 10 μM of 5-Aza with each MOI. Ratio group 5 included 0.25, 2.5, 5.0, 12.5, or 25 μM of 5-Aza with each MOI. CI’s were plotted against the affected fraction (Fa) ie. the percentage of cell death resulting from combination therapy. CI of <0.9 indicates synergy, CI between 0.9 and 1.1 is additive, and CI of >1.1 indicates antagonism.
rQNestin34.5 and demethylating agents lead to significantly increased survival in mice bearing orthotopic human gliomas
Based on the aforementioned findings, we sought to determine if the combination of oHSV and 5-Aza led to a significant increase in survival in an animal model of glioma. The rationale for carrying out the combination experiments was based on the in vitro data from previous figres showing that 5-Aza demethylated the nestin promoter/enhancer element leading to improved rQNestin34.5 replication. After orthotopic implantation of human G169 primary glioma cells in athymic nude mice, animals were treated at day 7 with either HBSS, 5-Aza (10 μM), oHSV (1×105 pfu/mouse), or the combination of 5-Aza (10 μM), and oHSV (1×105 pfu/mouse). The survival experiments include a heat-inactivated oHSV control but not replication-defective HSV vector, since we have previously shown that this vector alone lacks significant anticancer effects (25). Figure 4A shows that there was a significant increase in the survival of animals treated with the combination of 5-Aza and oHSV, compared to controls. Decitabine (5-Aza-2′-deoxycytidine) is being tested in clinical trials of humans suffering from a variety of cancers (www.clinicaltrials.gov). We thus asked whether the combination of Decitabine and oHSV was also beneficial. Figure 4B shows that indeed the combination of Decitabine (10 μM) and oHSV (1×105 pfu/mouse) led to a statistically significant increase in the survival of animals with orthotopic G169 gliomas. We then attempted to determine whether the combination of the oHSV and Decitabine was effective in a second human glioma model. After implanting human U87ΔEGFR glioma cells in the brains of athymic mice, we treated them with vehicle, oHSV alone, or oHSV and demethylating agent. Figure 4C shows that there was an overall significant increase in survivorship in treated animals when compared to controls. It should be noted that a pairwise comparison of the oHSV alone (1×105 pfu/mouse) vs. oHSV and 5-Aza (10 μM) treatment produced a survival difference that trended towards significance (P=0.062). Immunohistochemical analyses showed the presence of HSV antigen in oHSV treated animals, but with 5-Aza HSV antigen was visibly more widely distributed with increased necrosis (Supplemental figure 2). The data thus indicate that demethylating agents enhance the antiglioma effects of oHSV in vivo.
Discussion
Experimental attempts to improve the replication efficiency of OV in order to lyse more tumor cells require a detailed understanding of how the infecting virus and its host cell interact. In the context of the oncolytic HSV (oHSV), rQNestin34.5, where a heterologous promoter/enhancer is utilized to drive expression of a viral gene needed for robust replication it is not clear how the host cell would attempt to modify incoming viral DNA. In fact, in this report, we show that CpG islands in the nestin promoter/enhancer element become extensively methylated in glioma cells. In vitro, the addition of demethylating agents leads to demethylation of this promoter with improved replication of the virus and enhanced cytotoxicity. In fact, demethylating agents and rQNestin34.5 pharmacologically synergize in tumor lysis. In vivo, this leads to significantly improved survival of mice harboring orthotopic gliomas derived from a freshly explanted human tumor and a trend towards significantly improved survivorship in mice harboring glioma from a commonly established human glioma line. Therefore, the addition of demethylating agents to rQNestin34.5 virotherapy provides a beneficial therapeutic effect in a preclinical experimental setting.
In vitro, we also showed that the histone deacetylase inhibitor, VPA, led to demethylation of the nestin promoter. Previously, we had showed that VPA significantly improved OV replication and survival of mice with orthopic glioma xenografts (10, 12). The mechanism for this effect was reported by us to occur through VPA’s action on reducing STAT1’s activation of interferon signaling. We also have recently shown that VPA also enhances rQNestin34.5 replication and anticancer effects in vivo, by inhibiting antiviral immune responses such as the generation of Interferon γ by NK cells that rapidly infiltrate virally infected gliomas (26, 27). The finding of reducing the hypermethylation of the oHSV’s heterologous promoter uncovers another mechanism that plays a role in VPA’s improvement of rQNestin34.5 replication.
Recently, VPA has been reported to decrease DNA methyltransferases (DNMTs) protein levels and reduce DNMT enzyme activity (28). The role of epigenetics and the significance of aberrant gene regulation in the etiology of cancer is a well-established phenomenon. The hallmark of cancer epigenetics is aberrant DNA methylation consisting of global hypomethylation and regional hypermethylation of tumor suppressor genes (TSGs) by DNMTs (29, 30). Overexpression of DNMT1 and DNMT3B in gliomas leads to hypermethylation of various tumor suppressor genes, resulting in a lack of cell growth regulation and higher genomic instability. This hyper-activation of DNMTs may thus lead to reduced efficacy of oHSVs. In fact, there have been reports that the expression of transgenes in tumors after viral-mediated delivery decreased, but that 5-Aza and/or Trichostatin A reversed this, implying gene methylation as a silencing mechanism for oncolytic viruses in tumors (31). However, the role of methylation in silencing HSV genes is controversial. Kubat et al reported that the HSV1 genome was not hypermethylated after infection of rabbit skin cells (9). Our findings showed that the nestin promoter CpG islands were extensively methylated. Although this could be explained because it is heterologous, Figure 2 shows that the transcription of several oHSV genes was also enhanced by 5-Aza or VPA. One explanation may be that the enhanced transcription of the nestin promoter driven γ1 34.5 gene also led to increased transcription of other viral genes. The alternative explanation is that these epigenetic reagents demethylated CpG islands of endogenous HSV1 promoters, leading to the observed increased transcription. In fact, in support of the latter explanation, the methylation status of the endogenous ICP4 promoter in oHSV-infected glioma cells also showed extensive methylation with expected de-methylation by 5-Aza (data not shown).
Demethylating agents could also affect OV replication by reversing the hypermethylation status of promoter/enhancers of interferon and interferon response genes. However, this is unlikely to be operative in our model because we have observed that 5-Aza enhances rQNestin34.5 replication even in the human U87 glioma cell line that is interferon-insensitive (data not shown), because of deletion of chromosomal loci where such genes reside and not because of interferon gene promoter hypermethylation (32, 33) Instead, VSV action in breast cancer cells has been reported to be enhanced by DNA demethylating agents not because they demethylated the IRF5 and IRF7 promoters, but rather through another unknown mechanism (34). To determine in vivo effects of the combination therapy on human glioma animal models we had to utilize athymic mice. Possible effects of demethylating agents on T cells in immunocompetent animal models will thus require further experimentation, although we have minimized for this in our model by intratumoral, local administration of drug. In fact, to minimize possible systemic effects of demethylating agents and maximize effects on the oHSV itself, we also administered the two together intratumorally instead of administering the drug systemically. Local administration of agents in humans with malignant gliomas is possible via polymers, endovascular catherization or convection-enhanced delivery. Nevertheless, although the in vivo data confirms that the combination of oHSV and demethylating agent therapy was beneficial, it is possible that the mechanism for the observed in vivo benefit may be different than direct enhancement of rQNestin34.5 replication by demethylation of the Nestin promoter/enhancer.
The discovery of significant in vitro pharmacologic synergy in glioma cells when rQNestin34.5 was combined with 5-Aza extended the finding of a significant survival effect in mice with intracranial gliomas treated with the combination. When the affected cell fraction was low (less than 0.3), there was some evidence for antagonism. This could indicate a situation where low doses of demethylating agent may be stimulating glioma cell proliferation (i.e. by demethylation of oncogenes) while the replicative kinetics of the low initial dose of oHSV have not had sufficient time to generate sufficient numbers of viral progeny to “catch up” with the proliferating tumor cells.
Decitabine is being widely used in clinical trials for subjects afflicted with a variety of cancers and our findings may provide justification for further exploration of the combination of decitabine with rQNestin34.5. We are in the process of filing an investigational new drug (IND) use for rQNestin34.5 for a phase 1 clinical trial in recurrent glioma patients. The findings from this report indicate that further biodistribution and toxicity data in animals should be carried out to evaluate if Decitabine can be justifiably added to rQNestin34.5 in subsequent clinical trials, particularly since demethylating agents could reactivate latent viruses. In addition, the schedule of demethylating agent treatment with oHSV will need further detailed analysis.
There have been now several instances/reports of drugs that can be used to pharmacologically enhance viral oncolysis (35). The mechanism of action of such pharmacologic enhancement is dependent on the drugs’ mode of action. For instance, some will modulate the immune system to reduce immune cell-mediated clearance of injected OVs or OV-infected tumor cells. Other drugs will up-regulate tumor cell pathways that can be also utilized by the OV for improved replication. Still others will modulate the tumor microenvironment to facilitate OV distribution or survival. In this context, the findings in this report represent the first example of a drug that acts directly on the viral genome to improve its anticancer effect. As the field of epigenetic therapy progresses, it will be interesting to determine if other agents that act on other aspects of the epigenome will also modulate OV DNA to improve its replicative ability.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Oncolytic viruses (OV), such as those based on herpes simplex virus type 1 (HSV1), have and are being tested in clinical trials for malignant glioma. We have generated a novel tumor-specific OV (rQNestin34.5) and are in the process of testing it in a human clinical trial for recurrent malignant glioma. In this OV, a nestin promoter drives expression of a HSV1 viral gene needed for robust viral replication and glioma lysis. We have discovered that the nestin promoter becomes extensively methylated and silenced after glioma infection. Addition of demethylating drugs to rQNestin34.5 leads to re-expression of viral genes, enhanced viral replication and improved glioma lysis. This translates to significantly increased efficacy in animal models of gliomas. Therefore, demethylating agents, such as decitabine, may improve the clinical efficacy of rQNestin34.5 against human gliomas.
Acknowledgments
This work was supported by 7U01NS061811 (to E.A.C.), CA069246 (to E.A.C.), CA163205 (to E.A.C., B.K.), and CA150153, 1NS064607, and 050NS045758 (to B.K.)
Footnotes
The authors report no conflicts of interest.
Literature Cited
- 1.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–50. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 2.Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9:505–17. doi: 10.1586/ern.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohyeldin A, Chiocca EA. Gene and viral therapy for glioblastoma: a review of clinical trials and future directions. Cancer J. 2012;18:82–8. doi: 10.1097/PPO.0b013e3182458b13. [DOI] [PubMed] [Google Scholar]
- 4.Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27:4249–54. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre- and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergbauer M, Kalla M, Schmeinck A, Gobel C, Rothbauer U, Eck S, et al. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog. 2010;6:e1001114. doi: 10.1371/journal.ppat.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799:246–56. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowski DL, Thompson HW, Bloom DC. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. J Virol. 2009;83:8173–81. doi: 10.1128/JVI.00686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubat NJ, Tran RK, McAnany P, Bloom DC. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J Virol. 2004;78:1139–49. doi: 10.1128/JVI.78.3.1139-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–9. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 11.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–8. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 12.Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–55. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 13.Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. 2. Springer-Verlag; 2003. [Google Scholar]
- 14.Schiffer D, Annovazzi L, Caldera V, Mellai M. On the origin and growth of gliomas. Anticancer Res. 2010;30:1977–98. [PubMed] [Google Scholar]
- 15.Xie Z. Brain tumor stem cells. Neurochem Res. 2009;34:2055–66. doi: 10.1007/s11064-009-0079-5. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33:2407–15. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 17.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–73. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 18.Detich N, Bovenzi V, Szyf M. Valproate induces replication-independent active DNA demethylation. J Biol Chem. 2003;278:27586–92. doi: 10.1074/jbc.M303740200. [DOI] [PubMed] [Google Scholar]
- 19.Dong E, Chen Y, Gavin DP, Grayson DR, Guidotti A. Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics. 2010;5:730–5. doi: 10.4161/epi.5.8.13053. [DOI] [PubMed] [Google Scholar]
- 20.Milutinovic S, D’Alessio AC, Detich N, Szyf M. Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis. 2007;28:560–71. doi: 10.1093/carcin/bgl167. [DOI] [PubMed] [Google Scholar]
- 21.Reid G, Metivier R, Lin CY, Denger S, Ibberson D, Ivacevic T, et al. Multiple mechanisms induce transcriptional silencing of a subset of genes, including oestrogen receptor alpha, in response to deacetylase inhibition by valproic acid and trichostatin A. Oncogene. 2005;24:4894–907. doi: 10.1038/sj.onc.1208662. [DOI] [PubMed] [Google Scholar]
- 22.Van Nifterik KA, Van den Berg J, Slotman BJ, Lafleur MV, Sminia P, Stalpers LJ. Valproic acid sensitizes human glioma cells for temozolomide and gamma-radiation. J Neurooncol. 2012;107:61–7. doi: 10.1007/s11060-011-0725-z. [DOI] [PubMed] [Google Scholar]
- 23.Aghi M, Kramm CM, Chou TC, Breakefield XO, Chiocca EA. Synergistic anticancer effects of ganciclovir/thymidine kinase and 5-fluorocytosine/cytosine deaminase gene therapies. J Natl Cancer Inst. 1998;90:370–80. doi: 10.1093/jnci/90.5.370. [DOI] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa T, Chiocca EA. Comparative analyses of transgene delivery and expression in tumors inoculated with a replication-conditional or -defective viral vector. Cancer Res. 2001;61:5336–9. [PubMed] [Google Scholar]
- 26.Alvarez-Breckenridge CA, Yu J, Kaur B, Caligiuri MA, Chiocca EA. Deciphering the Multifaceted Relationship between Oncolytic Viruses and Natural Killer Cells. Advances in virology. 2012;2012:702839. doi: 10.1155/2012/702839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Breckenridge CA, Yu J, Price R, Wei M, Wang Y, Nowicki MO, et al. The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol. 2012;86:4566–77. doi: 10.1128/JVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Molecular pharmacology. 2009;75:342–54. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foltz G, Yoon JG, Lee H, Ryken TC, Sibenaller Z, Ehrich M, et al. DNA methyltransferase-mediated transcriptional silencing in malignant glioma: a combined whole-genome microarray and promoter array analysis. Oncogene. 2009;28:2667–77. doi: 10.1038/onc.2009.122. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran G, Shanmuganandam K, Bendre A, Muzumdar D, Goel A, Shiras A. Epigenetic regulation of DNA methyltransferases: DNMT1 and DNMT3B in gliomas. J Neurooncol. 2011;104:483–94. doi: 10.1007/s11060-010-0520-2. [DOI] [PubMed] [Google Scholar]
- 31.Bartoli A, Fettucciari K, Fetriconi I, Rosati E, Di Ianni M, Tabilio A, et al. Effect of trichostatin a and 5′-azacytidine on transgene reactivation in U937 transduced cells. Pharmacol Res. 2003;48:111–8. [PubMed] [Google Scholar]
- 32.James CD, He J, Carlbom E, Nordenskjold M, Cavenee WK, Collins VP. Chromosome 9 deletion mapping reveals interferon alpha and interferon beta-1 gene deletions in human glial tumors. Cancer Res. 1991;51:1684–8. [PubMed] [Google Scholar]
- 33.Miyakoshi J, Dobler KD, Allalunis-Turner J, McKean JD, Petruk K, Allen PB, et al. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990;50:278–83. [PubMed] [Google Scholar]
- 34.Li Q, Tainsky MA. Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One. 2011;6:e28683. doi: 10.1371/journal.pone.0028683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–40. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.