Abstract
Objectives
We obtained longitudinal, radial and circumferential strains in patients with dengue hemorrhagic fever (DhF) and thrombocytopenia using two-dimensional (2D) speckle tracking echocardiography to analyze left ventricular (LV) myocardial performance.
Methods
In this prospective study, 2D echocardiographic images of the left ventricle in the four-, three- and two-chamber views and parasternal short-axis views at the basal, mid and apical levels were obtained in 40 subjects: 20 patients (23 ± 8 years, 12 male) with DhF and thrombocytopenia and 20 healthy controls (23 ± 5 years, 11 male). Of the 20 patients, imaging was performed again in 19 at discharge after a hospital stay of 8 ± 1 days. Longitudinal, circumferential and radial strains were quantified and compared in an 18-segment model using a novel speckle tracking system.
Results
Left ventricular global ejection fraction was reduced in patients with DhF at presentation as compared with controls (51.25 ± 0.96% vs. 59.32 ± 1.26%; p = 0.032). Peak longitudinal strain in patients with DhF was significantly attenuated in the subendocardial region compared with normal controls (p < 0.001). A significant increase in circumferential strain for patients with DhF was evident only in the subepicardial region (p = 0.009). Patients with DhF showed significantly higher radial strain than controls (p < 0.001). On multivariate analysis, subendocardial longitudinal strain independently predicted the duration of hospital stay in patients with DhF.
Conclusion
Assessment of speckle tracking echocardiography-derived LV mechanics helps in understanding myocardial mechanics in patients with DhF and thrombocytopenia. Identification of reduced LV longitudinal strain helps in understanding the mechanism of reduced LV myocardial performance seen in patients with DhF.
Keywords: Dengue hemorrhagic fever, Speckle tracking echocardiography, Left ventricular function, Thrombocytopenia
1. Introduction
Dengue hemorrhagic fever (DhF) is one of the most common tropical diseases worldwide and globally associated with significant morbidity and mortality.1 It is caused by a flavivirus transmitted to humans by infected Aedes aegypti mosquitoes.2 Cardiac involvement in DhF has been reported3,4 but not studied adequately. The various cardiac conditions reported in patients with DhF are atrial fibrillation,5 heart block and left ventricular (LV) systolic and diastolic impairment.4 Left ventricular dysfunction has been reported to be transient as assessed by LV ejection fraction.6 For assessment of LV myocardial performance, ejection fraction, tissue Doppler imaging, Doppler strain and two-dimensional (2D) strain have been used widely.7,8 Subclinical changes in LV function can be identified by quantifying myocardial strain, a dimensionless measurement of deformation expressed as a fractional or percentage change from an object's original dimension. Two-dimensional speckle tracking echocardiography has recently emerged as a novel technique for rapid offline and bedside analysis of LV strains in the longitudinal, radial and circumferential directions.9–12 The modality is used to analyze myocardial motion as it tracks natural acoustic reflections and interference patterns of 2D echocardiographic images, and has been validated with measurements obtained by sonomicrometry and magnetic resonance imaging. Assessment of myocardial mechanics affecting LV function in patients with DhF and thrombocytopenia has not been studied in detail. The aim of this study was to assess the ability of using subtle differences in LV longitudinal, radial and circumferential strains to characterize features of LV dysfunction in patients with DhF and thrombocytopenia.
2. Methods
2.1. Study population
This was a prospective study conducted at a tertiary care hospital in central India from June to December 2011. The study population comprised 20 consecutive patients (23 ± 8 years, 12 male) seropositive for DhF presenting with thrombocytopenia (platelet count: 45,000 ± 6000 per μL) and 20 age- and sex-matched healthy volunteers from the community who acted as controls (23 ± 5 years, 11 male). Echocardiography was performed both at presentation and discharge after a hospital stay of 8 ± 1 days. One patient died in the hospital. Patients with established coronary artery disease with evidence of regional or global wall motion abnormalities, atrial fibrillation, valvular heart disease, diabetes mellitus, chronic obstructive airway disease and hypertrophic cardiomyopathy were excluded. Written informed consent was obtained from the patients and controls, and the study was approved by the institutional review board. The flow of participants through all stages of the study is depicted in Fig. 1. Patients were treated with intravenous fluids, platelet concentrate infusions according to platelet levels and supportive therapy. Patients were considered for discharge when their condition was stable, they were afebrile and platelet count was more than 150,000/μL.
Fig. 1.
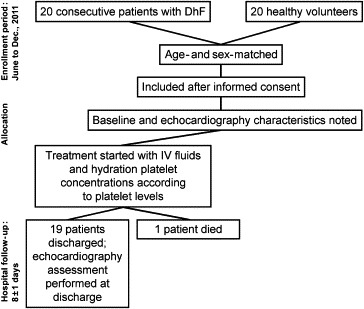
CONSORT diagram showing the flow of participants through each stage of the study. DhF: dengue hemorrhagic fever, IV: intravenous.
2.2. Echocardiography
A comprehensive transthoracic echocardiography examination was performed for all patients with DhF and thrombocytopenia at presentation and discharge (excluding the one patient who died) and members of the control group. Commercially available ultrasound transducer and equipment (S4-2 probe, HD7 ultrasound system, Philips Healthcare) were used. The same experienced operator performed all acquisitions with the patients in the left lateral position. Basic measurements included LV wall thickness by M-mode, LV diameter by 2D echocardiography and mitral inflow velocities by tissue Doppler imaging with settings per recommendations by the American Society of Echocardiography.13 Left ventricular volumes and LV ejection fraction were measured using the biplane method (modified Simpson's rule) as recommended by the American Society of Echocardiography.14 To determine the timing of cardiac events, mitral inflow and LV outflow were recorded using pulsed Doppler echocardiography. Two-dimensional echocardiographic images of the LV in the four-, three- and two-chamber views and parasternal short-axis views at the basal, mid and apical levels were acquired with same ultrasound machine. Three consecutive cardiac cycle loops were recorded at end expiration. The frame rate was kept between 70 and 100 Hz.15 Longitudinal, circumferential and radial strains were quantified in an 18-segment model using a novel speckle tracking system (2D Cardiac Performance Analysis, TomTec Imaging Systems, Munich, Germany). The system is a speckle tracking-based tool that can analyze 2D data from various ultrasound machines and is an extension of velocity vector imaging software, which has been previously validated with sonomicrometry8,9 and magnetic resonance imaging.10,11 The system, similar to velocity vector imaging, determines myocardial motion from a user-defined tracing along the endocardial border. Endocardial and automated subepicardial borders were traced throughout one cardiac cycle by successive application of a series of tracking steps (Fig. 2). Subendocardial and subepicardial longitudinal systolic strains were obtained from apical four-, three- and two-chamber views. Circumferential and radial strains were obtained from 6 segments in short-axis views of the LV. These images were then analyzed offline. The intra-observer variability for endocardial and epicardial longitudinal strains, endocardial and epicardial circumferential strains and radial strain by this method have been previously reported as 10 ± 7%, 8 ± 7%, 11 ± 10%, 25 ± 22%, and 24 ± 20%, respectively.16 Also interobserver variability reported for the same measurements were −13.6 ± 6.3%, −12.6 ± 7.9%, 16 ± 15%, 26 ± 21%, and 28 ± 29%, respectively.16
Fig. 2.
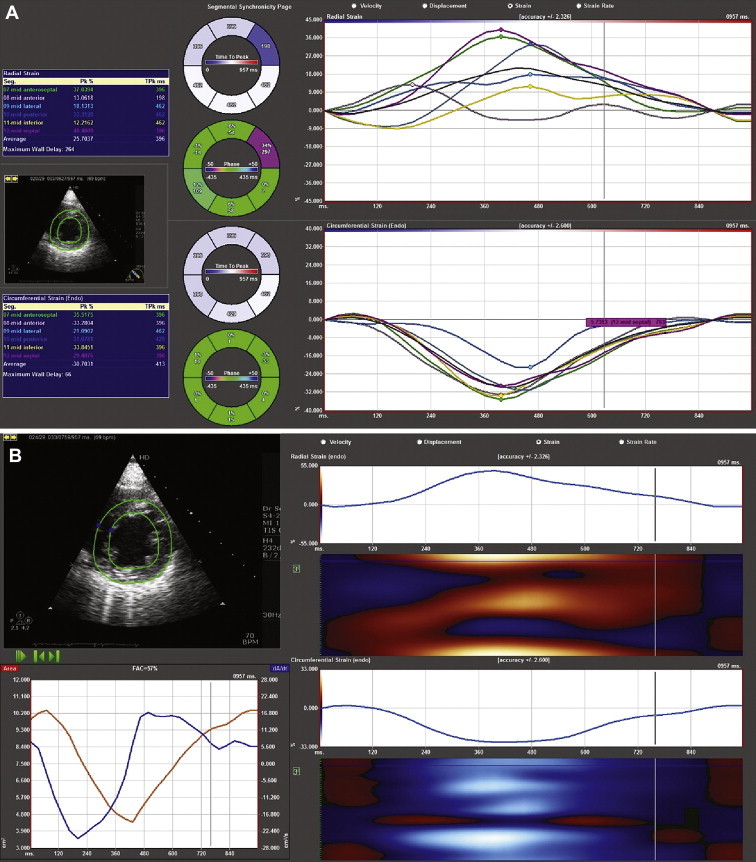
Two screen shots (A and B) showing the tracking of the endocardial border in the short-axis view to obtain strain values.
2.3. Statistics
Continuous variables were expressed as mean ± standard deviation. The differences between groups were analyzed using independent Student's t-tests (MedCalc 11.2, MedCalc Software Mariakerke, Belgium). Correlations between variables were determined using Pearson or Spearman correlation tests where appropriate. A p-value <0.05 was considered significant. Statistical significance was tested at 0.016 using one-way ANOVA and after applying Bonferroni multiple testing correction to control type 1 error. Univariate regression models were used to examine relationships between echocardiographic variables and duration of hospital stay. Multiple regressions with the investigator variable (duration of hospital stay) and echocardiographic strain variables as forced entry variables were used for the assessment of adjusted associations.
3. Results
The baseline clinical and echocardiography characteristics are shown in Table 1. There were no significant differences in age and gender between patients with DhF and controls. At presentation, patients with DhF had significantly larger LV end-systolic diameters (p = 0.009), which decreased to nonsignificant diameters at discharge as compared with the control group. There was no significant difference in LV size in diastole between the three groups. Patients with DhF at presentation had grade III diastolic dysfunction (ratio of early and late diastolic velocities [E/A] = 2.01 ± 12.13), which improved to grade II diastolic dysfunction (E/A = 1.36 ± 4.96) at discharge. However, there was no difference in the ratio of mitral early inflow velocity to early tissue Doppler annular velocity (E/e') between the three groups. Also, patients with DhF and thrombocytopenia had significantly larger left atrial volumes at discharge than the control group (p < 0.001). Left ventricular global ejection fraction was reduced in patients with DhF at presentation as compared with controls, although the difference was not statistically significant (51.25 ± 0.96 vs. 59.32 ± 1.26; p = 0.032). Left ventricular ejection fraction was improved at discharge.
Table 1.
Baseline and echocardiographic characteristics of healthy controls and patients with dengue hemorrhagic fever.
| Characteristic | Control (20) | DhF (20) |
p-value | Statistical significance* | |
|---|---|---|---|---|---|
| At presentation (20) | At discharge (19) | ||||
| Age (years) | 23.2 ± 5.38 | 23.38 ± 8.26 | 23.62 ± 7.82 | – | – |
| Men/Women (no.) | 11/9 | 12/8 | 11/8 | – | – |
| Height (cm) | 163.11 ± 12.29 | 156 ± 10.20 | 154 ± 9.86 | – | – |
| Weight (kg) | 58.33 ± 8.69 | 51.44 ± 8.84 | 51.38 ± 8.71 | 0.017 | NS |
| Heart rate (bpm) | 71.2 ± 10.7 | 92.4 ± 11.3 | 80.9 ± 18.8 | 0.012 | S |
| Systolic blood pressure (mmHg) | 120.0 ± 6.0 | 102.86 ± 4.86 | 104.34 ± 4.39 | 0.081 | NS |
| Diastolic blood pressure (mmHg) | 78.0 ± 4.0 | 75.2 ± 5.14 | 75.88 ± 4.92 | 0.462 | NS |
| Aorta (cm) | 2.36 ± 0.1 | 2.56 ± 0.29 | 2.52 ± 0.26 | 0.098 | NS |
| Left atrium (cm) | 2.63 ± 0.1 | 3.06 ± 0.48 | 2.98 ± 0.39 | 0.000 | S |
| LV end-diastolic dimension (cm) | 4.36 ± 0.14 | 4.33 ± 0.52 | 4.34 ± 0.58 | 0.870 | NS |
| LV end-systolic dimension (cm) | 2.75 ± 0.08 | 3.12 ± 0.49 | 2.76 ± 0.80 | 0.009 | S |
| Left atrial volume (cm3) | 23.3 ± 2.1 | 35.3 ± 3.2 | 34.2 ± 2.3 | 0.000 | HS |
| Global ejection fraction (%) | 59.32 ± 1.26 | 51.25 ± 0.96 | 56.34 ± 0.62 | 0.032 | NS |
| E (cm/s) | 72.2 ± 1.84 | 115.41 ± 15.27 | 93.65 ± 15.73 | 0.000 | HS |
| A (cm/s) | 63.6 ± 2.35 | 60.19 ± 17.34 | 58.12 ± 12.81 | 0.444 | NS |
| E/A | 1.16 ± 1.35 | 2.01 ± 12.13 | 1.36 ± 4.96 | 0.000 | HS |
| E/e′ | 6.75 ± 6.13 | 8.34 ± 4.23 | 9.77 ± 4.66 | 0.023 | NS |
| S′ (septal) (cm/s) | 13.47 ± 0.5 | 8.42 ± 1.62 | 9.59 ± 1.58 | 0.000 | HS |
| S′ (lateral) (cm/s) | 14.12 ± 0.56 | 12.48 ± 2.57 | 14.57 ± 2.09 | 0.024 | NS |
Values are mean ± standard deviation or p-value.
*Statistical significance was tested at 0.016 using one-way ANOVA and after applying Bonferroni multiple testing correction. Bolded are the two-dimensional echocardiographic parameters considered for multiple testing correction. S: significant, NS: not significant, HS: highly significant.
A: peak late diastolic velocity, DhF: dengue hemorrhagic fever, E: peak early diastolic velocity, e′: tissue Doppler early diastolic velocity, E/A: ratio of early and late diastolic velocities, E/e′: ratio of mitral early inflow velocity to early tissue Doppler annular velocity, LV: left ventricular, S′: peak systolic velocity of the mitral annular longitudinal movement.
Peak longitudinal strain in patients with DhF with thrombocytopenia was significantly attenuated in the subendocardial region compared with normal controls (−15.38 ± 5.18 vs. −20.17 ± 5.48, p < 0.001) (Table 2). The patients with DhF had significantly increased circumferential strain in the subepicardial region than controls (−12.75 ± 3.54 vs. −6.47 ± 1.80, p = 0.009). Patients with DhF and thrombocytopenia showed significantly higher radial strain than controls (36.86 ± 17.27 vs. 21.97 ± 10.37, p < 0.001). As LV strains are known to be load dependent, LV longitudinal strain was indexed to LV end-diastolic length obtained in the apical four-chamber view. Radial strain was indexed to LV end-diastolic length in the minor axis. Circumferential strain could not be indexed as it was difficult to identify perpendicular LV length to circumferential fibers in the 2D plane. We could identify a similar level of significance in LV longitudinal and radial strains as seen in nonindexed values (Table 3).
Table 2.
Comparison of left ventricular mechanics between healthy controls and patients with dengue hemorrhagic fever.
| Characteristic | Control group (20) | DhF (20) |
p-value | Statistical significance* | |
|---|---|---|---|---|---|
| At presentation (20) | At discharge (19) | ||||
| Longitudinal strain | |||||
| Subendocardial (%) | −20.17 ± 5.48 | −15.38 ± 5.18 | −19.24 ± 2.98 | <0.001 | HS |
| Subepicardial (%) | −17.68 ± 4.95 | −18.26 ± 5.43 | −17.30 ± 3.05 | 0.257 | NS |
| Circumferential strain | |||||
| Subendocardial (%) | −23.98 ± 8.08 | −22.18 ± 4.34 | −21.82 ± 5.17 | 0.414 | NS |
| Subepicardial (%) | −6.47 ± 1.80 | −12.75 ± 3.54 | −10.74 ± 2.81 | 0.009 | S |
| Radial (%) | 21.97 ± 10.37 | 36.86 ± 17.27 | 24.11 ± 5.98 | <0.001 | HS |
Values are mean ± stand deviation or p-value.
*Statistical significance was tested at 0.016 using one-way ANOVA and after applying Bonferroni multiple testing correction. DhF: dengue hemorrhagic fever, S: significant, NS: not significant, HS: highly significant.
Table 3.
Comparison of LV mechanics indexed to LV end-diastolic dimension between healthy controls and patients with DhF.
| Characteristic | Control group (20) | DhF (20) |
p-value | Statistical significance* | |
|---|---|---|---|---|---|
| At presentation (20) | At discharge (19) | ||||
| Longitudinal strain | |||||
| Subendocardial (%/cm) | −4.58 ± 1.48 | −3.46 ± 1.16 | −4.34 ± 0.78 | <0.001 | HS |
| Subepicardial (%/cm) | −4.03 ± 1.36 | −4.19 ± 1.13 | −4.09 ± 0.95 | 0.413 | NS |
| Radial (%/cm) | 5.11 ± 0.86 | 8.16 ± 3.29 | 5.32 ± 1.28 | <0.001 | HS |
Values are mean ± stand deviation or p-value.
*Statistical significance was tested at 0.016 using one-way ANOVA and after applying Bonferroni multiple testing correction. DhF: dengue hemorrhagic fever, LV: left ventricular, NS: not significant, HS: highly significant.
Impact of the five echocardiographic strain parameters, henceforth referred to as independent variables, on duration of hospital stay was studied using data from both groups. Univariate regression analysis revealed that only subendocardial longitudinal strain had a significant positive influence on the duration of hospital stay in patients with DhF (p < 0.05, r = 0.48) (Table 4).
Table 4.
Univariate regression with duration of hospital stay.
| Echocardiographic parameter (variable) | b | SE (b) | p-value | Adj R-square |
|---|---|---|---|---|
| SL Endo | −1.232 | 0.223 | 1.78E-06 | 0.48 |
| SL Epi | −0.434 | 0.183 | 0.038 | 0.09 |
| SL Rad | 0.096 | 0.037 | 0.238 | 0.03 |
| S Endo | −0.338 | 0.286 | 0.09 | 0.05 |
| S Epi | 0.462 | 0.403 | 0.211 | 0.03 |
S Endo: subendocardial circumferential strain, S Epi: subepicardial circumferential strain, SL Endo: subendocardial longitudinal strain, SL Epi: subepicardial longitudinal strain, SL Rad: radial strain.
Multivariate regression analysis was performed to consider all echocardiographic strain variables to predict duration of hospital stay. To determine if any co-linearity existed between independent variables, a correlation matrix with simple Pearson's correlation coefficient was created. The five echocardiographic parameters (Table 5) in the model resulted in an adjusted R-square value of 0.64, indicating a reasonably good linear correlation. The coefficient of subendocardial longitudinal strain showed a significant impact (p = 0.009) on duration of hospital stay with a coefficient value of −1.379. There was no significant correlation between other strain parameters and duration of hospital stay in multivariate analysis.
Table 5.
Multiple regression with duration of hospital stay.
| Echocardiographic parameter | b | SE (b) | p-value |
|---|---|---|---|
| SL Endo | −1.379 | 0.453 | 0.009 |
| SL Epi | 0.036 | 0.378 | 0.719 |
| SL Rad | 0.119 | 0.031 | 0.062 |
| S Endo | −0.145 | 0.211 | 0.085 |
| S Epi | 0.348 | 0.266 | 0.096 |
| R-square | 0.692 | ||
| Adj R-square | 0.646 | ||
| Model p-value | 0.000 | ||
S Endo: subendocardial circumferential strain, S Epi: subepicardial circumferential strain, SL Endo: subendocardial longitudinal strain, SL Epi: subepicardial longitudinal strain, SL Rad: radial strain.
4. Discussion
The first report of cardiac involvement in DhF was reported in 1972 by Obeyesekere and Hermon.17 They reported evidence of arrhythmia and depressed LV function during an epidemic of DhF. In the present study, conventional echocardiographic findings revealed increased LV dimensions in systole and left atrial volumes in patients with DhF presenting with thrombocytopenia and at the time of discharge. The findings in the present study coincide with findings in earlier studies in which larger left atrial volumes in patients with DhF suggested evidence of diastolic dysfunction.4 Salgado et al18 also reported evidence of diastolic dysfunction in patients with acute phase DhF. The filling pressures were significantly higher in patients with DhF presenting with thrombocytopenia and at the time of discharge. To our knowledge, filling pressure obtained by echocardiography has not been evaluated prior to the acute phase of DhF.
In the present study, LV ejection fraction obtained using conventional echocardiography measurements in patients with DhF presenting with thrombocytopenia was significantly reduced as compared with controls. Left ventricular ejection fraction recovered at the time of discharge. Similar findings have been reported in previous studies.3,19 The postulated underlying mechanisms for reduced LV ejection fraction are immune in origin, although myocarditis may be a contributory factor.20 Salgado et al18 postulated that the mechanism of cardiac dysfunction is direct infection of cardiac muscle cells by dengue virus.
Conventional 2D, M-mode and spectral Doppler echocardiography have several limitations in identifying and measuring early ventricular dysfunction. For example, measuring LV fractional shortening and LV ejection fraction are relatively simple, but inter- and intra-observer variability are high. Also, global LV ejection fraction and fractional shortening only reflect global cardiac contractility and do not take regional systolic abnormalities into consideration. We undertook this study to explain the need for understanding the myocardial mechanical properties in patients with DhF.
Global LV dysfunction has been reported previously in DhF with thrombocytopenia.19,21,22 Also, LV dysfunction occurs during acute phase of DhF and normalizes rapidly at the time of recovery3,23 as was seen in the present study. Left ventricular regional myocardial function in DhF has not been reported previously in the literature and, to our knowledge, this is the first comprehensive study to assess LV regional myocardial deformation properties in these patients.
In the present study, patients with DhF had significantly lower strain parameters in the subendocardial longitudinal segment as assessed by 2D speckle tracking echocardiography. This finding has not been reported in previous studies. This longitudinal fiber shortening normalized at the time of discharge as there was no significant difference as compared with the control group. The subendocardial longitudinally oriented myocardial fibers have shown to be particularly vulnerable to ischemia, leading to a dominant decrease in shortening in the longitudinal axis.24,25 Patients with DhF showed significantly higher radial thickening than the control and follow-up groups. Also, a significant reduction in circumferential strain was evident only in the subepicardial region in both patients with DhF presenting with thrombocytopenia and at discharge. This phenomenon may be helpful in explaining the compensatory mechanism produced by circumferential and radial fibers to maintain LV systolic function in this group.
Di Bella et al reported diffuse impairment of longitudinal strain and patchy reduction in circumferential epicardial strain in patients with myocarditis.26 Also, reversible cardiac dysfunction demonstrated by echocardiography has been reported in patients of H1NI influenza.27 Our results report for the first-time LV strain parameters in DhF.
4.1. Limitations
The present study is a single-center observational study. The size of the studied population is relatively small. Despite this limitation, the present data allow for the identification of both significant geometric and hemodynamic changes during DhF. Also, faster heart rates seen during DhF are not considered ideal for speckle tracking echocardiography. This is a true limitation of the study as the aim was to identify the LV myocardial performance by speckle tracking echocardiography during DhF.
5. Conclusion
Assessment of speckle tracking echocardiography-derived LV mechanics helps in understanding the myocardial mechanics in patients with DhF presenting with thrombocytopenia. Identification of reduced LV longitudinal strain helps in understanding the mechanism of reduced LV myocardial performance seen in patients with DhF.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors gratefully acknowledge Joe Grundle and Katie Klein of Aurora Cardiovascular Services for editorial preparation of the manuscript and Brian Miller and Brian Schurrer of Aurora Sinai Medical Center for their help with figures.
References
- 1.Karoli R., Fatima J., Siddiqi Z. Clinical profile of dengue infection at a teaching hospital in North India. J Infect Dev Ctries. 2012;6:551–554. doi: 10.3855/jidc.2010. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira M.G., Barreto M.L. Diagnosis management of dengue. BMJ. 2009;339:b4338. doi: 10.1136/bmj.b4338. Erratum in: BMJ 2009;339:b5522. [DOI] [PubMed] [Google Scholar]
- 3.Wali J.P., Biswas A., Chandra S. Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol. 1998;64:31–36. doi: 10.1016/s0167-5273(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub S., Griffiths A., Chau T.T. Cardiac function in Vietnamese patients with different dengue severity grades. Crit Care Med. 2012;40:477–483. doi: 10.1097/CCM.0b013e318232d966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmod M., Darul N.D., Mokhtar I. Atrial fibrillation as a complication of dengue hemorrhagic fever: non-self-limiting manifestation. Int J Infect Dis. 2009;13:e316–e318. doi: 10.1016/j.ijid.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Khongphatthanayothin A., Lertsapcharoen P., Supachokchaiwattana P. Myocardial depression in dengue hemorrhagic fever: prevalence and clinical description. Pediatr Crit Care Med. 2007;8:524–529. doi: 10.1097/01.PCC.0000288672.77782.D4. [DOI] [PubMed] [Google Scholar]
- 7.Marwick T.H. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G. Response to “non-Doppler two-dimensional strain imaging by echocardiography-from technical considerations to clinical applications”. J Am Soc Echocardiogr. 2007;20:1020. doi: 10.1016/j.echo.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Reisner S.A., Lysyansky P., Agmon Y. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Helle-Valle T., Crosby J., Edvardsen T. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 11.Rovner A., de las Fuentes L., Waggoner A.D. Characterization of left ventricular diastolic function in hypertension by use of Doppler tissue imaging and color M-mode techniques. J Am Soc Echocardiogr. 2006;19:872–879. doi: 10.1016/j.echo.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Nakai H., Takeuchi M., Nishikage T. Effect of aging on twist-displacement loop by 2-dimensional speckle tracking imaging. J Am Soc Echocardiogr. 2006;19:880–885. doi: 10.1016/j.echo.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Gottdiener J.S., Bednarz J., Devereux R. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Mor-Avi V., Lang R.M., Badano L.P. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 16.Caracciolo G., Eleid M.F., Abe H. Non-uniform recovery of left ventricular transmural mechanics in ST-segment elevation myocardial infarction. Cardiovasc Ultrasound. 2010;8:31. doi: 10.1186/1476-7120-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeyesekere I., Hermon Y. Myocarditis and cardiomyopathy after arbovirus infections (dengue and chikungunya fever) Br Heart J. 1972;34:821–827. doi: 10.1136/hrt.34.8.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado D.M., Eltit J.M., Mansfield K. Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J. 2010;29:238–242. doi: 10.1097/INF.0b013e3181bc3c5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabra S.K., Juneja R., Madhulika Myocardial dysfunction in children with dengue haemorrhagic fever. Natl Med J India. 1998;11:59–61. [PubMed] [Google Scholar]
- 20.Lateef A., Fisher D.A., Tambyah P.A. Dengue and relative bradycardia. Emerg Infect Dis. 2007;13:650–651. doi: 10.3201/eid1304.061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R., Kapoor S., Nagar R. A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow. India Southeast Asian J Trop Med Public Health. 1999;30:735–740. [PubMed] [Google Scholar]
- 22.Kalayanarooj S., Vaughn D.W., Nimmannitya S. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 23.Malheiros S.M., Oliveira A.S., Schmidt B. Muscle biopsy findings in 15 patients. Arq Neuropsiquiatr. 1993;51:159–164. doi: 10.1590/s0004-282x1993000200001. [DOI] [PubMed] [Google Scholar]
- 24.Jones C.J., Raposo L., Gibson D.G. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J. 1990;63:215–220. doi: 10.1136/hrt.63.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcos-Alberca P., García-Fernández M.A., Ledesma M.J. Intramyocardial analysis of regional systolic and diastolic function in ischemic heart disease with Doppler tissue imaging: role of the different myocardial layers. J Am Soc Echocardiogr. 2002;15:99–108. doi: 10.1067/mje.2002.120634. [DOI] [PubMed] [Google Scholar]
- 26.Di Bella G., Gaeta M., Pingitore A. Myocardial deformation in acute myocarditis with normal left ventricular wall motion – a cardiac magnetic resonance and 2-dimensional strain echocardiographic study. Circ J. 2010;74:1205–1213. doi: 10.1253/circj.cj-10-0017. [DOI] [PubMed] [Google Scholar]
- 27.Martin S.S., Hollingsworth C.L., Norfolk S.G. Reversible cardiac dysfunction associated with pandemic 2009 influenza A (H1N1) Chest. 2010;137:1195–1197. doi: 10.1378/chest.10-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]


