Abstract
Aims
Evaluation of safety and efficacy of ProNOVA XR, a new generation of polymer-free sirolimus eluting stents (SES), utilizing a pharmaceutical excipient for timed release of sirolimus from the XR platform.
Methods and results
Safety and efficacy of ProNOVA XR coronary stent system was examined in EURONOVA prospective, single arm, multi-center registry of 50 patients with de novo native coronary lesions up to 28 mm in length in arteries between 2.25 and 4 mm.
At 6-month, in-stent late lumen loss by QCA was 0.45 ± 0.41 mm and in-stent neointimal volume obstruction in the IVUS sub-study was 14 ± 11%. One-year clinical follow-up revealed a favorable safety profile, with 2% of in-hospital MACE and 6.4% of MACE from hospital discharge up to 12 months (including 1 cardiac death >30 days after stent implantation and 2 TLRs). According to the ARC definition, there was no definite or probable stent thrombosis and 1 possible stent thrombosis (2%) up to 12 months of clinical follow-up.
Conclusions
In this preliminary evaluation, ProNOVA XR polymer-free sirolimus eluting stent system appeared safe with an early promise of adequate effectiveness in the treatment of de novo coronary lesions in up to 12 months of clinical, angiographic and IVUS follow-up.
Keywords: DES, IVUS, QCA
Abbreviations: PF, polymer-free; EEM, external elastic membrane
1. Introduction
Percutaneous coronary interventions (PCI) with drug eluting stents (DES) are considered most effective and secure way of treatment for de novo single vessel coronary artery disease.1 DESs eluting the two most commonly utilized anti-proliferative medications, paclitaxel and sirolimus, have shown to be overwhelmingly superior to the bare metal stents (BMS) in reducing restenosis and target vessel revascularization, as proven in multiple randomized trials.2–4 However, most of currently used DES employ a polymer coating as a drug carrier, and the permanent presence of these durable polymers has been associated with increased risk of late and very late thrombosis and local inflammatory responses.5–7 On the other hand, the use of bioresorbable polymers has been most recently shown to be associated with decreased definite stent thrombosis when compared to durable polymer DES and lower revascularization rates in bifurcation lesions.8–10 Consequently, these observations have stimulated the development of novel stent systems employing biodegradable polymers as drug carriers, or completely polymer-free DES.11 The ProNOVA XR stent is representative of these new generation polymer-free sirolimus eluting stents (SES). The aim of the present Euronova XR I study was a preliminary assessment of the safety and efficacy of the ProNOVA XR Polymer-Free Drug Eluting Stent System in the treatment of consecutive patients with de novo coronary artery lesions in the real-world use setting.
2. Methods
2.1. Device description
The XR Stent platform is manufactured from an L605 cobalt-chromium alloy, with 65-micron thin stent struts and employs a pharmaceutical excipient – polylactic glycolic acid (PLGA) – for the timed release delivery of sirolimus from the XR stent platform. The formulation of PLGA used in the ProNOVA XR stent was tailored such that the polymer is absorbed once the drug release is completed. Hence the stent is polymer-free upon release of the drug, which is maintained uniformly up to 30 days and after this time less than 25% of the drug remains on the surface of the stent. The release kinetic is presented in Fig. 1.
Fig. 1.
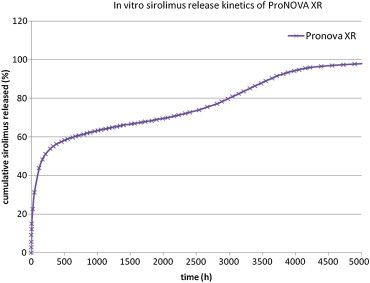
Sirolimus release kinetics of the ProNOVA XR stent.
2.2. Study design and Patient population
EURONOVA XR I Study was a prospective, single arm, multi-center registry evaluating performance, safety and efficacy of the ProNOVA XR DES in the real-world use setting. A total of consecutive 50 patients with de novo native coronary artery lesions, who were admitted for PCI at 4 investigational sites in Poland, were enrolled in the study. This study was conducted in accordance with Declaration of Helsinki and the protocol approval was obtained from the Local Ethics Committee of the Jagiellonian University, Krakow, Poland (Principal Investigator's site). The study was registered in NCT (NCT01151033).
Patients older than 18 years of age, with de novo lesions (no prior stent implant; no brachytherapy), with reference vessel diameter between 2.25 mm and 4.0 mm and target lesion ≤28 mm in length assessed by visual estimate and with evidence of myocardial ischemia (e.g. stable or unstable angina, silent ischemia) were eligible for inclusion provided that written informed consent prior to any study related procedure was obtained from the patient or the patient's legally authorized representative. Exclusion criteria were: other medical illnesses, known history of substance abuse, limited life expectancy <1 year, contraindications to dual antiplatelet therapy, participation in another study, nursing or pregnancy.
2.3. Procedure and medications
Procedural success was defined as successful delivery and deployment of the study stent at the intended target lesion and successful withdrawal of the stent delivery system with attainment of final residual stenosis of less than 50% of the target lesion by QCA (or by visual estimate if QCA was unavailable), without use of a device outside the assigned treatment strategy and without adverse cardiovascular events.
Patients were pretreated with loading doses of aspirin (≥75 mg) and clopidogrel (≥300 mg), between 12 and 6 h prior to the index procedure if possible, followed by maintenance dosages of clopidogrel (75 mg daily) for a minimum of one-year and aspirin (75 mg daily) for a minimum of 5 years. Either unfractionated heparin or bivalirudin were used for procedural anticoagulation. The use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the investigator.
2.4. Data collection and core laboratory analyses
All data were submitted to the independent core laboratory (KCRI, Krakow, Poland). The core laboratory was blinded to the clinical data and procedural information.
Coronary angiograms, obtained at baseline, immediately after the procedure, and at follow-up were digitally recorded and assessed Sanders Data Systems QCAPlus software (Palo Alto, CA, USA). Measurements were performed on the angiograms after maximum vasodilatation with nitroglycerin and the average of multiple projections were analyzed with the help of an automated edge-detection system. The contrast-filled, non-tapered catheter tip was used for calibration (≥6 French guiding catheter).
All angiographic measurements of the target lesion were obtained in the “in-stent” zone, within 5 mm proximal and distal margins to each stent edge, and over the entire segment (“in-segment” zone). Quantitative QCA parameters included the reference vessel diameter (RVD), minimal luminal diameter (MLD), percent diameter stenosis (difference between reference vessel diameter and minimal luminal diameter/reference diameter × 100), and late lumen loss (LLL, difference between minimal luminal diameter after the procedure and minimal luminal diameter at follow-up). Binary restenosis was defined as stenosis of 50% or greater of the minimal luminal diameter in the target lesion.
Intravascular ultrasonographic (IVUS) sub-study included 33 of the 50 enrolled patients. IVUS examinations were performed after stent implantation and at follow-up using the commercially available Atlantis® SR Pro Imaging Catheter (Boston Scientific, Natick, MA, USA). Quantitative analysis, according to the guidelines of the American College of Cardiology (ACC) was performed off-line by two experienced analysts using a dedicated software package echoPlaque 3 (Indec Medical Systems, Santa Clara, CA, USA).12
Quantitative measures obtained every 0.5 mm of the stent length included the external elastic membrane (EEM) (mm2), lumen cross-sectional area (CSA) (mm2), and stent CSA (mm2) in the stented and 5 mm proximal and distal reference segments. Incomplete stent apposition (ISA) was defined as the lack of contact between at least 1 strut and the underlying arterial wall intima that did not overlap a side branch with evidence of blood flow behind the strut. Stent malapposition was defined as a separation of at least 1 stent strut not in contact with the intimal surface of the arterial wall that was not overlapping a side branch, was not present immediately after stent implantation, and had evidence of blood speckling behind the strut.13 Stent expansion index was defined as the ratio of minimal stent CSA divided by the mean proximal and distal reference lumen areas. Stent underexpansion was defined as stent expansion <80% (stent expansion index <0.80). Neointimal area was calculated as stent area minus lumen area. Volumes were calculated by assuming a 0.5 mm thickness of each cross-section with no change in planimetric measurements within that 0.5 mm thickness, and adding the consecutive slices to obtain the volume over the entire stent length.
2.5. Follow-up
Clinical evaluation was scheduled at 1, 6, and 12 months with assessment of angina status, collection of data regarding adverse events, and use of concomitant medications. Follow-up angiography was performed at 6 months. IVUS imaging was obtaining at the 6-month follow-up of 33 patients enrolled to IVUS sub-study. Patients were enrolled to IVUS sub-study in all centers where IVUS imaging was available.
2.6. Study endpoints
The primary endpoint of the study was angiographic in-stent LLL at 6 months after stent implantation. The secondary clinical endpoints were: device success, lesion success, procedural success and assessed at 30 days, 6 and 12 months, target lesion revascularization (TLR), target vessel revascularization (TVR), cardiac death, all deaths (cardiac and non-cardiac), myocardial infarction (Q-wave and non Q-wave), definite, probable, and possible stent thrombosis and occurrence of MACE (defined as composite of death, MI and TLR) or MACCE (defined as composite of cardiac death, MI and TLR). Secondary angiographic endpoints assessed at 6 months follow-up by QCA were: in-stent and in-segment percent diameter stenosis (% DS), in-stent and in-segment binary restenosis rate, in-stent and in-segment MLD. Secondary IVUS endpoints from IVUS sub-study assessed at 6 months follow-up were: in-stent and in-segment LLL, in-stent neointimal volume obstruction, rate of incomplete stent apposition.
2.7. Statistical analysis
Continuous variables were presented as means and standard deviations. A probability <0.05 was considered to be statistically significant. Statistical analysis was performed using the JMP software, version 9.0.0 (SAS Institute Cary, NC, USA).
For angiographic parameters, one sided paired t-test or Wilcoxon signed-rank test (depending on normality) was used. Because measurements were dependent and direction of difference was known, only the significance of observed differences was investigated.
The primary endpoint and all study endpoints were analyzed on the per-treatment evaluable population (patients who had no major protocol deviations). Patients lost to follow-up were not included in the denominator for calculations of binary endpoints.
3. Results
3.1. Procedural results and angiographic outcomes
A total of 50 study patients were randomized and enrolled at 4 investigational sites between January 2009 and February 2010. The demographic characteristics of patients enrolled to Euronova XR I Study are shown in Table 1. Patient flow in the study is presented in Fig. 2. Procedure success was achieved in 49 patients (98%; one patient had clinically indicated in-hospital TLR).
Table 1.
Demographic characteristic of enrolled patients.
| On admission (n = 50) | |
|---|---|
| Age | 63.0 ± 9.25 years |
| Male gender | 39 (78%) |
| Current smokers | 14 (28%) |
| Diabetes | 12 (24%) |
| Hypertension | 45 (90%) |
| Hyperlipidemia | 43 (86%) |
| Prior intervention | 34 (68%) |
| Prior MI | 38 (76%) |
| Stable angina | 37 (74%) |
| Prior PCI | 35 (70%) |
| NSTE ACS | 13 (26%) |
Fig. 2.
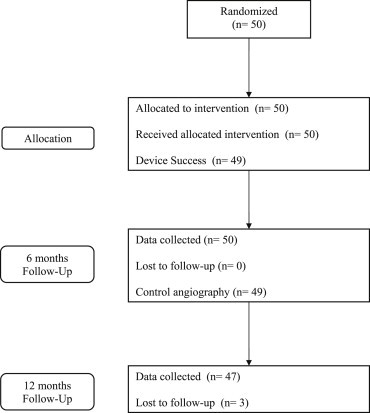
Patient flow in the study.
Results of QCA are shown in Tables 2 and 3. The primary endpoint of the study was an angiographic in-stent LLL at 6 months after stent implantation, which was 0.45 ± 0.41 mm. In-stent binary restenosis was observed in 4.1% of the treated lesions. By IVUS, in 33 patients enrolled to IVUS sub-study, in-stent neointimal volume obstruction at 6 months was 14.11 ± 11.45%. Specific information on other IVUS variables is summarized in Table 4.
Table 2.
Baseline QCA and procedural data.
| Target vessel | n (%) | |
|---|---|---|
| LAD | 23 (44) | |
| Cx | 11 (21) | |
| RCA | 16 (31) | |
| IM | 2 (4) | |
| Lesion class (AHA/ACC) | ||
| A | 7 (13) | 22 (42) |
| B1 | 15 (29) | |
| B2 | 24 (46) | 30 (58) |
| C | 6 (12) | |
| Vessel and lesion | ||
| Reference vessel diameter (mm; ±SD) | 3.00 ± 0.45 | |
| Lesion length (mm; ±SD) | 18.07 ± 9.55 | |
| Minimal Luminal Diameter (mm) | 1.06 ± 0.38 | |
| Diameter stenosis (%) | 65.08 ± 11.34 | |
| Predilatation (n = 32) | ||
| Balloon length (mm; ±SD) | 16.90 ± 3.27 | |
| Balloon diameter (mm; ±SD) | 2.60 ± 0.42 | |
| Implantation pressure (atm; ±SD) | 11.80 ± 1.84 | |
| Stent | ||
| Stent length (mm; ±SD) | 19.76 ± 5.63 | |
| Stent diameter (mm; ±SD) | 3.14 ± 0.34 | |
| Implantation pressure (atm; ±SD) | 14.29 ± 1.55 | |
| No of stents per lesion | 1.13 | |
| Postdilatation (n = 36) | ||
| Balloon length (mm; ±SD) | 13.60 ± 3.48 | |
| Balloon diameter (mm; ±SD) | 3.50 ± 0.43 | |
| Implantation pressure (atm; ±SD) | 16.00 ± 3.09 | |
| Dissection (all) | 8 | |
| Type A | 4 | |
| Type B | 4 | |
| SB closure | 0 | |
| Embolization | 0 | |
Table 3.
Follow-up QCA data.
| Proximal edge (n = 47) | In-stent (n = 49) | Distal edge (n = 49) | In-segment (n = 49) | |
|---|---|---|---|---|
| Reference vessel diameter (mm) | ||||
| After procedure | 2.98 ± 0.37 (n = 52) | |||
| At 6 m FU | 2.93 ± 0.36 (n = 49) | |||
| Minimal luminal diameter (mm) | ||||
| After procedure | 2.71 ± 0.45 | 2.62 ± 0.34 | 2.33 ± 0.42 | 2.28 ± 0.39 |
| At 6 m FU | 2.38 ± 0.47 | 2.16 ± 0.39 | 2.27 ± 0.42 | 2.01 ± 0.39 |
| p value (one sided paired T-test or Wilcoxon signed-rank test, depends on normality) | <0.001 | <0.001 | 0.03396 | <0.001 |
| Late loss (mm) | ||||
| At 6 m FU | 0.33 ± 0.36 | 0.45 ± 0.41 | 0.06 ± 0.36 | 0.28 ± 0.45 |
| Diameter stenosis (% DS) | ||||
| After procedure | 10.51 ± 10.11 | 12.96 ± 9.52 | 22.86 ± 10.26 | 24.37 ± 9.42 |
| At 6 m FU | 19.04 ± 13.76 | 26.20 ± 11.74 | 22.84 ± 11.07 | 31.67 ± 11.80 |
| p value (one sided paired T-test or Wilcoxon signed-rank test, depends on normality) | <0.001 | <0.001 | 0.2852 | 0.000788 |
| Binary restenosis | ||||
| At 6 m FU | 1/47 (2.1%) | 2/49 (4.1%) | 0/49 (0%) | 3/49 (6.1%) |
Table 4.
IVUS outcomes.
| Baseline (n = 33) | 6-Month follow-up (n = 33) | p = | |
|---|---|---|---|
| Vessel volume (mm3) | 300.7 ± 119.9 | 322.5 ± 128.6 | 0.48 |
| Stent volume (mm3) | 136.8 ± 49.0 | 146.8 ± 52.1 | 0.43 |
| Luminal volume (mm3) | 136.8 ± 49.0 | 127.8 ± 48.0 | 0.46 |
| In-stent neo-intimal volume (mm3) | – | 20.4 ± 15.9 | – |
| In-stent volume obstruction (%) | – | 14.11 ± 11.45 | – |
| LA mean ref. proks. (mm2) | 8.24 ± 3.31 | 7.91 ± 2.75 | 0.51 |
| LA mean ref dist (mm2) | 5.94 ± 1.89 | 6.26 ± 2.06 | 0.79 |
| MLA in-stent (mm2) | 5.45 ± 1.46 | 4.68 ± 1.41 | 0.03 |
| MLA in-stent <5.5 mm2 (n =, %) | n = 6, 18.1% | – | – |
| Stent expansion ratio | 0.81 | – | – |
| % Stent expansion <80% (n = , %) | n = 5, 15.2% | – | – |
| %PB ref. proks. (%) | 48.42 ± 11.15 | 50.21 ± 10.00 | |
| %PB ref. dist (%) | 39.81 ± 13.50 | 38.97 ± 12.89 | |
| Edge dissection (n =, %) | n = 3, 9.4% | n = 0, 0% | 0.07 |
| Acute stent malapposition (%) | n = 8, 24.2% | n = 6, 18.1% | 0.55 |
| Late acquired stent malapposition (%) | – | n = 5, 15.2% | – |
3.2. Clinical outcomes
Clinical follow-up revealed a favorable safety profile, with 2% of in-hospital MACE (one urgent repeat PCI 24 h after the procedure due to recurrence of symptoms related to stent edge dissection) and 6.4% of MACE from hospital discharge up to 12 months (including 1 cardiac death and 2 TLRs). One patient was admitted to local hospital 7 months after index procedure due to exacerbation of heart failure and died during that hospitalization. Control coronary angiography was not performed, so this event is classified as possible stent thrombosis. Two other patients were hospitalized due to unstable angina in 5th and 7th month of observation and both of them had restenosis in study stent diagnosed in control coronarography and treated by PCI procedure. Detailed information regarding one-year clinical follow-up is presented in Table 5.
Table 5.
One-year clinical follow-up.
| From admission to 12 month FU (n = 47) | |
|---|---|
| Cardiac death | 1 (2.1%) |
| Myocardial Infarction | 0 |
| Reintervention – TLR | 2 (4.3%) |
| Major adverse cardiac events (defined as: death, myocardial infarction, TLR) | 6.4% (3 – one cardiac death, two TLR) |
| Stent thrombosis (ARC) | |
| Definite | 0 (0%) |
| Probable | 0 (0%) |
| Possible | 1 (2.1%) |
A typical case example is shown in Fig. 3. There is an evident LAD lesion in angiography (1) treated by ProNOVA stent (angiographic and IVUS post-implant results 2, 2a) and seen again at follow-up with favorable outcome (3, 3a, 3b, 3c).
Fig. 3.
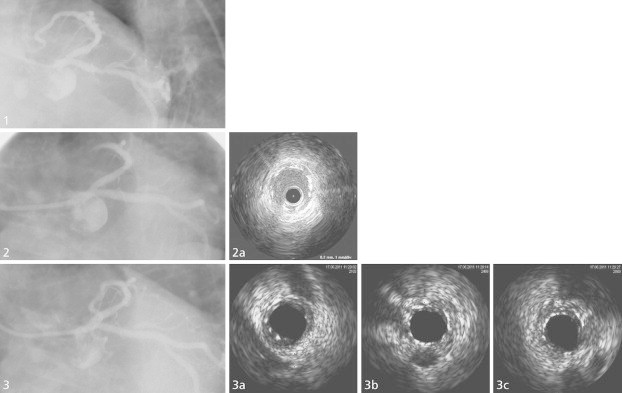
Representative case from the study. PCI of a circumflex artery with critical stenosis in proximal segment before stenting (1), after ProNOVA XR implantation (2 and 2a); and at 6-month angiographic follow-up (3, 3a and 3b).
4. Discussion
This study aimed at establishing a preliminary safety and efficacy profile of a novel polymer-free sirolimus eluting stent ProNOVA XR. In a prospective 4-center registry of patients with de novo lesions followed-up angiographically at 6 months and clinically until 12 months, standard indices of safety and efficacy suggested a favorable response to the new device. The study's primary endpoint of LLL was 0.45 ± 0.41 mm. This represents a reduction from the typical LLL in the bare metal stents, as exemplified by the SIRIUS pivotal trial of the sirolimus eluting stent (Cypher) which showed 1.00 ± 0.70 mm LLL in the control arm.14 Such in-stent LLL is comparable to that reported across different studies of paclitaxel-eluting stents in the total of 2692 patients (0.40 mm) in a recent meta-analysis.15 It also appears somewhat lower than the average calculated for the zotarolimus-eluting stents (0.56 mm), and similar to that reported in the “other” category (0.46 mm) in the same meta-analysis (this category consisted of pooled 919 patients who were treated with Yukon polymer-free sirolimus containing stent), with or without estrogen coating, biodegradable sirolimus polymer stent and Costar (absorbable polymer eluting paclitaxel).
Of note, despite the LLL being numerically higher than that consistently reported for the durable polymer-based sirolimus eluting stents such as Cypher, or for newer everolimus-eluting stents (Xience/Promus), the TLR remained low at 4%. When events related to the target vessel are summarized, the target vessel failure (not originally set as an endpoint per protocol) would be 10.6% (5/47 patients), of which 2 were TLR, two were TVR and one death which occurred at 197 days in a remote hospital and based on limited documentation was adjudicated as possible stent thrombosis, however, autopsy was not performed. These rates are similar to those reported in the 1-year follow-up of the SIRIUS trial (TLR 4.9%, target vessel failure 9.8%).16
When compared with other studies of polymer-free sirolimus eluting stent, the ProNOVA XR appeared similar in outcomes. Slightly higher LLL was reported for ChoiceDES in ISAR-TEST 1 in 9-month FU (0.48 ± 0.61 mm),17 and lower in subsequent ISAR-TEST 2 (0.23 ± 0.50 mm).18 Hydroxyapatite-based release of sirolimus from the VESTAsync system demonstrated LLL of 0.36 ± 0.23 mm.19 However, in terms of clinical outcomes, the ProNOVA XR stent demonstrated lower TLR than other investigated polymer-free platforms, 4.2% in comparison to 9.3% and 6.8% achieved in ISAR-TEST 1 and ISAR-TEST 2, respectively. Similarly, percentage of in-segment binary restenosis was lower than in the ISAR studies – 6.1% for ProNOVA and from 11% up to 14.2% for ISAR-TEST 2 and 1 respectively. In all these 3 studies, the investigated population had similar distribution of lesion types, with noticeable predominance of more complex lesion (B2 and C over 50%), and the present study featured longer baseline lesion length than the 3 comparator studies, which is a predisposing factor for DES restenosis.20
4.1. Limitations of the study
The study was non-randomized, without a control comparator treatment and in a limited number of patients. Also, given the theoretical benefit of a better artery healing in absence of permanent polymer coating on our investigative stent, it would also require a longer follow-up to more adequately examine the possible positive impact of the particular stent design on the adverse clinical events such as stent thrombosis and possibility to shorten the antiplatelet therapy. However, it is customary for a first-in-man study to have this feasibility single arm design and expand to larger randomized investigation if the initial results are positive.
In summary, even though the study sample was small, results of this preliminary study demonstrate that the polymer-free sirolimus eluting ProNOVA XR stent system is safe, with outcomes similar to other polymer-free drug eluting stents. This preliminary assessment encourages further investigation.
Conflicts of interest
All authors have none to declare.
Footnotes
Clinical Study was performed in Department of Interventional Cardiology, Jagiellonian University, Krakow, Poland, in Department of Invasive Cardiology, Electrotherapy and Angiology, Intercard, Nowy Targ, Poland, in Department of Invasive Cardiology, Electrotherapy and Angiology, Intercard, Nowy Sącz, Poland and in Krakow Cardiovascular Research Institute, Krakow, Poland.
References
- 1.Maisel W.H. Unanswered questions – drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–984. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 2.Spaulding C., Daemen J., Boersma E., Cutlip D.E., Serruys P.W. A pooled analysis of data comparing sirolimus-eluting stents with bare metal stents. N Engl J Med. 2007;356:989–997. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]
- 3.Stone G.W., Moses J.W., Ellis S.G. Safety and efficacy of sirolimus and paclitaxel-eluting stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 4.Stettler C., Wandel S., Allemann S. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 5.Feres F., Costa J.R., Jr., Abizaid A. Very late thrombosis after drug-eluting stents. Catheter Cardiovasc Interv. 2006;68:83–88. doi: 10.1002/ccd.20692. [DOI] [PubMed] [Google Scholar]
- 6.Pfisterer M., Brunner-La Rocca H.P., Buser P.T. BASKET-LATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Daemen J., Wenaweser P., Tsuchida K. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 8.Byrne R.A., Kastrati A., Massberg S. ISAR-TEST 4 Investigators. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2011;58:1325–1331. doi: 10.1016/j.jacc.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Chevalier B., Serruys P.W., Silber S. Randomised comparison of Nobori, biolimus A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the Nobori 1 trial. EuroIntervention. 2007;2:426–434. [PubMed] [Google Scholar]
- 10.Garg S., Wykrzykowska J., Serruys P.W. The outcome of bifurcation lesion stenting using a biolimus-eluting stent with a bio-degradable polymer compared to a sirolimus-eluting stent with a durable polymer. EuroIntervention. 2011;6:928–935. doi: 10.4244/EIJV6I8A162. [DOI] [PubMed] [Google Scholar]
- 11.Abizaid A., Ribamar Costa J., Jr. New drug-eluting stents an overview on biodegradable and polymer-free next-generation stent systems. Circ Cardiovasc Interv. 2010;3:384–393. doi: 10.1161/CIRCINTERVENTIONS.109.891192. [DOI] [PubMed] [Google Scholar]
- 12.Mintz G.S., Nissen S.E., Anderson W.D. ACC Clinical Expert Consensus Document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS) J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 13.Kang S.J., Mintz G.S., Park D.W. Late and very late drug-eluting stent malapposition, serial 2-Year quantitative IVUS analysis. Circ Cardiovasc Interventions. 2010;3:335–340. doi: 10.1161/CIRCINTERVENTIONS.109.916502. [DOI] [PubMed] [Google Scholar]
- 14.Moses J.W., Leon M.B., Popma J.J., SIRIUS Investigators Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 15.Brener S.J., Prasad A.J., Khan Z., Sacchi T.J. The relationship between late lumen loss and restenosis among various drug-eluting stents: a systematic review and meta-regression analysis of randomized clinical trials. Atherosclerosis. 2011;214:158–162. doi: 10.1016/j.atherosclerosis.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Holmes D.R., Jr., Leon M.B., Moses J.W. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109:634–640. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]
- 17.Mehilli J., Kastrati A., Wessely R. Randomized trial of a nonpolymer-based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late lumen loss. Circulation. 2006;113:273–279. doi: 10.1161/CIRCULATIONAHA.105.575977. [DOI] [PubMed] [Google Scholar]
- 18.Byrne R.A., Mehilli J., Iijima R. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30:923–931. doi: 10.1093/eurheartj/ehp044. [DOI] [PubMed] [Google Scholar]
- 19.Costa J.R., Jr., Abizaid A., Costa R. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Interv. 2009;2:422–427. doi: 10.1016/j.jcin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Stolker J.M., Kennedy K.F., Lindsey J.B. Predicting restenosis of drug-eluting stents placed in real-world clinical practice: derivation and validation of a risk model from the EVENT registry. Circ Cardiovasc Interv. 2010;3:327–334. doi: 10.1161/CIRCINTERVENTIONS.110.946939. [DOI] [PubMed] [Google Scholar]


