Abstract
Background
The status of left ventricle in sickle cell anemia presenting in sickle crisis and follow up has been minimally studied in past. To determine the left ventricular (LV) myocardial performance in these patients, we performed the study to assess two dimensional strains imaging which allowed a rapid and an accurate analysis of global and regional LV myocardial performance in longitudinal, radial, and circumferential directions.
Methods
In this prospective study, 2-dimensional echocardiography (2DE) images of the LV were obtained in 52 subjects which included 32 patients (23 ± 8yrs, 16 male) with homozygous sickle cell anemia (SCA) in sickle cell crisis and 20 healthy controls (23 ± 5 yrs, 11 male) using apical 4-chamber and parasternal short-axis at the basal, mid, and apical levels. Of these 32 patients, 2DE was performed again in 18 patients in follow up (8 months ± 5 days). Longitudinal, circumferential and radial strains (LS, CS and RS respectively) were quantified and compared in an 18-segment model using a novel speckle tracking system (2D Cardiac Performance Analysis, TomTec Imaging System, Munich, Germany).
Results
There was no significant difference in LV ejection fraction between both the groups (59.32 ± 12.6 vs. 52.3 ± 7.9; p-value > 0.05). In comparison with normal controls and follow up of sickle cell patients, peak LS was significantly attenuated in the subendocardial and subepicardial regions during sickle cell crisis (p < 0.05). However, a significant reduction in circumferential strain was evident only in subepicardial region (p < 0.001). Also patients in sickle cell crisis showed significantly higher radial strain parameters than controls (p < 0.001).
Conclusion
Patients with SCA presenting in sickle cell crisis have reduced longitudinal shortening. LV myocardial performance remains unaltered due to relatively preserved circumferential shortening and increased radial thickening.
Keywords: Sickle cell crisis, Speckle tracking, Left ventricular function
1. Background
Individuals with homozygous sickle cell anemia (SCA) (SS pattern) show frequent evidence of cardiovascular system abnormalities which include abnormal systolic and diastolic ventricular function.1,2 Low haemoglobin levels in sickle cell anemia are associated with an elevated cardiac output at rest, cardiomegaly, and frequently, heart murmurs, due to an increase in stroke volume.3,4 It has been postulated that excessive work load chronically imposed on heart by the cardiac output, along with the tendency of the sickle cells to occlude vessels in the systemic, pulmonary and coronary circulation during crisis phase may result in LV dysfunction.5 LV diastolic dysfunction occurs earlier in the development of overall cardiac dysfunction, and its presence is an independent risk factor for mortality among adults with SCA.6
For the assessment of LV myocardial performance ejection fraction (EF), tissue Doppler imaging (TDI), Doppler strain and 2D strain have been used widely.7,8 Subclinical changes in LV function in SCA can be identified by quantifying myocardial strain, a dimensionless measurement of deformation, expressed as a fractional or percentage change from an object's original dimension. 2D speckle tracking has recently emerged as a novel echocardiographic technique for rapid, offline, bedside analysis of regional LV strains in the longitudinal, radial, and circumferential directions.9–12 This technique analyzes myocardial motion by tracking natural acoustic reflections and interference patterns seen in 2D echocardiography images and has been validated with measurements obtained by sonomicrometry and magnetic resonance imaging. Assessment of regional LV function during the phase of sickle cell crisis has not been studied in detail. The aim of this study was to assess the ability of subtle differences in the LV strain patterns in the longitudinal, radial and circumferential directions to characterize features of subclinical LV dysfunction in patients presenting with sickle cell crisis.
2. Methods
2.1. Study population
In the present study, 32 sickle cell patients (SS pattern) (23 ± 8yrs, 16 male) presenting with sickle cell crisis and 20 healthy (AA pattern) controls (23 ± 5 yrs, 11 male) were included. Of these 32 patients, 18 patients (25 ± 4yrs, 8 male) of sickle cell anemia could come for follow up after 8 months ± 5 days. Patients with established coronary artery disease, echocardiography evidence of regional or global wall motion abnormalities, atrial fibrillation, valvular heart disease, diabetes mellitus, chronic obstructive airway disease and hypertrophic cardiomyopathy were excluded. Written informed consent was obtained from the patients and the study was approved by the institutional review board.
2.2. Echocardiography
A complete 2DE was performed in all patients of sickle cell presenting in crisis and on follow up and also control group. A commercially available ultrasound transducer and equipment (S4-2 probe, HD7, Philips) was used. The same experienced operator performed all acquisitions with the patients in the left lateral position. Basic measurements included LV wall thickness by M-mode and LV diameter by 2D. LV volumes and LVEF were measured using the modified biplane Simpson method as recommended by the American Society of Echocardiography.13 To determine the timing of cardiac events, mitral inflow and LV outflow were recorded using pulsed Doppler echocardiography. 2DE images of the LV were acquired in apical 4-chamber and parasternal short-axis at the basal, mid, and apical levels with same ultrasound machine. Three consecutive cardiac cycles loops were recorded at end expiration. The frame rate was kept between 70 Hz to 100 Hz. Longitudinal (LS), circumferential (CS) and radial strains (RS) were quantified in an 18-segment model using a novel speckle tracking system (2D Cardiac Performance Analysis, TomTec Imaging System, Munich, Germany). This is an offline myocardial performance analysis system which uses dicom files from any echocardiography machine and can track the 2D speckles in the myocardium. Once tracked, it generates waveforms from which various strain values can be derived.
2.3. Statistics
Continuous variables were expressed as mean ± SD. The differences between groups were analyzed by independent student t tests (MedCalc 11.2 software MariaKerke, Belgium). Annova and paired t – test were used to assess the level of significance in the follow up group. Correlations between variables were tested by Pearson or Spearman correlation tests where appropriate. A p-value < 0.05 was considered to be significant. Univariate regression models were used to examine the relations between echocardiography variables and EF. For the assessment of adjusted associations, we used multiple regression with the investigator variable (EF) and echocardiography variables as forced entry variables.
3. Results
The study population consisted of 52 subjects, including 20 healthy (AA pattern) controls (23 ± 5 yrs, 11 male) and 32 sickle cell patients (SS pattern) (23 ± 8 yrs, 16 male) presenting with sickle cell crisis. Of these 32 patients, 18 patients could come in follow up period of 8 months ± 5 days. There were no significant differences in age and gender between sickle crisis patients, control subjects and sickle cell patients during follow up. The baseline clinical and echocardiography characteristics are shown in Table 1. Patients in sickle cell crisis had significant larger LV systolic diameters (p < 0.05) as compared to control group, which decreased to non-significant diameters on follow up. There was no significant difference in LV size in diastole in all the three groups. Patients in sickle cell crisis had significant higher ratio of E/A and E/e′ as compared to the control group (p < 0.05). E/e′ was also significantly higher during follow up, but E/A reduced to non-significant level during follow up. Also patients in sickle cell crisis and during follow up had significant larger left atrial (LA) volumes than the control group (p < 0.05). There were no significant differences in LV global EF between the groups.
Table 1.
Summary statistics for different echocardiographic parameters and their comparison in control and sickle cell groups.
| Characteristic | Mean ± SD |
p-value |
||||
|---|---|---|---|---|---|---|
| Control (20) | Sickle cell (32) |
Control (20) vs. |
Control (20) vs. |
Sickle cell: during crisis (32) vs. |
||
| During Crisis (32)c | During follow up (18) | During Crisis (32)a | During follow up (18)a | During follow up (18)b | ||
| Age (years) | 23.2 ± 5.38 | 25.38 ± 8.38 | 25.38 ± 8.38 | – | – | – |
| Men/women (No.) | 11/9 | 8/10 | 8/10 | – | – | – |
| Height (cm) | 163.11 ± 12.29 | 146 ± 12.20 | 146 ± 12.20 | – | – | – |
| Weight (kg) | 58.33 ± 8.69 | 41.44 ± 8.84 | 41.38 ± 8.81 | <0.001* | <0.001* | 0.331 |
| Systolic blood pressure (mmHg) | 120.0 ± 6.0 | 108.66 ± 5.86 | 109.44 ± 5.39 | <0.001* | <0.001* | 0.202 |
| Diastolic blood pressure (mmHg) | 78.0 ± 4.0 | 75 ± 5.14 | 75.88 ± 4.92 | 0.234 | 0.494 | 0.177 |
| Aorta (cm) | 2.36 ± 0.1 | 2.46 ± 0.29 | 2.52 ± 0.26 | 0.146 | <0.05** | 0.063 |
| Left atrium (cm) | 2.63 ± 0.1 | 3.14 ± 0.48 | 2.96 ± 0.39 | <0.001* | <0.05** | 0.073 |
| LV end – diastolic dimension (cm) | 4.36 ± 0.14 | 4.33 ± 0.52 | 4.24 ± 0.58 | 0.870 | <0.001* | 0.145 |
| LV end-systolic dimension (cm) | 2.75 ± 0.08 | 3.02 ± 0.49 | 2.86 ± 0.80 | <0.05** | 0.123 | 0.18 |
| LA volume (cm3) | 23.3 ± 2.1 | 36.3 ± 3.2 | 34.2 ± 2.3 | <0.05* | <0.05* | 0.852 |
| Global ejection fraction (%) | 59.32 ± 12.64 | 52.25 ± 9.61 | 55.34 ± 6.21 | 0.074 | 0.245 | <0.05** |
| E peak velocity (cm/s) | 72.2 ± 1.84 | 119.61 ± 25.27 | 83.65 ± 15.73 | <0.001* | <0.05** | <0.05** |
| A peak velocity (cm/s) | 63.6 ± 2.35 | 59.19 ± 17.34 | 57.12 ± 12.81 | 0.344 | 0.063 | 0.465 |
| E/A | 1.16 ± 1.35 | 2.11 ± 12.13 | 1.36 ± 4.96 | <0.001* | 0.512 | 0.433 |
| E/e′ | 6.75 ± 6.13 | 8.12 ± 4.23 | 9.77 ± 4.66 | <0.05* | <0.05* | 0.324 |
| e′ (Septal) (cm/s) | 11.72 ± 0.8 | 11.92 ± 2.66 | 8.66 ± 1.88 | 0.799 | <0.001* | <0.05** |
| A′ (septal) (cm/s) | 11.0 ± 0.4 | 8.86 ± 2.16 | 8.36 ± 1.64 | <0.001* | <0.001* | 0.207 |
| S′ (septal) (cm/s) | 13.47 ± 0.5 | 9.42 ± 1.62 | 9.59 ± 1.58 | <0.001* | <0.001* | 0.083 |
| e′ (lateral) (cm/s) | 12.44 ± 0.6 | 18.33 ± 3.32 | 14.30 ± 2.81 | <0.001* | <0.05** | <0.05** |
| A′ (lateral) (cm/s) | 10.84 ± 1.93 | 9.88 ± 2.43 | 10.21 ± 2.00 | 0.386 | 0.604 | 0.083 |
| S′ (lateral) (cm/s) | 14.12 ± 0.56 | 12.18 ± 2.57 | 13.57 ± 2.09 | <0.05** | 0.260 | <0.05** |
*p < 0.0001; **p < 0.05.
Statistical significance tested at 5% level using t-test for independent samples.
Statistical significance tested at 5% level using paired t-test.
Includes same cases as appearing in follow up.
In comparison with normal controls, peak LS was significantly attenuated in the subendocardial regions during sickle cell crisis (p < 0.05) (Table 2). There was no significant difference in peak LS during follow up and control group (p = 0.422). A significant reduction in circumferential strain was observed in subepicardial region only in sickle cell patients presenting in crisis which persisted during follow up also (p < 0.001). However, circumferential strain was higher in subendocardial region in crisis and follow up, but this change was not significant. Patients in sickle cell crisis showed significantly higher radial strain parameters than controls (p < 0.001) (Graph 1), which came to non-significant levels during follow up.
Table 2.
Comparison of left ventricular mechanics between control and sickle cell groups.
| Characteristic | Mean ± SD |
p-value |
||||
|---|---|---|---|---|---|---|
| Control group (20) | Sickle cell (32) |
Control (20) vs. |
Control (20) vs. |
Sickle cell: |
||
| During Crisis (32)c | During follow up (18) | During crisis (32)a | During follow up (18)a | During Crisis (32) vs. During follow up (18)b | ||
| Longitudinal strain | ||||||
| Subendocardial (%) | −20.17 ± 5.48 | −16.40 ± 4.78 | −19.04 ± 1.98 | <0.05** | 0.422 | <0.05** |
| Subepicardial (%) | −17.68 ± 4.95 | −19.13 ± 6.59 | −18.80 ± 4.05 | 0.477 | 0.474 | 0.679 |
| Circumferential strain | ||||||
| Subendocardial (%) | −23.98 ± 8.08 | −21.02 ± 5.43 | −20.74 ± 4.07 | 0.214 | 0.143 | 0.622 |
| Subepicardial (%) | −6.47 ± 1.80 | −14.85 ± 4.54 | −11.74 ± 2.81 | <0.001* | <0.001* | <0.001** |
| Radial (%) | 21.97 ± 10.37 | 41.86 ± 22.27 | 27.11 ± 5.98 | <0.001* | 0.082 | <0.001** |
*p < 0.0001; **p < 0.05.
Statistical significance tested at 5% level using t-test for independent samples.
Statistical significance tested at 5% level using paired t-test.
Includes same cases as appearing in follow up.
Graph 1.
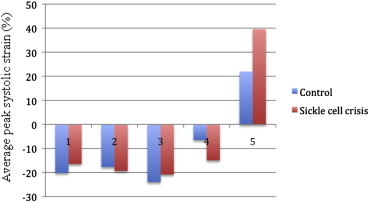
Showing comparison of LV mechanics between control (blue) and sickle cell patients during crisis (red). 1 – Subendocardial Longitudinal strain, 2- Subepicardial Longitudinal strain, 3- Subendocardial circumferential strain, 4- Subepicardial circumferential strain, 5- Radial strain.
The impact of six echo strain parameters, henceforth, referred as independent variables (IVs), was studied on LVEF using data from both the groups. Univariate regression analysis revealed that only subendocardial longitudinal strain during sickle cell crisis had a significant positive influence on EF (p < 0.05, R = 0.38) (Table 3).
Table 3.
Univariate regression with EF.
| Echo parameter (variable) | b | SE(b) | p-value | Adj R-square |
|---|---|---|---|---|
| SL Endo | −1.328 | 0.244 | 1.97E-06 | 0.38 |
| SL Epi | −0.645 | 0.272 | 0.022 | 0.09 |
| SL Rad | 0.098 | 0.068 | 0.158 | 0.02 |
| S Endo | −0.369 | 0.205 | 0.08 | 0.05 |
| S Epi | 0.359 | 0.241 | 0.144 | 0.02 |
| S Rad | −0.039 | 0.075 | 0.604 | 0.006 |
Ejection fraction (EF); Standard error (SE).
Multivariate regression analysis was done to know the prediction accuracy of EF considering all echocardiography strain variables. The results obtained are shown in Table 4, which revealed that echocardiography strain variables like subendocardial longitudinal strain, radial and circumferential strain were all significantly affecting independently the LVEF (R = 0.67, p < 0.001). There was no significant correlation between subepicardial longitudinal strain and EF in multivariate analysis.
Table 4.
Multiple regression with EF.
| Echo parameter | b | SE (b) | p-value |
|---|---|---|---|
| SL Endo | −1.213 | 0.464 | 0.012 |
| SL Epi | 0.019 | 0.405 | 0.961 |
| SL Rad | 0.273 | 0.051 | 0.000 |
| S Endo | −0.550 | 0.220 | 0.016 |
| S Epi | 0.578 | 0.263 | 0.034 |
| S Rad | −0.160 | 0.063 | 0.015 |
| Intercept | 23.903 | 4.757 | 0.000 |
| R-square: | 0.669 | ||
| Adj R-Square: | 0.620 | ||
| Model p-value: | 0.000 |
Ejection fraction (EF); Standard error (SE).
4. Discussion
In this study, conventional echocardiography findings revealed, increased LV dimensions in systole and LA volumes in sickle cell patients both during crisis and follow up. The findings in the present study are in conjunction to findings in the earlier studies where they have reported larger LA volumes in SCA suggesting evidence of diastolic dysfunction.14,15 Also the filling pressures were significantly higher in sickle cell anemia during crisis and follow up than the control group.
Also in the present study, LVEF by conventional echocardiography measurements in patients in sickle cell crisis was normal. Conventional 2-dimensional, M-mode, and spectral Doppler echocardiography have several limitations in identifying and measuring early ventricular dysfunction. For example, measuring LV fractional shortening (FS) and LVEF were relatively simple, but inter-observer and intra-observer variability were high.16 Also, the global LVEF and FS only reflect the global cardiac contractility and do not take regional systolic abnormalities into consideration. To explain this need of understanding the regional myocardial deformation properties, our study was undertaken.
Cardiovascular abnormalities are apparent in SCA.17 To our knowledge, this is the first comprehensive study to assess the LV regional myocardial deformation properties in sickle cell anemia patients presenting with sickle cell crisis and during follow up. The impact of SCA on LV systolic function is conflicting depending on the echocardiography method used.14,18,19 Lamers et al20 found abnormal LV systolic function in children with SCD using preload-independent end-systolic LV wall stress–velocity measurements. In contrast, Covitz et al21 reported normal LV function based on normal LV fractional shortening, which is a preload dependent parameter, in a large study of 191 children and young adults with SCD. Denenberg et al22 were the first to report about the cardiac function in sickle cell anemia. They demonstrated by echocardiography that heart failure in SCA was due to a pseudo normalization of the indices of the ejection phase (EF and FS) by load alterations. These indices were the only ones used to assess the systolic function in patients with SCA.
In patients with abnormal diastolic LV filling, LV systolic function is commonly considered normal if the global EF and FS are normal.23–25 Heart failure due to LV dysfunction in sickle cell patients is a late finding.19 LV dysfunction in SCA may be the result of myocardial ischemia, fibrosis, iron deposition and ventricular hypertrophy.25
Ischemic myocardial changes not related to coronary atherosclerotic disease have been found in autopsy specimens of adults with SCD.25 Myocardial ischemia has been documented in children with SCD. Older children with SCD tend to have more extensive ischemic defects than younger patients.26 Haywood27 reported that myocardial dysfunction may be related to long-term recurrent myocardial microinjuries and metabolic tissue alterations aggravated by sickle cell-related vaso-occlusive crises, including acute chest syndrome.
Evidence for a specific myocardial lesion called the myocardial factor attributable to the sickle cell disease is ambiguous.28 Our study focuses on the regional myocardial systolic dysfunction in patients presenting in sickle cell crisis despite normal global systolic parameters by the use of 2D speckle tracking echocardiography. In recent years, 2D speckle tracking echocardiography is increasingly being used in the assessment of global and regional ventricular function. Being angle-independent, reproducible and being able to provide quantitative data further increases the utility of 2D speckle tracking echocardiography in evaluation of ventricular functions in clinical settings.26,27 The tracking system is based on gray-scale B-mode images and is obtained by automatic measurement of the distance between two pixels of an LV segment during the cardiac cycle, independent of the angle of insonation. The integration of 2D speckle tracking with real-time cardiac ultrasound imaging overcomes some of the limitations of previous work in the field and has the potential to provide a unified framework to more accurately quantify the regional and global function of the LV. 2D speckle tracking holds promise to reduce inter- and intra-observer variability in assessing regional LV function and improve patient care while reducing health care costs by early identification of subclinical disease.
In the present study, patients in sickle cell crisis had significantly lower strain parameters assessed by 2D speckle tracking inspite of having preserved LV global EF.
Our study reveals no significant difference in LV volumes between patients in sickle cell crisis and control groups which is unlike previous studies.27 The possible reason for this difference was that our patient group were in the phase of sickle cell crisis.
The longitudinal fiber shortening was significantly less in sickle cell patients presenting in crisis in the present study than the corresponding segments in the control group. This longitudinal fiber shortening normalized in the follow up group of sickle cell anemia as there was no significant difference as compared to control group. This finding that has been reported for the first time by us in sickle cell anemia presenting in crisis.
The subendocardial longitudinal-oriented myocardial fibers have shown to be particularly vulnerable to ischemia leading to a dominant decrease in shortening in the longitudinal axis.28–31 Patients in sickle cell crisis showed significantly higher radial thickening than control and follow up group. Also a significant reduction in circumferential strain was evident only in subepicardial region in both sickle cell crisis and follow up. This phenomenon may be helpful in explaining the compensatory mechanism produced by circumferential and radial fibers to maintain LV systolic function in this group.
Study limitations
The present study is a single-center observational study. It is limited by the relative small sample size. Also, the LS was assessed in apical four chamber view.
5. Conclusion
Patients with SCA presenting in sickle cell crisis have reduced longitudinal shortening which gets normalized on follow up when they are not in crisis phase. However, LV myocardial performance remains unaltered due to relatively preserved circumferential shortening and increased radial thickening. In the detection of subclinical LV dysfunction in patients presenting with sickle cell crisis, 2D speckle tracking echocardiography appears to be useful.
Conflicts of interest
All authors have none to declare.
References
- 1.Ng M.L., Liegman J., Anslovar J., Gross S. Cardiovascular findings in children with sickle cell anemia. Dis Chest. 1967;52:78–799. doi: 10.1378/chest.52.6.788. [DOI] [PubMed] [Google Scholar]
- 2.Raj A.B., Condurache T., Bertolone S., Williams D., Sobczyk W. Quantitative assessment of ventricular function in sickle cell disease: effect of ling term erythocytapheresis. Pediatr Blood Cancer. 2005;45:976–981. doi: 10.1002/pbc.20521. [DOI] [PubMed] [Google Scholar]
- 3.Varat M.A., Adolph R.J., Fowler N.O. Cardiovascular effects of anemia. Am Heart J. 1972;83:415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 4.Uzsoy N.K. Cardiovascular findings in patients with sickle cell anemia. Am J Cardiol. 1964;13:320–328. doi: 10.1016/0002-9149(64)90447-3. [DOI] [PubMed] [Google Scholar]
- 5.Platt O.S., Brambilla D.J., Rosse W.F. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 6.Sachdev V., Machado R.F., Shizukuda Y. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007 Jan 30;49(4):472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marwick T.H. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G. Response to “non-doppler two-dimensional strain imaging byechocardiography-from technical considerations to clinical applications”. J Am Soc Echocardiogr. 2007;20:1020. doi: 10.1016/j.echo.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Reisner S.A., Lysyansky P., Agmon Y., Mutlak D., Lessick J., Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Helle-Valle T., Crosby J., Edvardsen T. New non-invasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 11.Rovner A., de las Feuntas L., Waggoner A.D. Characterization of left ventricular diastolic dysfunction in hypertension by use of Doppler tissue imaging and color M –mode techniques. J Am Soc Echocardiogr. 2006;19:872–879. doi: 10.1016/j.echo.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Nakai H., Takeuchi M., Nishikage T., Kokumai M., Otani S., Lang R.M. Effect of aging on twist-displacement loop by 2-dimensional speckle tracking imaging. J Am Soc Echocardiogr. 2006;19:880–885. doi: 10.1016/j.echo.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Caldas M.C., Meira Z.A., Barbosa M.M. Evaluation of 107 patients with sickle cell anemia through tissue Doppler and myocardial performance index. J Am Soc Echocardiogr. 2008;21:1163–1167. doi: 10.1016/j.echo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Zilberman M.V., Du W., Das S., Sarnaik S.A. Evaluation of left ventricular diastolic function in pediatric sickle cell disease patients. Am J Hematol. 2007;82:433–438. doi: 10.1002/ajh.20866. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz S.E., Easley K.A., Orav E.J. Reliability of multicenter pediatric echocardiography measurements of left ventricular structure and function: the prospective P(2)C(2) HIV study. Circulation. 2001;104:310–316. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamers L., Ensing G., Pignatelli R. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J Am Coll Cardiol. 2006 June 6;47(11):2283–2288. doi: 10.1016/j.jacc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Akgul F., Yalçin F., Babayiğit C., Seyfeli E., Seydaliyeva T., Gali E. Right ventricular and pulmonary function in sickle cell disease patients with pulmonary hypertension. Pediatr Cardiol. 2006;27:440–446. doi: 10.1007/s00246-006-1257-8. [DOI] [PubMed] [Google Scholar]
- 19.Batra A.S., Acherman R.J., Wong W.Y. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol. 2002;70:306–312. doi: 10.1002/ajh.10154. [DOI] [PubMed] [Google Scholar]
- 20.Lamers L., Ensing G., Pignatelli R. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J Am Coll Cardiol. 2006;47:2283–2288. doi: 10.1016/j.jacc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Covitz W., Espeland M., Gallagher D., Hellenbrand W., Leff S., Talner N. The heartin sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD) Chest. 1995;108:1214–1219. doi: 10.1378/chest.108.5.1214. [DOI] [PubMed] [Google Scholar]
- 22.Denenberg B.S., Criner G., Jones R., Spann J.F. Cardiac function in sickle cell anemia. Am J Cardiol. 1983;51:1674–1678. doi: 10.1016/0002-9149(83)90208-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Cao T., Duan Y., Yuan L., Wang Z. Velocity vector imaging in assessing myocardial systolic function of hypertensive patients with left ventricular hypertrophy. Can J Cardiol. 2007;23(12):957–961. doi: 10.1016/s0828-282x(07)70857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edvardsen T., Skulstad H., Uhrheim S., Ihlen H. Regional myocardial function during acute myocardial ischemia assessed by strain Doppler echocardiography. J Am Coll Cardiol. 2001;37:726–730. doi: 10.1016/s0735-1097(00)01160-8. [DOI] [PubMed] [Google Scholar]
- 25.Martin C.R., Johnson C.S., Cobb C., Tatter D., Haywood L.J. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428–432. [PMC free article] [PubMed] [Google Scholar]
- 26.de Montalembert M., Maunoury C., Acar P., Brousse V., Sidi D., Lenoir G. Myocardial ischemia in children with sickle cell disease. Arch Dis Child. 2004;89:359–362. doi: 10.1136/adc.2003.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haywood L.J. Cardiovascular function and dysfunction in sickle cell anemia. J Natl Med Assoc. 2009;101:24–30. doi: 10.1016/s0027-9684(15)30807-5. [DOI] [PubMed] [Google Scholar]
- 28.Vogel M., Anderson L.J., Holden S. Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur Heart J. 2003;24:113–119. doi: 10.1016/s0195-668x(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 29.Dandel M., Hetzer R. Echocardiographic strain and strain rate imaging clinical applications. Int J Cardiol. 2009;132:11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 30.Jones C., Raposo L., Gibson D. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J. 1990;63:215–220. doi: 10.1136/hrt.63.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcos-Alberca P., Garcia-Fernandez M., Ledesma M.J. Intramyocardial analysis of regional systolic and diastolic function in ischemic heart disease with Doppler tissue imaging: role of the different myocardial layers. J Am Soc Echocardiogr. 2002;15:99–108. doi: 10.1067/mje.2002.120634. [DOI] [PubMed] [Google Scholar]


